Digestate as Sustainable Nutrient Source for Microalgae—Challenges and Prospects
Abstract
:Featured Application
Abstract
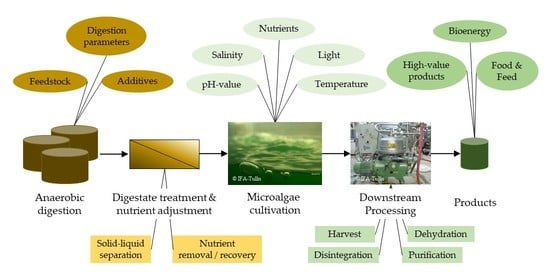
1. Introduction
2. Cultivation Conditions
2.1. Temperature, pH, and Salinity
2.2. Light
2.3. Carbon Sources
2.4. Nutrient Requirements
2.5. Digestate as Nutrient Source
3. Origin of Digestate
4. Digestate Processing
4.1. Solid–Liquid Separation
4.2. Nutrient Removal and Recovery
5. Cultivation in Digestate
5.1. Removal of Solids and Increasing Light Permeability
5.2. Adjustment of Nutrients
5.3. Organic Carbon in Digestate
6. Downstream Processing
6.1. Harvesting Techniques
6.2. Disintegration of Cells
6.3. Dehydration of Cells
6.4. Purification of Products
7. Use of Microalgae Biomass
7.1. Bioenergy Production
7.2. Applications in the Food and Feed Industry
7.3. High-Value Products and Microalgae Biorefienery
8. Thoughts on Economics and Sustainability
9. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andersen, R.A. The Microalgal Cell. In Handbook of Microalgal Culture: Applied Phycology and Biotechnology; Richmond, A., Hu, Q., Eds.; John Wiley & Sons, Ltd.: Oxford, UK, 2013; pp. 3–20. ISBN 978-1-118-56716-6. [Google Scholar]
- De Morais, M.G.; Vaz, B.d.S.; de Morais, E.G.; Costa, J.A.V. Biologically Active Metabolites Synthesized by Microalgae. BioMed Res. Int. 2015, 2015, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acién Fernández, F.G.; Fernández Sevilla, J.M.; Molina Grima, E. Costs analysis of microalgae production. In Biofuels from Algae; Elsevier: Amsterdam, The Netherlands, 2019; pp. 551–566. ISBN 978-0-444-64192-2. [Google Scholar]
- Dos Santos Fernandes de Araujo, R.; Lusser, M.; Sanchez Lopez, J.; Avraamides, M. Brief on Algae Biomass Production. 2019. Available online: https://ec.europa.eu/jrc/en/publication/brochures-leaflets/brief-algae-biomass-production (accessed on 1 November 2020).
- Nowicka-Krawczyk, P.; Mühlsteinová, R.; Hauer, T. Detailed Characterization of the Arthrospira Type Species Separating Commercially Grown Taxa into the New Genus Limnospira (Cyanobacteria). Sci. Rep. 2019, 9, 694. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, J.C.; Sydney, E.B.; Assú Tessari, L.F.; Soccol, C.R. Culture media for mass production of microalgae. In Biofuels from Algae; Elsevier: Amsterdam, The Netherlands, 2019; pp. 33–50. ISBN 978-0-444-64192-2. [Google Scholar]
- Liu, J.; Hu, Q. Chlorella: Industrial Production of Cell Mass and Chemicals. In Handbook of Microalgal Culture: Applied Phycology and Biotechnology; Richmond, A., Hu, Q., Eds.; John Wiley & Sons, Ltd.: Oxford, UK, 2013; pp. 329–338. ISBN 978-1-118-56716-6. [Google Scholar]
- Wang, C.-Y.; Fu, C.-C.; Liu, Y.-C. Effects of Using Light-Emitting Diodes on the Cultivation of Spirulina platensis. Biochem. Eng. J. 2007, 37, 21–25. [Google Scholar] [CrossRef]
- Borowitzka, M.A. Dunaliella: Biology, Production, and Markets. In Handbook of Microalgal Culture: Applied Phycology and Biotechnology; Richmond, A., Hu, Q., Eds.; John Wiley & Sons, Ltd.: Oxford, UK, 2013; pp. 359–368. ISBN 978-1-118-56716-6. [Google Scholar]
- Han, D.; Li, Y.; Hu, Q. Biology and Commercial Aspects of Haematococcus pluvialis. In Handbook of Microalgal Culture: Applied Phycology and Biotechnology; Richmond, A., Hu, Q., Eds.; John Wiley & Sons, Ltd.: Oxford, UK, 2013; pp. 388–405. ISBN 978-1-118-56716-6. [Google Scholar]
- Ghimire, A.; Kumar, G.; Sivagurunathan, P.; Shobana, S.; Saratale, G.D.; Kim, H.W.; Luongo, V.; Esposito, G.; Munoz, R. Bio-Hythane Production from Microalgae Biomass: Key Challenges and Potential Opportunities for Algal Bio-Refineries. Bioresour. Technol. 2017, 241, 525–536. [Google Scholar] [CrossRef] [PubMed]
- Saratale, R.G.; Kuppam, C.; Mudhoo, A.; Saratale, G.D.; Periyasamy, S.; Zhen, G.; Koók, L.; Bakonyi, P.; Nemestóthy, N.; Kumar, G. Bioelectrochemical Systems Using Microalgae—A Concise Research Update. Chemosphere 2017, 177, 35–43. [Google Scholar] [CrossRef]
- Morales-Amaral, M.d.M.; Gómez-Serrano, C.; Acién, F.G.; Fernández-Sevilla, J.M.; Molina-Grima, E. Production of Microalgae Using Centrate from Anaerobic Digestion as the Nutrient Source. Algal Res. 2015, 9, 297–305. [Google Scholar] [CrossRef]
- Navarro-López, E.; Ruíz-Nieto, A.; Ferreira, A.; Acién, F.G.; Gouveia, L. Biostimulant Potential of Scenedesmus Obliquus Grown in Brewery Wastewater. Molecules 2020, 25, 664. [Google Scholar] [CrossRef] [Green Version]
- Ranglová, K.; Lakatos, G.E.; Câmara Manoel, J.A.; Grivalský, T.; Suárez Estrella, F.; Acién Fernández, F.G.; Molnár, Z.; Ördög, V.; Masojídek, J. Growth, Biostimulant and Biopesticide Activity of the MACC-1 Chlorella Strain Cultivated Outdoors in Inorganic Medium and Wastewater. Algal Res. 2020, 102136. [Google Scholar] [CrossRef]
- Zhu, L.; Yan, C.; Li, Z. Microalgal Cultivation with Biogas Slurry for Biofuel Production. Bioresour. Technol. 2016, 220, 629–636. [Google Scholar] [CrossRef]
- Drosg, B.; Fuchs, W.; Seadi, T.A.; Madsen, M.; Linke, B. Nutrient Recovery by Biogas Digestate Processing. 40. Available online: http://www.iea-biogas.net/files/daten-redaktion/download/Technical%20Brochures/NUTRIENT_RECOVERY_RZ_web1.pdf (accessed on 13 November 2020).
- Marcilhac, C.; Sialve, B.; Pourcher, A.-M.; Ziebal, C.; Bernet, N.; Béline, F. Digestate Color and Light Intensity Affect Nutrient Removal and Competition Phenomena in a Microalgal-Bacterial Ecosystem. Water Res. 2014, 64, 278–287. [Google Scholar] [CrossRef]
- Borowitzka, M.A. Culturing microalgae in outdoor ponds. In Algal Culturing Techniques; Andersen, R.A., Ed.; Elsevier Academic Press: Burlington, MA, USA, 2005; pp. 205–218. ISBN 978-0-12-088426-1. [Google Scholar]
- Daliry, S.; Hallajsani, A.; Mohammadi Roshandeh, J.; Nouri, H.; Golzary, A. Investigation of Optimal Condition for Chlorella Vulgaris Microalgae Growth. GJESM 2017, 3. [Google Scholar] [CrossRef]
- Belay, A. Biology and Industrial Production of Arthrospira (Spirulina). In Handbook of Microalgal Culture: Applied Phycology and Biotechnology; Richmond, A., Hu, Q., Eds.; John Wiley & Sons, Ltd.: Oxford, UK, 2013; pp. 339–358. ISBN 978-1-118-56716-6. [Google Scholar]
- Hosseini Tafreshi, A.; Shariati, M. Dunaliella Biotechnology: Methods and Applications. J. Appl. Microbiol. 2009, 107, 14–35. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.-W.; Choi, H.I.; Sim, S.J. Acidic Cultivation of Haematococcus Pluvialis for Improved Astaxanthin Production in the Presence of a Lethal Fungus. Bioresour. Technol. 2019, 278, 138–144. [Google Scholar] [CrossRef]
- Farhat, N.; Rabhi, M.; Falleh, H.; Jouini, J.; Abdelly, C.; Smaoui, A. Optimization of Salt Concentrations for a Higher Carotenoid Production in Dunaliella Salina (Chlorophyceae). J. Phycol. 2011, 47, 1072–1077. [Google Scholar] [CrossRef] [PubMed]
- Blair, M.F.; Kokabian, B.; Gude, V.G. Light and Growth Medium Effect on Chlorella Vulgaris Biomass Production. J. Environ. Chem. Eng. 2014, 2, 665–674. [Google Scholar] [CrossRef]
- Bajwa, K.; Bishnoi, N.R.; Kirrolia, A.; Sharma, J.; Gupta, S. Comparison of Various Growth Media Composition for Physio-Biochemical Parameters of Biodiesel Producing Microalgal Species (Chlorococcum Aquaticum, Scenedesmus Obliquus, Nannochloropsis Oculata and Chlorella Pyrenoidosa). Eur. J. Biotechnol. Biosci. 2017, 5, 5. [Google Scholar]
- Borowitzka, M.A. Appendix: Algal growth media and sources of algal cultures. In Micro-Algal Biotechnology; Borowitzka, M.A., Borowitzka, L.J., Eds.; Cambridge University Press: Cambridge, UK, 1988; pp. 456–465. [Google Scholar]
- Mortimer, C.E.; Müller, U. Chemie: Das Basiswissen der Chemie, 10th ed.; Georg Thieme Verlag: Stuttgart, Germany, 2010; ISBN 978-3-13-484310-1. [Google Scholar]
- Markou, G.; Vandamme, D.; Muylaert, K. Microalgal and Cyanobacterial Cultivation: The Supply of Nutrients. Water Res. 2014, 65, 186–202. [Google Scholar] [CrossRef] [Green Version]
- Torzillo, G.; Vonshak, A. Environmental Stress Physiology with Reference to Mass Cultures. In Handbook of Microalgal Culture: Applied Phycology and Biotechnology; Richmond, A., Hu, Q., Eds.; John Wiley & Sons, Ltd.: Oxford, UK, 2013; pp. 90–113. ISBN 978-1-118-56716-6. [Google Scholar]
- Venkata Mohan, S.; Devi, M.P. Salinity Stress Induced Lipid Synthesis to Harness Biodiesel during Dual Mode Cultivation of Mixotrophic Microalgae. Bioresour. Technol. 2014, 165, 288–294. [Google Scholar] [CrossRef]
- Pal, D.; Khozin-Goldberg, I.; Cohen, Z.; Boussiba, S. The Effect of Light, Salinity, and Nitrogen Availability on Lipid Production by Nannochloropsis sp. Appl. Microbiol. Biotechnol. 2011, 90, 1429–1441. [Google Scholar] [CrossRef]
- Yan, C.; Zhang, L.; Luo, X.; Zheng, Z. Effects of Various LED Light Wavelengths and Intensities on the Performance of Purifying Synthetic Domestic Sewage by Microalgae at Different Influent C/N Ratios. Ecol. Eng. 2013, 51, 24–32. [Google Scholar] [CrossRef]
- Wang, S.-K.; Stiles, A.R.; Guo, C.; Liu, C.-Z. Microalgae Cultivation in Photobioreactors: An Overview of Light Characteristics. Eng. Life Sci. 2014, 14, 550–559. [Google Scholar] [CrossRef] [Green Version]
- De Mooij, T.; De Vries, G.; Latsos, C.; Wijffels, R.H.; Janssen, M. Impact of Light Color on Photobioreactor Productivity. Algal Res. 2016, 15, 32–42. [Google Scholar] [CrossRef] [Green Version]
- Ho, S.-H.; Chen, C.-Y.; Chang, J.-S. Effect of Light Intensity and Nitrogen Starvation on CO2 Fixation and Lipid/Carbohydrate Production of an Indigenous Microalga Scenedesmus Obliquus CNW-N. Bioresour. Technol. 2012, 113, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Gruber-Brunhumer, M.; Nussbaumer, M.; Jerney, J.; Ludwig, I.; Zohar, E.; Lang, I.; Bochmann, G.; Schagerl, M.; Obbard, J.P.; Fuchs, W.; et al. Two-Stage Cultivation of N-Rich and N-Deprived Acutodesmus Obliquus Biomass: Influence of Cultivation and Dewatering Methods on Microalgal Biomass Used in Anaerobic Digestion. Algal Res. 2016, 17, 105–112. [Google Scholar] [CrossRef]
- Schulze, P.S.C.; Barreira, L.A.; Pereira, H.G.C.; Perales, J.A.; Varela, J.C.S. Light Emitting Diodes (LEDs) Applied to Microalgal Production. Trends Biotechnol. 2014, 32, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Xia, A.; Murphy, J.D. Microalgal Cultivation in Treating Liquid Digestate from Biogas Systems. Trends Biotechnol. 2016, 34, 264–275. [Google Scholar] [CrossRef]
- Malapascua, J.R.F.; Jerez, C.G.; Sergejevová, M.; Figueroa, F.L.; Masojídek, J. Photosynthesis Monitoring to Optimize Growth of Microalgal Mass Cultures: Application of Chlorophyll Fluorescence Techniques. Aquat. Biol. 2014, 22, 123–140. [Google Scholar] [CrossRef] [Green Version]
- Grivalský, T.; Ranglová, K.; da Câmara Manoel, J.A.; Lakatos, G.E.; Lhotský, R.; Masojídek, J. Development of Thin-Layer Cascades for Microalgae Cultivation: Milestones (Review). Folia Microbiol. 2019, 64, 603–614. [Google Scholar] [CrossRef]
- Grobbelaar, J.U. Inorganic Algal Nutrition. In Handbook of Microalgal Culture: Applied Phycology and Biotechnology; Richmond, A., Hu, Q., Eds.; John Wiley & Sons, Ltd.: Oxford, UK, 2013; pp. 123–133. ISBN 978-1-118-56716-6. [Google Scholar]
- Gao, F.; Yang, H.-L.; Li, C.; Peng, Y.-Y.; Lu, M.-M.; Jin, W.-H.; Bao, J.-J.; Guo, Y.-M. Effect of Organic Carbon to Nitrogen Ratio in Wastewater on Growth, Nutrient Uptake and Lipid Accumulation of a Mixotrophic Microalgae Chlorella sp. Bioresour. Technol. 2019, 282, 118–124. [Google Scholar] [CrossRef]
- Meixner, K.; Fritz, I.; Daffert, C.; Markl, K.; Fuchs, W.; Drosg, B. Processing Recommendations for Using Low-Solids Digestate as Nutrient Solution for Poly-ß-Hydroxybutyrate Production with Synechocystis salina. J. Biotechnol. 2016, 240, 61–67. [Google Scholar] [CrossRef]
- Borowitzka, M.A. Algal physiology and large-scale outdoor cultures of microalgae. In The Physiology of Microalgae; Borowitzka, M.A., Beardall, J., Raven, J.A., Eds.; Developments in Applied Phycology; Springer International Publishing: Cham, Switzerland, 2016; pp. 601–652. ISBN 978-3-319-24945-2. [Google Scholar]
- Barampouti, E.M.; Mai, S.; Malamis, D.; Moustakas, K.; Loizidou, M. Exploring Technological Alternatives of Nutrient Recovery from Digestate as a Secondary Resource. Renew. Sustain. Energy Rev. 2020, 134, 110379. [Google Scholar] [CrossRef]
- Al Seadi, T.; Drosg, B.; Fuchs, W.; Rutz, D.; Janssen, R. Biogas digestate quality and utilization. In The Biogas Handbook: Science, Production and Applications; Woodhead Publishing Series in Energy; Wellinger, A., Murphy, J., Baxter, D., Eds.; Woodhead Publishing: Cambridge, UK, 2013; pp. 267–301. ISBN 978-0-85709-498-8. [Google Scholar]
- Fuchs, W.; Drosg, B. Technologiebewertung von Gärrestbehandlungs- und Verwertungskonzepten; Eigenverlag der Universität für Bodenkultur Wien: Tulln, Austria, 2010; ISBN 978-3-900962-86-9. [Google Scholar]
- Lu, Q.; Chen, P.; Addy, M.; Zhang, R.; Deng, X.; Ma, Y.; Cheng, Y.; Hussain, F.; Chen, C.; Liu, Y.; et al. Carbon-Dependent Alleviation of Ammonia Toxicity for Algae Cultivation and Associated Mechanisms Exploration. Bioresour. Technol. 2018, 249, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, F.; Silkina, A.; Fuentes-Grünewald, C.; Wood, E.E.; Ndovela, V.L.S.; Oatley-Radcliffe, D.L.; Lovitt, R.W.; Llewellyn, C.A. Valorising Nutrient-Rich Digestate: Dilution, Settlement and Membrane Filtration Processing for Optimisation as a Waste-Based Media for Microalgal Cultivation. Waste Manag. 2020, 118, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gabauer, W.; Li, Z.; Ortner, M.; Fuchs, W. Improving Exploitation of Chicken Manure via Two-Stage Anaerobic Digestion with an Intermediate Membrane Contactor to Extract Ammonia. Bioresour. Technol. 2018, 268, 811–814. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.; Mayr, H.; Hopfner-Sixt, K.; Amon, T. Detailed Monitoring of Two Biogas Plants and Mechanical Solid–Liquid Separation of Fermentation Residues. J. Biotechnol. 2009, 142, 56–63. [Google Scholar] [CrossRef]
- Guilayn, F.; Jimenez, J.; Rouez, M.; Crest, M.; Patureau, D. Digestate Mechanical Separation: Efficiency Profiles Based on Anaerobic Digestion Feedstock and Equipment Choice. Bioresour. Technol. 2019, 274, 180–189. [Google Scholar] [CrossRef]
- Meixner, K.; Fuchs, W.; Valkova, T.; Svardal, K.; Loderer, C.; Neureiter, M.; Bochmann, G.; Drosg, B. Effect of Precipitating Agents on Centrifugation and Ultrafiltration Performance of Thin Stillage Digestate. Sep. Purif. Technol. 2015, 145, 154–160. [Google Scholar] [CrossRef]
- David, G.; Negrell, C.; Vachoud, L.; Ruiz, E.; Delalonde, M.; Wisniewski, C. An Environmental Application of Functionalized Chitosan: Enhancement of the Separation of the Solid and Liquid Fractions of Digestate from Anaerobic Digestion. Pure and Appl. Chem. 2016, 88, 1155–1166. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.S.; Robinson, J.; Chong, M.F. A Review on Application of Flocculants in Wastewater Treatment. Process Saf. Environ. Prot. 2014, 92, 489–508. [Google Scholar] [CrossRef]
- Bousek, J.; Scroccaro, D.; Sima, J.; Weissenbacher, N.; Fuchs, W. Influence of the Gas Composition on the Efficiency of Ammonia Stripping of Biogas Digestate. Bioresour. Technol. 2016, 203, 259–266. [Google Scholar] [CrossRef] [Green Version]
- Drosg, B. Energy Recovery in Grain Bioethanol Production by Anaerobic Digestion of Stillage Fractions; Universität für Bodenkultur Wien: Wien, Austria, 2012. [Google Scholar]
- Qiu, B.; Fan, S.; Tang, X.; Qi, B.; Deng, L.; Wang, W.; Liu, J.; Wang, Y.; Xiao, Z. Simultaneous Recovery of Phosphorus and Nitrogen from Liquid Digestate by Vacuum Membrane Distillation with Permeate Fractional Condensation. Chin. J. Chem. Eng. 2020, 28, 1558–1565. [Google Scholar] [CrossRef]
- Jiang, Y.; Pu, X.; Zheng, D.; Zhu, T.; Wang, S.; Deng, L.; Wang, W. Cultivation of Lipid-Producing Microalgae in Struvite-Precipitated Liquid Digestate for Biodiesel Production. Biotechnol. Biofuels 2018, 11, 101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veronesi, D.; Ida, A.; Imporzano, G.D.; Adani, F. Microalgae Cultivation: Nutrient Recovery from Digestate for Producing Algae Biomass. Chem. Eng. Trans. 2015, 43, 1201–1206. [Google Scholar] [CrossRef]
- Hollinshead, W.D.; Varman, A.M.; You, L.; Hembree, Z.; Tang, Y.J. Boosting D-Lactate Production in Engineered Cyanobacteria Using Sterilized Anaerobic Digestion Effluents. Bioresour. Technol. 2014, 169, 462–467. [Google Scholar] [CrossRef]
- Lorenz, M.; Friedl, T.; Day, J.G. Perpetual maintenance of actively metabolizing microalgal cultures. In Algal Culturing Techniques; Andersen, R.A., Ed.; Elsevier Academic Press: Burlington, MA, USA, 2005; pp. 145–156. ISBN 978-0-12-088426-1. [Google Scholar]
- Zmora, O.; Grosse, D.J.; Zou, N.; Samocha, T.M. Microalga for Aquaculture: Practical Implications. In Handbook of Microalgal Culture—Applied Phycology and Biotechnology; Richmond, A., Hu, Q., Eds.; John Wiley & Sons, Ltd.: Oxford, UK, 2013; pp. 628–652. ISBN 978-1-118-56716-6. [Google Scholar]
- Zittelli, G.C.; Biondi, N.; Rodolfi, L.; Tredici, M.R. Photobioreactors for Mass Production of Microalgae. In Handbook of Microalgal Culture—Applied Phycology and Biotechnology; Richmond, A., Hu, Q., Eds.; John Wiley & Sons, Ltd.: Oxford, UK, 2013; pp. 225–266. ISBN 978-1-118-56716-6. [Google Scholar]
- Molina Grima, E.; Acién Fernández, F.G.; Robles Medina, A. Downstream Processing of Cell Mass and Products. In Handbook of Microalgal Culture: Applied Phycology and Biotechnology; Richmond, A., Hu, Q., Eds.; John Wiley & Sons, Ltd.: Oxford, UK, 2013; pp. 267–309. ISBN 978-1-118-56716-6. [Google Scholar]
- Muylaert, K.; Bastiaens, L.; Vandamme, D.; Gouveia, L. Harvesting of microalgae: Overview of process options and their strengths and drawbacks. In Microalgae-Based Biofuels and Bioproducts: From Feedstock Cultivation to End-products; Gonzalez-Fernandez, C., Muñoz, R., Eds.; Woodhead Publishing: Duxford, UK, 2017; pp. 113–132. ISBN 978-0-08-101027-3. [Google Scholar]
- Masojídek, J.; Torzillo, G. Mass Cultivation of Freshwater Microalgae. In Reference Module in Earth Systems and Environmental Sciences; Elsevier: Amsterdam, The Netherlands, 2014; ISBN 978-0-12-409548-9. [Google Scholar]
- Evodos Dynamic Settlers. Available online: https://www.evodos.eu/technology/ (accessed on 22 December 2020).
- Vandamme, D.; Pontes, S.C.V.; Goiris, K.; Foubert, I.; Pinoy, L.J.J.; Muylaert, K. Evaluation of Electro-Coagulation-Flocculation for Harvesting Marine and Freshwater Microalgae. Biotechnol. Bioeng. 2011, 108, 2320–2329. [Google Scholar] [CrossRef] [Green Version]
- Cerff, M.; Morweiser, M.; Dillschneider, R.; Michel, A.; Menzel, K.; Posten, C. Harvesting Fresh Water and Marine Algae by Magnetic Separation: Screening of Separation Parameters and High Gradient Magnetic Filtration. Bioresour. Technol. 2012, 118, 289–295. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, Y. Synergistic Integration of Electrocoagulation and Algal Cultivation to Treat Liquid Anaerobic Digestion Effluent and Accumulate Algal Biomass. Process Biochem. 2016, 51, 89–94. [Google Scholar] [CrossRef] [Green Version]
- Souza, F.L.; Cotillas, S.; Saéz, C.; Cañizares, P.; Lanza, M.R.; Seco, A.; Rodrigo, M.A. Removal of Algae from Biological Cultures: A Challenge for Electrocoagulation? J. Chem. Technol. Biotechnol. 2016, 91, 82–87. [Google Scholar] [CrossRef]
- D’Hondt, E.; Martín-Juárez, J.; Bolado, S.; Kasperoviciene, J.; Koreiviene, J.; Sulcius, S.; Elst, K.; Bastiaens, L. Cell disruption technologies. In Microalgae-Based Biofuels and Bioproducts: From Feedstock Cultivation to End-Products; Gonzalez-Fernandez, C., Muñoz, R., Eds.; Woodhead Publishing: Duxford, UK, 2017; pp. 133–154. ISBN 978-0-08-101027-3. [Google Scholar]
- Amaro, H.M.; Sousa-Pinto, I.; Malcata, F.X.; Catarina Guedes, A. Microalgal fatty acids—From harvesting until extraction. In Microalgae-Based Biofuels and Bioproducts: From Feedstock Cultivation to End-Products; Gonzalez-Fernandez, C., Muñoz, R., Eds.; Woodhead Publishing: Duxford, UK, 2017; pp. 369–400. ISBN 978-0-08-101027-3. [Google Scholar]
- Ventura, S.P.M.; Nobre, B.P.; Ertekin, F.; Hayes, M.; García-Vaquero, M.; Vieira, F.; Koc, M.; Gouveia, L.; Aires-Barros, M.R.; Palavra, A.M.F. Extraction of value-added compounds from microalgae. In Microalgae-Based Biofuels and Bioproducts: From Feedstock Cultivation to End-Products; Gonzalez-Fernandez, C., Muñoz, R., Eds.; Woodhead Publishing: Duxford, UK, 2017; pp. 461–484. ISBN 978-0-08-101027-3. [Google Scholar]
- Directive 2008/98/EC of the European Parliament and of the Council of 19 November 2008 on Waste and Repealing Certain Directives; European Parliament, Council of the European Union, EU: Brussels, Belgium, 2008.
- Koutra, E.; Economou, C.N.; Tsafrakidou, P.; Kornaros, M. Bio-Based Products from Microalgae Cultivated in Digestates. Trends Biotechnol. 2018, 36, 819–833. [Google Scholar] [CrossRef]
- Meixner, K.; Kovalcik, A.; Sykacek, E.; Gruber-Brunhumer, M.; Zeilinger, W.; Markl, K.; Haas, C.; Fritz, I.; Mundigler, N.; Stelzer, F.; et al. Cyanobacteria Biorefinery—Production of Poly(3-Hydroxybutyrate) with Synechocystis Salina and Utilisation of Residual Biomass. J. Biotechnol. 2018, 265, 46–53. [Google Scholar] [CrossRef]
- Gouveia, L.; Oliveira, A.C.; Congestri, R.; Bruno, L.; Soares, A.T.; Menezes, R.S.; Filho, N.R.A.; Tzovenis, I. Biodiesel from microalgae. In Microalgae-Based Biofuels and Bioproducts: From Feedstock Cultivation to End-products; Gonzalez-Fernandez, C., Muñoz, R., Eds.; Woodhead Publishing: Duxford, UK, 2017; pp. 235–258. ISBN 978-0-08-101027-3. [Google Scholar]
- Martín-Juárez, J.; Markou, G.; Muylaert, K.; Lorenzo-Hernando, A.; Bolado, S. Breakthroughs in bioalcohol production from microalgae: Solving the hurdles. In Microalgae-Based Biofuels and Bioproducts: From Feedstock Cultivation to End-Products; Gonzalez-Fernandez, C., Muñoz, R., Eds.; Woodhead Publishing: Duxford, UK, 2017; pp. 183–208. ISBN 978-0-08-101027-3. [Google Scholar]
- Ganesh Saratale, R.; Kumar, G.; Banu, R.; Xia, A.; Periyasamy, S.; Dattatraya Saratale, G. A Critical Review on Anaerobic Digestion of Microalgae and Macroalgae and Co-Digestion of Biomass for Enhanced Methane Generation. Bioresour. Technol. 2018, 262, 319–332. [Google Scholar] [CrossRef]
- Nurk, L.; Graβ, R.; Pekrun, C.; Wachendorf, M. Methane Yield and Feed Quality Parameters of Mixed Silages from Maize (Zea mays L.) and Common Bean (Phaseolus vulgaris L.). Bioenerg. Res. 2017, 10, 64–73. [Google Scholar] [CrossRef]
- Gruber-Brunhumer, M.; Montgomery, L.; Nussbaumer, M.; Schoepp, T.; Zohar, E.; Ludwig, I.; Fuchs, W.; Drosg, B. Effects of Partial Maize Silage Substitution with Microalgae on Viscosity and Biogas Yields in Continuous AD Trials. J. Biotechnol. 2019, 295, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Duan, N.; Zhang, Y.; Liu, Z.; Li, B.; Zhang, D.; Lu, H.; Dong, T. Co-Digestion of Chicken Manure and Microalgae Chlorella 1067 Grown in the Recycled Digestate: Nutrients Reuse and Biogas Enhancement. Waste Manag. 2017, 70, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.; Skomedal, H.; Skjånes, K.; Mazur-Marzec, H.; Toruńska-Sitarz, A.; Catala, M.; Isleten Hosoglu, M.; García-Vaquero, M. Microalgal proteins for feed, food and health. In Microalgae-Based Biofuels and Bioproducts: From Feedstock Cultivation to End-products; Gonzalez-Fernandez, C., Muñoz, R., Eds.; Woodhead Publishing: Duxford, UK, 2017; pp. 347–368. ISBN 978-0-08-101027-3. [Google Scholar]
- Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 Laying down the General Principles and Requirements of Food Law, Establishing the European Food Safety Authority and Laying down Procedures in Matters of Food Safety; European Parliament, Council of the European Union, EU: Brussels, Belgium, 2002.
- Commission Regulation (EC) No 852/2003 of 16 May 2003 Amending Regulation (EC) No 1445/95 on Rules of Application for Import and Export Licences in the Beef and Veal Sector; European Parliament, Council of the European Union, EU: Brussels, Belgium, 2003; Volume 123.
- Regulation (EC) No 853/2004 of the European Parliament and of the Council of 29 April 2004 Laying down Specific Hygiene Rules for Food of Animal Origin; European Parliament, Council of the European Union, EU: Brussels, Belgium, 2004; Volume 139.
- Regulation (EC) No 258/97 of the European Parliament and of the Council of 27 January 1997 Concerning Novel Foods and Novel Food Ingredients; European Parliament, Council of the European Union, EU: Brussels, Belgium, 1997; Volume 043.
- Regulation (EC) No 767/2009 of the European Parliament and of the Council of 13 July 2009 on the Placing on the Market and Use of Feed, Amending European Parliament and Council Regulation (EC) No 1831/2003 and Repealing Council Directive 79/373/EEC, Commission Directive 80/511/EEC, Council Directives 82/471/EEC, 83/228/EEC, 93/74/EEC, 93/113/EC and 96/25/EC and Commission Decision 2004/217/EC; European Parliament, Council of the European Union, EU: Brussels, Belgium, 2009.
- Directive 2002/32/EC of the European Parliament and of the Council of 7 May 2002 on Undesirable Substances in Animal Feed—Council Statement; European Parliament, Council of the European Union, EU: Brussels, Belgium, 2002.
- Ronga, D.; Biazzi, E.; Parati, K.; Carminati, D.; Carminati, E.; Tava, A. Microalgal Biostimulants and Biofertilisers in Crop Productions. Agronomy 2019, 9, 192. [Google Scholar] [CrossRef] [Green Version]
- Sivagurunathan, P.; Kuppam, C.; Mudhoo, A.; Saratale, G.D.; Kadier, A.; Zhen, G.; Chatellard, L.; Trably, E.; Kumar, G. A Comprehensive Review on Two-Stage Integrative Schemes for the Valorization of Dark Fermentative Effluents. Crit. Rev. Biotechnol. 2018, 38, 868–882. [Google Scholar] [CrossRef] [PubMed]
- Shobana, S.; Kumar, G.; Bakonyi, P.; Saratale, G.D.; Al-Muhtaseb, A.H.; Nemestóthy, N.; Bélafi-Bakó, K.; Xia, A.; Chang, J.-S. A Review on the Biomass Pretreatment and Inhibitor Removal Methods as Key-Steps towards Efficient Macroalgae-Based Biohydrogen Production. Bioresour. Technol. 2017, 244, 1341–1348. [Google Scholar] [CrossRef] [PubMed]
- Samantaray, S.; Bhati, R.; Mallick, N. Cyanobacterial polyhydroxyalkanoates: An alternative source for plastics. In Cyanobacteria: An Economic Perspective; Sharma, N.K., Rai, A.K., Stal, L.J., Eds.; John Wiley & Sons, Ltd.: Oxford, UK, 2014; pp. 227–244. ISBN 978-1-118-40223-8. [Google Scholar]
- Kovalcik, A.; Meixner, K.; Mihalic, M.; Zeilinger, W.; Fritz, I.; Fuchs, W.; Kucharczyk, P.; Stelzer, F.; Drosg, B. Characterization of Polyhydroxyalkanoates Produced by Synechocystis Salina from Digestate Supernatant. Int. J. Biol. Macromol. 2017. [Google Scholar] [CrossRef]
- Acién, F.G.; Molina, E.; Fernández-Sevilla, J.M.; Barbosa, M.; Gouveia, L.; Sepúlveda, C.; Bazaes, J.; Arbib, Z. Economics of microalgae production. In Microalgae-Based Biofuels and Bioproducts: From Feedstock Cultivation to End-products; Gonzalez-Fernandez, C., Muñoz, R., Eds.; Woodhead Publishing: Duxford, UK, 2017; pp. 485–504. ISBN 978-0-08-101027-3. [Google Scholar]
- Gouveia, L.; Graça, S.; Sousa, C.; Ambrosano, L.; Ribeiro, B.; Botrel, E.P.; Neto, P.C.; Ferreira, A.F.; Silva, C.M. Microalgae Biomass Production Using Wastewater: Treatment and Costs. Algal Res. 2016, 16, 167–176. [Google Scholar] [CrossRef]
- Chia, S.R.; Chew, K.W.; Show, P.L.; Yap, Y.J.; Ong, H.C.; Ling, T.C.; Chang, J.-S. Analysis of Economic and Environmental Aspects of Microalgae Biorefinery for Biofuels Production: A Review. Biotechnol. J. 2018, 1700618. [Google Scholar] [CrossRef]
- Slade, R.; Bauen, A. Micro-Algae Cultivation for Biofuels: Cost, Energy Balance, Environmental Impacts and Future Prospects. Biomass Bioenergy 2013, 53, 29–38. [Google Scholar] [CrossRef] [Green Version]
- Stiles, W.A.V.; Styles, D.; Chapman, S.P.; Esteves, S.; Bywater, A.; Melville, L.; Silkina, A.; Lupatsch, I.; Fuentes Grünewald, C.; Lovitt, R.; et al. Using Microalgae in the Circular Economy to Valorise Anaerobic Digestate: Challenges and Opportunities. Bioresour. Technol. 2018, 267, 732–742. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Unit | Chlorella | Arthrospira | Dunaliella | Haematococcus | |
|---|---|---|---|---|---|
| Temperature | [°C] | 25–30 [19,20] | 30–38 [21] | 30–40 [19] | 18–22 [19] |
| pH | [-] | 6.5–7.5 [19] | 9–10 [21] | 9–11 [22] | 7 [23] |
| Max. salinity | [% (w/v) NaCl] | 1 [19] | <3 [19] | 20–35 [19,24] | 1 [19] |
| Typical cultivation medium | [-] | BBM b [19,25]/ BG-11 c [26] | Zarrouk [19,21] | Modified Johnson [19,22] | BBM b [10,19] |
| N a | [mM] | 2.94/17.63 [6] | 29.40 [6] | 9.89 [27] | 2.94 [6] |
| P a | [mM] | 1.72/0.23 [6] | 2.87 [6] | 0.26 [27] | 1.72 [6] |
| K a | [mM] | 2.15/0.46 [6] | 17.2 [6] | 12.83 [27] | 2.15 [6] |
| N/P ratio a | [-] | 1.71/76.78 [6] | 10.24 [6] | 38.46 [27] | 1.71 [6] |
| Origin | Raw Material | pH [-] | TS a [%] | VS b [%] | NH4-N c [g kg−1] | TKN d [g kg−1] | P e [g kg−1] | References |
|---|---|---|---|---|---|---|---|---|
| Agricultural residues/ renewable materials | n.d. f | 7.5–8.4 | 6.41–24 | 4.42–18.5 | 0.03–4.1 | 0.09–5.04 | 0.46–5.76 | [46] * |
| Co-digestion manure + crops and/or industrial waste | 5.6–8.3 | 1.5–24 | 0.93–18.5 | 0.01–1.63 | 0.02–12.1 | 0.002–2.4 | [46] * | |
| Corn silage, manure, agricultural residues | 7.7–8.1 | 6.1–8.3 | 4.4–6.3 | 4.9–6.1 | 7.6–9.6 | n.d. f | [17] | |
| Crop digestion with manure | 7.7–8.0 | 6.5–8.6 | 4.8–6.4 | 2.3–4.2 | 4.3–6.1 | n.d. f | [17] | |
| Crop digestion | 7.4–7.9 | 6.2–8.6 | 4.8–6.2 | 1.5–2.5 | 3.6–5.2 | n.d. f | [17] | |
| Crop digestion | 7.2–7.9 | 7.8–9.0 | 5.7–6.7 | 1.3–3.6 | 4.6–6.3 | n.d. f | [17] | |
| Corn and grass silage | 7.6–8.0 | 6.6–9.3 | 4.8–6.9 | 1.3–2.4 | 3.6–4.9 | n.d. f | [17] | |
| Manure | 7.3–8.6 | 2.2–9.2 | 1.49–6.9 | 0.06–0.93 | 0.01–0.57 | 0.007–0.2 | [46]* | |
| Industrial residues | Brewers’ spent grains | 7.3–7.5 | 5.3–5.8 | 4.7–5.3 | 1.9–2.3 | 2.3–3.1 | n.d. f | [17] |
| Slaughterhouse waste | 7.9–8.3 | 2.2–4.9 | 1.6–3.9 | 5.3–7.7 | 6.4–8.1 | n.d. f | [17] | |
| Thin stillage—bioethanol by-product | 7.7–8.1 | 1.7–2.8 | 0.9–1.6 | 2.2–2.8 | 3.0–4.3 | n.d. f | [17] | |
| Food waste/residues | n.d. f | 7.9–8.3 | 1.4–7.88 | 0.56–5.78 | 0.01–0.67 | 0.01–098 | 0.002–0.1 | [46] * |
| Bio waste | 7.6–8.1 | 2.5–4.7 | 1.4–2.7 | 1.5–5.6 | 3.0–6.8 | n.d. f | [17] | |
| Bio and food waste, blood | 8.0–8.3 | 3.9–4.1 | 2.4–2.8 | 5.1–7.2 | 6.4–8.1 | n.d. f | [17] | |
| Bio and food waste | 7.3–7.9 | 1.6–3.3 | 1.0–1.7 | 0.6–1.5 | 1.4–2.3 | n.d. f | [17] | |
| Bio and food waste, blood, food industry residues | 7.8–8.2 | 5.6–8.1 | 3.0–4.5 | 3.1–4.1 | 4.2–6.7 | n.d. f | [17] | |
| Manure, slaughterhouse, bio, food, and kitchen waste | 8.0–8.3 | 5.7–7.2 | 4.1–5.6 | 6.8–8.6 | 8.4–10.8 | n.d. f | [17] | |
| Kitchen food waste | 8.0 | 5.9 | n.d. f | 4.02 | n.d. f | 0.67 | [50] |
| Unit | Liquid | Solids | |
|---|---|---|---|
| Mass | [%] | 80–90 | 10–20 |
| TS a | [%] | 40–50 | 50–60 |
| VS b | [%] | 35–45 | 55–65 |
| Ash | [%] | 50–60 | 40–50 |
| TN c | [%] | 65–75 | 25–35 |
| NH4-N d | [%] | 70–80 | 20–30 |
| P e | [%] | 35–45 | 55–65 |
| K f | [%] | 70–80 | 20–30 |
| C g | [%] | 30–40 | 60–70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bauer, L.; Ranglová, K.; Masojídek, J.; Drosg, B.; Meixner, K. Digestate as Sustainable Nutrient Source for Microalgae—Challenges and Prospects. Appl. Sci. 2021, 11, 1056. https://0-doi-org.brum.beds.ac.uk/10.3390/app11031056
Bauer L, Ranglová K, Masojídek J, Drosg B, Meixner K. Digestate as Sustainable Nutrient Source for Microalgae—Challenges and Prospects. Applied Sciences. 2021; 11(3):1056. https://0-doi-org.brum.beds.ac.uk/10.3390/app11031056
Chicago/Turabian StyleBauer, Lisa, Karolína Ranglová, Jiří Masojídek, Bernhard Drosg, and Katharina Meixner. 2021. "Digestate as Sustainable Nutrient Source for Microalgae—Challenges and Prospects" Applied Sciences 11, no. 3: 1056. https://0-doi-org.brum.beds.ac.uk/10.3390/app11031056