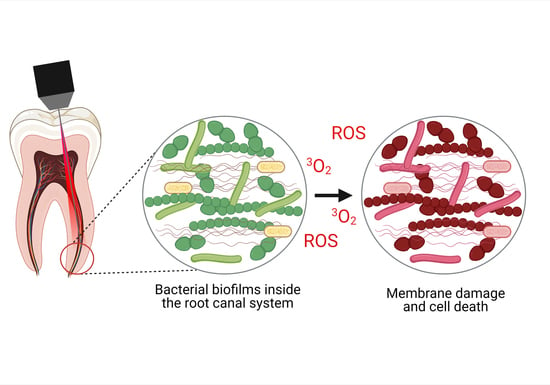
Advancing Photodynamic Therapy for Endodontic Disinfection with Nanoparticles: Present Evidence and Upcoming Approaches
Abstract
:1. Introduction
2. Overview of Chemo-Mechanical Disinfection and Current Intracanal Medications
3. Antimicrobial Photodynamic Therapy (aPDT)
4. aPDT Performance Based on In Vitro Studies
5. aPDT Performance in Clinical Studies
- Phenothiazine photosensitizers, mostly methylene blue (MBO) is the chosen PS.
- The concentrations of MB range from 25 to 100 μg/mL.
- Toluidine blue ortho (TBO) at 0.1 mg/mL concentration was used for one study.
- The most frequently used light source was a diode laser at 660 nm.
- Energy doses range from 1.4 to 200 J/cm2.
6. Nanostructures-Based Photosensitizers (PSs) to Overcome the Drawback of Conventional Endodontic Therapy: Present and Future Approaches
6.1. PS Loaded in Polymeric Nanoparticles
- Higher PS per mass content can be achieved when PS are conjugated with nanoparticles, leading to a higher ROS production.
- Reduced ability of the target microorganism to pump molecules out of the cell, which leads to reduced resistance against agents.
- Prospect of targeting the microorganisms due to the improved relationship between nanoparticles and bacteria because of the electronic charge of nanoparticles surfaces.
- The PS achieve higher stability when combined with nanoparticles.
- Lower physical quenching due to PS aggregation. Most PS form aggregates in the aqueous medium when they are in their free form, leading to self-quenching when they are excited and reduced ROS generation.
- Possibility of controlled release of ROS after photoactivation.
6.2. Nanoparticles as an Active PS
6.3. PS in Nanoemulsions
6.4. Quantum Dots in Antimicrobial Photodynamic Therapy
6.5. The Conjugates of PS and Nanodiamonds
6.6. The Conjugates of PS and Magnetic Nanoparticles
6.7. The Conjugates of PS and Liposomes
7. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Martinho, F.C.; Gomes, B.P.F.A. Quantification of Endotoxins and Cultivable Bacteria in Root Canal Infection before and after Chemomechanical Preparation with 2.5% Sodium Hypochlorite. J. Endod. 2008, 34, 268–272. [Google Scholar] [CrossRef]
- Prada, I.; Micó-Muñoz, P.; Giner-Lluesma, T.; Micó-Martínez, P.; Collado-Castellano, N.; Manzano-Saiz, A. Influence of Microbiology on Endodontic Failure. Literature Review. Med. Oral Patol. Oral Cir. Bucal 2019, 24, e364–e372. [Google Scholar] [CrossRef] [PubMed]
- Sjögren, U.; Figdor, D.; Persson, S.; Sundqvist, G. Influence of Infection at the Time of Root Filling on the Outcome of Endodontic Treatment of Teeth with Apical Periodontitis. Int. Endod. J. 1997, 30, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.G.; Walton, R.E. The Effect of Intramuscular Injection of Steroid on Posttreatment Endodontic Pain. J. Endod. 1984, 10, 584–588. [Google Scholar] [CrossRef]
- Rosen, E.; Elbahary, S.; Haj-Yahya, S.; Jammal, L.; Shemesh, H.; Tsesis, I. The Invasion of Bacterial Biofilms into the Dentinal Tubules of Extracted Teeth Retrofilled with Fluorescently Labeled Retrograde Filling Materials. Appl. Sci. 2020, 10, 6996. [Google Scholar] [CrossRef]
- Love, R.M. Enterococcus faecalis—A Mechanism for Its Role in Endodontic Failure. Int. Endod. J. 2001, 34, 399–405. [Google Scholar] [CrossRef]
- Distel, J.W.; Hatton, J.F.; Gillespie, M.J. Biofilm Formation in Medicated Root Canals. J. Endod. 2002, 28, 689–693. [Google Scholar] [CrossRef]
- Evans, M.; Davies, J.K.; Sundqvist, G.; Figdor, D. Mechanisms Involved in the Resistance of Enterococcus faecalis to Calcium Hydroxide. Int. Endod. J. 2002, 35, 221–228. [Google Scholar] [CrossRef]
- Jett, B.D.; Huycke, M.M.; Gilmore, M.S. Virulence of Enterococci. Clin. Microbiol. Rev. 1994, 7, 462–478. [Google Scholar] [CrossRef]
- Vivacqua-Gomes, N.; Gurgel-Filho, E.D.; Gomes, B.P.F.A.; Ferraz, C.C.R.; Zaia, A.A.; Souza-Filho, F.J. Recovery of Enterococcus faecalis after Single- or Multiple-Visit Root Canal Treatments Carried out in Infected Teeth Ex Vivo. Int. Endod. J. 2005, 38, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Waltimo, T.; Trope, M.; Haapasalo, M.; Ørstavik, D. Clinical Efficacy of Treatment Procedures in Endodontic Infection Control and One Year Follow-Up of Periapical Healing. J. Endod. 2005, 31, 863–866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basrani, B.; Tjäderhane, L.; Santos, J.M.; Pascon, E.; Grad, H.; Lawrence, H.P.; Friedman, S. Efficacy of Chlorhexidine- and Calcium Hydroxide–Containing Medicaments against Enterococcus faecalis in Vitro. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2003, 96, 618–624. [Google Scholar] [CrossRef]
- Melo, M.A.; Rolim, J.P.; Zanin, I.C.; Silva, J.J.; Paschoal, A.R.; Ayala, A.P.; Rodrigues, L.K. A Comparative Study of the Photosensitizer Penetration into Artificial Caries Lesions in Dentin Measured by the Confocal Raman Microscopy. Photochem. Photobiol. 2014, 90, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Samaranayake, L.P.; Yip, H.-K. Molecular Evaluation of Residual Endodontic Microorganisms after Instrumentation, Irrigation and Medication with Either Calcium Hydroxide or Septomixine. Oral Dis. 2004, 10, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, J.F.; Guimarães-Pinto, T.; Rôças, I.N. Effects of Chemomechanical Preparation with 2.5% Sodium Hypochlorite and Intracanal Medication with Calcium Hydroxide on Cultivable Bacteria in Infected Root Canals. J. Endod. 2007, 33, 800–805. [Google Scholar] [CrossRef]
- Peters, O.A.; Schönenberger, K.; Laib, A. Effects of Four Ni–Ti Preparation Techniques on Root Canal Geometry Assessed by Micro Computed Tomography. Int. Endod. J. 2001, 34, 221–230. [Google Scholar] [CrossRef]
- Melo, M.A.; Rolim, J.P.; Passos, V.F.; Lima, R.A.; Zanin, I.C.; Codes, B.M.; Rocha, S.S.; Rodrigues, L.K. Photodynamic antimicrobial chemotherapy and ultraconservative caries removal linked for management of deep caries lesions. Photodiagnosis Photodyn Ther. 2015, 12, 581–586. [Google Scholar] [CrossRef] [Green Version]
- Gomes, B.P.F.A.; Martinho, F.C.; Vianna, M.E. Comparison of 2.5% Sodium Hypochlorite and 2% Chlorhexidine Gel on Oral Bacterial Lipopolysaccharide Reduction from Primarily Infected Root Canals. J. Endod. 2009, 35, 1350–1353. [Google Scholar] [CrossRef]
- Estrela, C.; Sydney, G.B.; Bammann, L.L.; Felippe Júnior, O. Mechanism of Action of Calcium and Hydroxyl Ions of Calcium Hydroxide on Tissue and Bacteria. Braz. Dent. J. 1995, 6, 85–90. [Google Scholar]
- Siqueira, J.F.; Rôças, I.N.; Favieri, A.; Lima, K.C. Chemomechanical Reduction of the Bacterial Population in the Root Canal after Instrumentation and Irrigation with 1%, 2.5%, and 5.25% Sodium Hypochlorite. J. Endod. 2000, 26, 331–334. [Google Scholar] [CrossRef]
- Baras, B.H.; Melo, M.A.S.; Thumbigere-Math, V.; Tay, F.R.; Fouad, A.F.; Oates, T.W.; Weir, M.D.; Cheng, L.; Xu, H.H.K. Novel Bioactive and Therapeutic Root Canal Sealers with Antibacterial and Remineralization Properties. Materials 2020, 13, 1096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souza, M.A.; Tumelero Dias, C.; Zandoná, J.; Paim Hoffmann, I.; Sanches Menchik, V.H.; Palhano, H.S.; Bertol, C.D.; Rossato-Grando, L.G.; Cecchin, D.; de Figueiredo, J.A.P. Antimicrobial Activity of Hypochlorite Solutions and Reciprocating Instrumentation Associated with Photodynamic Therapy on Root Canals Infected with Enterococcus faecalis—An in Vitro Study. Photodiagnosis Photodyn. Ther. 2018, 23, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Orstavik, D.; Haapasalo, M. Disinfection by Endodontic Irrigants and Dressings of Experimentally Infected Dentinal Tubules. Endod. Dent. Traumatol. 1990, 6, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Fonzar, F.; Mollo, A.; Venturi, M.; Pini, P.; Fabian Fonzar, R.; Trullenque-Eriksson, A.; Esposito, M. Single versus Two Visits with 1-Week Intracanal Calcium Hydroxide Medication for Endodontic Treatment: One-Year Post-Treatment Results from a Multicentre Randomised Controlled Trial. Eur. J. Oral Implant. 2017, 10, 29–41. [Google Scholar]
- Erdem Hepsenoglu, Y.; Eyuboglu, T.F.; Özcan, M. Postoperative Pain Intensity after Single- versus Two-Visit Nonsurgical Endodontic Retreatment: A Randomized Clinical Trial. J. Endod. 2018, 44, 1339–1346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shuping, G.B.; Ørstavik, D.; Sigurdsson, A.; Trope, M. Reduction of Intracanal Bacteria Using Nickel-Titanium Rotary Instrumentation and Various Medications. J. Endod. 2000, 26, 751–755. [Google Scholar] [CrossRef] [PubMed]
- Abbott, P.V.; Hume, W.R.; Pearman, J.W. Antibiotics and Endodontics. Aust. Dent. J. 1990, 35, 50–60. [Google Scholar] [CrossRef]
- WHO. World Health Organization Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Tennert, C.; Feldmann, K.; Haamann, E.; Al-Ahmad, A.; Follo, M.; Wrbas, K.-T.; Hellwig, E.; Altenburger, M.J. Effect of Photodynamic Therapy (PDT) on Enterococcus faecalis Biofilm in Experimental Primary and Secondary Endodontic Infections. BMC Oral Health 2014, 14, 132. [Google Scholar] [CrossRef] [Green Version]
- Abrahamse, H.; Hamblin, M.R. New Photosensitizers for Photodynamic Therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Xuan, Y.; Koide, Y.; Zhiyentayev, T.; Tanaka, M.; Hamblin, M. Type I and Type II Mechanisms of Antimicrobial Photodynamic Therapy: An in Vitro Study on Gram-Negative and Gram-Positive Bacteria. Lasers Surg. Med. 2012, 44, 490–499. [Google Scholar] [CrossRef] [Green Version]
- Teófilo, M.Í.S.; de Carvalho Russi, T.M.A.Z.; de Barros Silva, P.G.; Balhaddad, A.A.; Melo, M.A.S.; Rolim, J.P.M.L. The Impact of Photosensitizers Selection on Bactericidal Efficacy Of PDT against Cariogenic Biofilms: A Systematic Review and Meta-Analysis. Photodiagnosis Photodyn. Ther. 2020, 102046. [Google Scholar] [CrossRef]
- Balhaddad, A.A.; AlQranei, M.S.; Ibrahim, M.S.; Weir, M.D.; Martinho, F.C.; Xu, H.H.K.; Melo, M.A.S. Light Energy Dose and Photosensitizer Concentration Are Determinants of Effective Photo-Killing against Caries-Related Biofilms. Int. J. Mol. Sci. 2020, 21, 7612. [Google Scholar] [CrossRef] [PubMed]
- Hardee, M.W.; Miserendino, L.J.; Kos, W.; Walia, H. Evaluation of the Antibacterial Effects of Intracanal Nd: YAG Laser Irradiation. J. Endod. 1994, 20, 377–380. [Google Scholar] [CrossRef]
- Melo, M.A.S.; De-paula, D.M.; Lima, J.P.M.; Borges, F.M.C.; Steiner-oliveira, C.; Nobre-dos-santos, M.; Zanin, I.C.J.; Barros, E.B.; Rodrigues, L.K.A. In vitro photodynamic antimicrobial chemotherapy in dentine contaminated by cariogenic bacteria. Laser Phys. 2010, 20, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Balhaddad, A.A.; Garcia, I.M.; Ibrahim, M.S.; Rolim, J.P.M.L.; Gomes, E.A.B.; Martinho, F.C.; Collares, F.M.; Xu, H.; Melo, M.A.S. Prospects on Nano-Based Platforms for Antimicrobial Photodynamic Therapy Against Oral Biofilms. Photobiomodul. Photomed. Laser Surg. 2020, 38, 481–496. [Google Scholar] [CrossRef] [PubMed]
- Zohre, R.; Ali, Y.; Mostafa, J.; Samaneh, R. Nondrug Antimicrobial Techniques: Electromagnetic Fields and Photodynamic Therapy. Biomed. Pharmacol. J. 2015, 8, 147–155. [Google Scholar] [CrossRef]
- Plotino, G.; Grande, N.M.; Mercade, M. Photodynamic Therapy in Endodontics. Int. Endod. J. 2019, 52, 760–774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melo, M.A.S. Photodynamic Antimicrobial Chemotherapy as a Strategy for Dental Caries: Building a More Conservative Therapy in Restorative Dentistry. Photomed. Laser Surg. 2014, 32, 589–591. [Google Scholar] [CrossRef]
- Tavares, A.; Carvalho, C.M.B.; Faustino, M.A.; Neves, M.G.P.M.S.; Tomé, J.P.C.; Tomé, A.C.; Cavaleiro, J.A.S.; Cunha, A.; Gomes, N.C.M.; Alves, E.; et al. Antimicrobial Photodynamic Therapy: Study of Bacterial Recovery Viability and Potential Development of Resistance after Treatment. Mar. Drugs 2010, 8, 91–105. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Qin, R.; Zaat, S.A.J.; Breukink, E.; Heger, M. Antibacterial Photodynamic Therapy: Overview of a Promising Approach to Fight Antibiotic-Resistant Bacterial Infections. J. Clin. Transl. Res. 2015, 1, 140–167. [Google Scholar]
- Pfitzner, A.; Sigusch, B.W.; Albrecht, V.; Glockmann, E. Killing of Periodontopathogenic Bacteria by Photodynamic Therapy. J. Periodontol. 2004, 75, 1343–1349. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.; Lai, C.-H. Bactericidal Effects of Different Laser Wavelengths on Periodontopathic Germs in Photodynamic Therapy. Lasers Med. Sci. 2003, 18, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Seal, G.J.; Ng, Y.-L.; Spratt, D.; Bhatti, M.; Gulabivala, K. An in Vitro Comparison of the Bactericidal Efficacy of Lethal Photosensitization or Sodium Hyphochlorite Irrigation on Streptococcus Intermedius Biofilms in Root Canals. Int. Endod. J. 2002, 35, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Silva Garcez, A.; Núñez, S.C.; Lage-Marques, J.L.; Jorge, A.O.C.; Ribeiro, M.S. Efficiency of NaOCl and Laser-Assisted Photosensitization on the Reduction of Enterococcus faecalis in Vitro. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 102, e93–e98. [Google Scholar] [CrossRef] [PubMed]
- Garcez, A.S.; Ribeiro, M.S.; Tegos, G.P.; Núñez, S.C.; Jorge, A.O.C.; Hamblin, M.R. Antimicrobial Photodynamic Therapy Combined with Conventional Endodontic Treatment to Eliminate Root Canal Biofilm Infection. Lasers Surg. Med. 2007, 39, 59–66. [Google Scholar] [CrossRef] [Green Version]
- Foschi, F.; Fontana, C.R.; Ruggiero, K.; Riahi, R.; Vera, A.; Doukas, A.G.; Pagonis, T.C.; Kent, R.; Stashenko, P.P.; Soukos, N.S. Photodynamic Inactivation of Enterococcus faecalis in Dental Root Canals in Vitro. Lasers Surg. Med. 2007, 39, 782–787. [Google Scholar] [CrossRef]
- Nunes, M.R.; Mello, I.; Franco, G.C.N.; de Medeiros, J.M.F.; Dos Santos, S.S.F.; Habitante, S.M.; Lage-Marques, J.L.; Raldi, D.P. Effectiveness of Photodynamic Therapy against Enterococcus faecalis, with and without the Use of an Intracanal Optical Fiber: An in Vitro Study. Photomed. Laser Surg. 2011, 29, 803–808. [Google Scholar] [CrossRef] [Green Version]
- Saber, S.E.-D.M.; El-Hady, S.A. Development of an Intracanal Mature Enterococcus faecalis Biofilm and Its Susceptibility to Some Antimicrobial Intracanal Medications; an in Vitro Study. Eur. J. Dent. 2012, 6, 43–50. [Google Scholar]
- Miranda, R.G.; Santos, E.B.; Souto, R.M.; Gusman, H.; Colombo, A.P.V. Ex Vivo Antimicrobial Efficacy of the EndoVac System plus Photodynamic Therapy Associated with Calcium Hydroxide against Intracanal Enterococcus faecalis. Int. Endod. J. 2013, 46, 499–505. [Google Scholar] [CrossRef]
- Ahangari, Z.; Mojtahed Bidabadi, M.; Asnaashari, M.; Rahmati, A.; Tabatabaei, F.S. Comparison of the Antimicrobial Efficacy of Calcium Hydroxide and Photodynamic Therapy Against Enterococcus faecalis and Candida Albicans in Teeth with Periapical Lesions; An In Vivo Study. J. Lasers Med. Sci. 2017, 8, 72–78. [Google Scholar] [CrossRef] [Green Version]
- Asnaashari, M.; Ashraf, H.; Rahmati, A.; Amini, N. A Comparison between Effect of Photodynamic Therapy by LED and Calcium Hydroxide Therapy for Root Canal Disinfection against Enterococcus faecalis: A Randomized Controlled Trial. Photodiagnosis Photodyn. Ther. 2017, 17, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Rabello, D.G.D.; Corazza, B.J.M.; Ferreira, L.L.; Santamaria, M.P.; Gomes, A.P.M.; Martinho, F.C. Does Supplemental Photodynamic Therapy Optimize the Disinfection of Bacteria and Endotoxins in One-Visit and Two-Visit Root Canal Therapy? A Randomized Clinical Trial. Photodiagnosis Photodyn. Ther. 2017, 19, 205–211. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, C.C.; Chaves Júnior, S.P.; Pereira, G.L.D.; Fontes, K.B.F.d.C.; Antunes, L.A.A.; Póvoa, H.C.C.; Antunes, L.S.; Iorio, N.L.P.P. Antimicrobial Photodynamic Therapy Associated with Conventional Endodontic Treatment: A Clinical and Molecular Microbiological Study. Photochem. Photobiol. 2018, 94, 351–356. [Google Scholar] [CrossRef]
- De Miranda, R.G.; Colombo, A.P.V. Clinical and Microbiological Effectiveness of Photodynamic Therapy on Primary Endodontic Infections: A 6-Month Randomized Clinical Trial. Clin. Oral Investig. 2018, 22, 1751–1761. [Google Scholar] [CrossRef] [PubMed]
- Barciela, B.; da Silva Limoeiro, A.G.; Bueno, C.E.; Fernandes, S.L.; Mandarini, D.R.; Boer, N.C.; Camara Fernandes, K.G.; Rocha, D.G. In Vivo Evaluation of Painful Symptomatology after Endodontic Treatment with or without the Use of Photodynamic Therapy. J. Conserv. Dent. 2019, 22, 332–335. [Google Scholar] [CrossRef] [PubMed]
- Coelho, M.S.; Vilas-Boas, L.; Tawil, P.Z. The Effects of Photodynamic Therapy on Postoperative Pain in Teeth with Necrotic Pulps. Photodiagnosis Photodyn. Ther. 2019, 27. [Google Scholar] [CrossRef]
- Hu, D.; Pan, M.; Yu, Y.; Sun, A.; Shi, K.; Qu, Y.; Qian, Z. Application of Nanotechnology for Enhancing Photodynamic Therapy via Ameliorating, Neglecting, or Exploiting Tumor Hypoxia. View 2020, 1, e6. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on Nanoparticles and Nanostructured Materials: History, Sources, Toxicity and Regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef] [Green Version]
- Harris, F.; Chatfield, L.K.; Phoenix, D.A. Phenothiazinium Based Photosensitisers–Photodynamic Agents with a Multiplicity of Cellular Targets and Clinical Applications. Curr. Drug Targets 2005, 6, 615–627. [Google Scholar] [CrossRef]
- Kishen, A. Advanced Therapeutic Options for Endodontic Biofilms. Endod. Top. 2010, 22, 99–123. [Google Scholar] [CrossRef]
- Shrestha, A.; Kishen, A. Antibacterial Nanoparticles in Endodontics: A Review. J. Endod. 2016, 42, 1417–1426. [Google Scholar] [CrossRef]
- Abou Neel, E.A.; Bozec, L.; Perez, R.A.; Kim, H.-W.; Knowles, J.C. Nanotechnology in Dentistry: Prevention, Diagnosis, and Therapy. Int. J. Nanomed. 2015, 10, 6371–6394. [Google Scholar] [CrossRef] [Green Version]
- Leung, K.C.-F.; Seneviratne, C.J.; Li, X.; Leung, P.C.; Lau, C.B.S.; Wong, C.-H.; Pang, K.Y.; Wong, C.W.; Wat, E.; Jin, L. Synergistic Antibacterial Effects of Nanoparticles Encapsulated with Scutellaria Baicalensis and Pure Chlorhexidine on Oral Bacterial Biofilms. Nanomaterials 2016, 6, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerrini, L.; Alvarez-Puebla, R.A.; Pazos-Perez, N. Surface Modifications of Nanoparticles for Stability in Biological Fluids. Materials 2018, 11, 1154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zamboni, C.G.; Kozielski, K.L.; Vaughan, H.J.; Nakata, M.M.; Kim, J.; Higgins, L.J.; Pomper, M.G.; Green, J.J. Polymeric Nanoparticles as Cancer-Specific DNA Delivery Vectors to Human Hepatocellular Carcinoma. J. Control. Release 2017, 263, 18–28. [Google Scholar] [CrossRef]
- Balhaddad, A.A.; Kansara, A.A.; Hidan, D.; Weir, M.D.; Xu, H.H.K.; Melo, M.A.S. Toward Dental Caries: Exploring Nanoparticle-Based Platforms and Calcium Phosphate Compounds for Dental Restorative Materials. Bioact. Mater. 2019, 4, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Jazayeri, H.E.; Lee, S.-M.; Kuhn, L.; Fahimipour, F.; Tahriri, M.; Tayebi, L. Polymeric Scaffolds for Dental Pulp Tissue Engineering: A Review. Dent. Mater. 2020, 36, e47–e58. [Google Scholar] [CrossRef]
- Camacho-Alonso, F.; Julián-Belmonte, E.; Chiva-García, F.; Martínez-Beneyto, Y. Bactericidal Efficacy of Photodynamic Therapy and Chitosan in Root Canals Experimentally Infected with Enterococcus faecalis: An In Vitro Study. Photomed. Laser Surg. 2017, 35, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, A.; Kishen, A. Polycationic Chitosan-Conjugated Photosensitizer for Antibacterial Photodynamic Therapy. Photochem. Photobiol. 2012, 88, 577–583. [Google Scholar] [CrossRef]
- Pagonis, T.C.; Chen, J.; Fontana, C.R.; Devalapally, H.; Ruggiero, K.; Song, X.; Foschi, F.; Dunham, J.; Skobe, Z.; Yamazaki, H.; et al. Nanoparticle-Based Endodontic Antimicrobial Photodynamic Therapy. J. Endod. 2010, 36, 322–328. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, C.; Tsujimoto, Y.; Yamamoto, Y. The Effect of Irradiation Wavelengths and the Crystal Structures of Titanium Dioxide on the Formation of Singlet Oxygen for Bacterial Killing. J. Clin. Biochem. Nutr. 2012, 51, 128–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sehmi, S.K.; Noimark, S.; Pike, S.D.; Bear, J.C.; Peveler, W.J.; Williams, C.K.; Shaffer, M.S.P.; Allan, E.; Parkin, I.P.; MacRobert, A.J. Enhancing the Antibacterial Activity of Light-Activated Surfaces Containing Crystal Violet and ZnO Nanoparticles: Investigation of Nanoparticle Size, Capping Ligand, and Dopants. ACS Omega 2016, 1, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Aydın, H.; Er, K.; Kuştarcı, A.; Akarsu, M.; Gençer, G.M.; Er, H.; Felek, R. Antibacterial Activity of Silver Nanoparticles Activated by Photodynamic Therapy in Infected Root Canals. Dent. Med. Probl. 2020, 57, 393–400. [Google Scholar] [CrossRef]
- Diogo, P.; F. Faustino, M.A.; P. M. S. Neves, M.G.; Palma, P.J.; P. Baptista, I.; Gonçalves, T.; Santos, J.M. An Insight into Advanced Approaches for Photosensitizer Optimization in Endodontics—A Critical Review. J. Funct. Biomater. 2019, 10, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McClements, D.J. Nanoemulsions versus Microemulsions: Terminology, Differences, and Similarities. Soft Matter 2012, 8, 1719–1729. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; González, C.; Maestro, A.; Solè, I.; Pey, C.M.; Nolla, J. Nano-Emulsions: New Applications and Optimization of Their Preparation. Curr. Opin. Colloid Interface Sci. 2008, 13, 245–251. [Google Scholar] [CrossRef]
- Ribeiro, A.P.D.; Andrade, M.C.; Bagnato, V.S.; Vergani, C.E.; Primo, F.L.; Tedesco, A.C.; Pavarina, A.C. Antimicrobial Photodynamic Therapy against Pathogenic Bacterial Suspensions and Biofilms Using Chloro-Aluminum Phthalocyanine Encapsulated in Nanoemulsions. Lasers Med. Sci. 2015, 30, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Schuenck-Rodrigues, R.A.; de Oliveira de Siqueira, L.B.; Dos Santos Matos, A.P.; da Costa, S.P.; da Silva Cardoso, V.; Vermelho, A.B.; Colombo, A.P.V.; Oliveira, C.A.; Santos-Oliveira, R.; Ricci-Júnior, E. Development, Characterization and Photobiological Activity of Nanoemulsion Containing Zinc Phthalocyanine for Oral Infections Treatment. J. Photochem. Photobiol. B 2020, 211, 112010. [Google Scholar] [CrossRef]
- Garcia, I.M.; Leitune, V.C.B.; Kist, T.L.; Takimi, A.; Samuel, S.M.W.; Collares, F.M. Quantum Dots as Nonagglomerated Nanofillers for Adhesive Resins. J. Dent. Res. 2016, 95, 1401–1407. [Google Scholar] [CrossRef] [PubMed]
- Meulenkamp, E.A. Synthesis and Growth of ZnO Nanoparticles. J. Phys. Chem. B 1998, 102, 5566–5572. [Google Scholar] [CrossRef]
- Viana, O.S.; Ribeiro, M.S.; Fontes, A.; Santos, B.S. Quantum Dots in Photodynamic Therapy. In Redox-Active Therapeutics; Batinić-Haberle, I., Rebouças, J.S., Spasojević, I., Eds.; Oxidative Stress in Applied Basic Research and Clinical Practice; Springer International Publishing: Cham, Germany, 2016; pp. 525–539. ISBN 978-3-319-30705-3. [Google Scholar]
- Zhang, Y.; Liu, Y.; Li, C.; Chen, X.; Wang, Q. Controlled Synthesis of Ag2S Quantum Dots and Experimental Determination of the Exciton Bohr Radius. J. Phys. Chem. C 2014, 118, 4918–4923. [Google Scholar] [CrossRef]
- Xu, W.; Liu, W.; Schmidt, J.F.; Zhao, W.; Lu, X.; Raab, T.; Diederichs, C.; Gao, W.; Seletskiy, D.V.; Xiong, Q. Correlated Fluorescence Blinking in Two-Dimensional Semiconductor Heterostructures. Nature 2017, 541, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Efros, A.L.; Nesbitt, D.J. Origin and Control of Blinking in Quantum Dots. Nat. Nanotechnol. 2016, 11, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Garcia, I.M.; Souza, V.S.; Scholten, J.D.; Collares, F.M. Quantum Dots of Tantalum Oxide with an Imidazolium Ionic Liquid as Antibacterial Agent for Adhesive Resin. J. Adhes. Dent. 2020, 22, 207–214. [Google Scholar] [CrossRef]
- Garcia, I.M.; Leitune, V.C.B.; Visioli, F.; Samuel, S.M.W.; Collares, F.M. Influence of Zinc Oxide Quantum Dots in the Antibacterial Activity and Cytotoxicity of an Experimental Adhesive Resin. J. Dent. 2018, 73, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Garcia, I.M.; Souza, V.S.; Hellriegel, C.; Scholten, J.D.; Collares, F.M. Ionic Liquid-Stabilized Titania Quantum Dots Applied in Adhesive Resin. J. Dent. Res. 2019, 98, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Alves, L.P.; Pilla, V.; Murgo, D.O.A.; Munin, E. Core-Shell Quantum Dots Tailor the Fluorescence of Dental Resin Composites. J. Dent. 2010, 38, 149–152. [Google Scholar] [CrossRef]
- Pourhajibagher, M.; Parker, S.; Chiniforush, N.; Bahador, A. Photoexcitation Triggering via Semiconductor Graphene Quantum Dots by Photochemical Doping with Curcumin versus Perio-Pathogens Mixed Biofilms. Photodiagnosis Photodyn. Ther. 2019, 28, 125–131. [Google Scholar] [CrossRef]
- Owusu, E.G.A.; MacRobert, A.J.; Naasani, I.; Parkin, I.P.; Allan, E.; Yaghini, E. Photoactivable Polymers Embedded with Cadmium-Free Quantum Dots and Crystal Violet: Efficient Bactericidal Activity against Clinical Strains of Antibiotic-Resistant Bacteria. ACS Appl. Mater. Interfaces 2019, 11, 12367–12378. [Google Scholar] [CrossRef] [Green Version]
- Hui, Y.Y.; Cheng, C.-L.; Chang, H.-C. Nanodiamonds for Optical Bioimaging. J. Phys. D Appl. Phys. 2010, 43, 374021. [Google Scholar] [CrossRef]
- McGuinness, L.P.; Yan, Y.; Stacey, A.; Simpson, D.A.; Hall, L.T.; Maclaurin, D.; Prawer, S.; Mulvaney, P.; Wrachtrup, J.; Caruso, F.; et al. Quantum Measurement and Orientation Tracking of Fluorescent Nanodiamonds inside Living Cells. Nat. Nanotechnol. 2011, 6, 358–363. [Google Scholar] [CrossRef]
- Protopapa, P.; Kontonasaki, E.; Bikiaris, D.; Paraskevopoulos, K.M.; Koidis, P. Reinforcement of a PMMA Resin for Fixed Interim Prostheses with Nanodiamonds. Dent. Mater. J. 2011, 30, 222–231. [Google Scholar] [CrossRef] [Green Version]
- Cao, W.; Zhang, Y.; Wang, X.; Li, Q.; Xiao, Y.; Li, P.; Wang, L.; Ye, Z.; Xing, X. Novel Resin-Based Dental Material with Anti-Biofilm Activity and Improved Mechanical Property by Incorporating Hydrophilic Cationic Copolymer Functionalized Nanodiamond. J. Mater. Sci. Mater. Med. 2018, 29, 162. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-K.; Kim, S.V.; Limansubroto, A.N.; Yen, A.; Soundia, A.; Wang, C.-Y.; Shi, W.; Hong, C.; Tetradis, S.; Kim, Y.; et al. Nanodiamond-Gutta Percha Composite Biomaterials for Root Canal Therapy. ACS Nano 2015, 9, 11490–11501. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, G.R.; López-Abarrategui, C.; de la Serna Gómez, I.; Dias, S.C.; Otero-González, A.J.; Franco, O.L. Antimicrobial Magnetic Nanoparticles Based-Therapies for Controlling Infectious Diseases. Int. J. Pharm. 2019, 555, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Arruebo, M.; Fernández-Pacheco, R.; Ibarra, M.R.; Santamaría, J. Magnetic Nanoparticles for Drug Delivery. Nano Today 2007, 2, 22–32. [Google Scholar] [CrossRef]
- Ashkbar, A.; Rezaei, F.; Attari, F.; Ashkevarian, S. Treatment of Breast Cancer in Vivo by Dual Photodynamic and Photothermal Approaches with the Aid of Curcumin Photosensitizer and Magnetic Nanoparticles. Sci. Rep. 2020, 10, 21206. [Google Scholar] [CrossRef]
- Sun, X.; Wang, L.; Lynch, C.D.; Sun, X.; Li, X.; Qi, M.; Ma, C.; Li, C.; Dong, B.; Zhou, Y.; et al. Nanoparticles Having Amphiphilic Silane Containing Chlorin E6 with Strong Anti-Biofilm Activity against Periodontitis-Related Pathogens. J. Dent. 2019, 81, 70–84. [Google Scholar] [CrossRef]
- Ghosh, S.; Carter, K.A.; Lovell, J.F. Liposomal Formulations of Photosensitizers. Biomaterials 2019, 218, 119341. [Google Scholar] [CrossRef]
- Skupin-Mrugalska, P.; Piskorz, J.; Goslinski, T.; Mielcarek, J.; Konopka, K.; Düzgüneş, N. Current Status of Liposomal Porphyrinoid Photosensitizers. Drug Discov. Today 2013, 18, 776–784. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, L.; Cao, H.; Li, Q.; Li, Y.; Han, M.; Wang, H.; Li, J. Photodynamic Therapy with Liposomes Encapsulating Photosensitizers with Aggregation-Induced Emission. Nano Lett. 2019, 19, 1821–1826. [Google Scholar] [CrossRef] [PubMed]
- Düzgüneş, N.; Piskorz, J.; Skupin-Mrugalska, P.; Goslinski, T.; Mielcarek, J.; Konopka, K. Photodynamic Therapy of Cancer with Liposomal Photosensitizers. Ther. Deliv. 2018, 9, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Longo, J.P.F.; Leal, S.C.; Simioni, A.R.; de Fátima Menezes Almeida-Santos, M.; Tedesco, A.C.; Azevedo, R.B. Photodynamic Therapy Disinfection of Carious Tissue Mediated by Aluminum-Chloride-Phthalocyanine Entrapped in Cationic Liposomes: An in Vitro and Clinical Study. Lasers Med. Sci. 2012, 27, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Ko, A.; Yee, M.; Skupin-Mrugalska, P.; Düzgünes, N. Photodynamic Therapy of Porphyromonas Gingivalis via Liposome-Encapsulated Sensitizers. J. Calif. Dent. Assoc. 2013, 41, 827–830. [Google Scholar] [PubMed]
- Kranz, S.; Guellmar, A.; Völpel, A.; Gitter, B.; Albrecht, V.; Sigusch, B.W. Photodynamic Suppression of Enterococcus faecalis Using the Photosensitizer MTHPC. Lasers Surg. Med. 2011, 43, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Ossmann, A.; Kranz, S.; Andre, G.; Völpel, A.; Albrecht, V.; Fahr, A.; Sigusch, B.W. Photodynamic Killing of Enterococcus faecalis in Dentinal Tubules Using MTHPC Incorporated in Liposomes and Invasomes. Clin. Oral Investig. 2015, 19, 373–384. [Google Scholar] [CrossRef]
- Ramos, U.D.; Suaid, F.; Wikesjö, U.M.E.; Susin, C.; Vital, P.C.; de Souza, S.L.S.; Messora, M.R.; Palioto, D.B.; Novaes, A.B. Microbiologic Effect of Two Topical Anti-Infective Treatments on Ligature-Induced Peri-Implantitis: A Pilot Study in Dogs. J. Periodontol. 2018, 89, 995–1002. [Google Scholar] [CrossRef]






| Chemical Class | Photosensitizer | Spectra Absorption | Basic Chemical Structure |
|---|---|---|---|
| Phthalocyanines | Zinc phthalocyanine | 600–700 nm |  |
| Aluminum disulphonated phthalocyanine (AlPcS2) | 675 nm |  | |
| Phenothiazines | Methylene blue | 600–650 nm |  |
| Toluidine blue ortho | 632–638 nm |  | |
| Porphyrin platform | Porphyrin | 632 nm |  |
| Rose Bengal | 500 nm |  | |
| Chlorophyll platform Chloryns | Chloryn e6 | 645–675 nm |  |
| Author | Target Tooth | PS | Light Parameters | Protocol | Main Outcome |
|---|---|---|---|---|---|
| Ahangari Z et al. (2017) [51] | Root canal treated molars with periapical lesion | MB (50 mg/mL) | Diode laser (808 nm; 0.2 W) | The PS intracanal application; 5 min + 10 s irradiation | Both aPDT and calcium hydroxide therapies significantly reduced the CFUs counts of E. faecalis and C. albicans, with no significant difference between the two approaches. |
| Asnaashari M et al. (2017) [52] | Root canal treated molars with periapical lesion but with no existing pain, swelling, or any systematic diseases | TBO (0.1 mg/mL) | Red LED (630 nm; 2–4 mW; 1.2–4.4 J/cm2) | The PS intracanal application; 5 min + 60 s irradiation | The microbiological sampling revealed that aPDT could disinfect the canals in a single visit. aPDT was associated with a lower number of colonies compared to the calcium hydroxide group. |
| Rabello DGD et al. (2017) [53] | Root canal treated teeth (single root) with apical periodontitis | MB (0.1 mg/mL) | Diode Laser (660 nm; 60 mW; 129 J/cm2) | The PS intracanal application; 1 min + 2 min irradiation | In the single-visit treatment, aPDT significantly reduced the bacterial load inside the root canals.In the two-visit treatment, aPDT was used following calcium hydroxide, and no additional benefits from using the aPDT were observed. Using the aPDT did not complement the reduction of endotoxins inside the canals, while calcium hydroxide therapy was significantly reduced. |
| da Silva C.C. et al. (2018) [54] | Non-treated single-rooted teeth diagnosed with necrotic pulp and apical periodontitis | MB (100 μg/mL) | Indium-gallium-aluminum-phosphide laser (660 nm; 100 mW; 7 J/cm2) | The PS intracanal application; 5 min + 2 × 40 s irradiation at the apical level +1 × 30 s irradiation By light tip movement | aPDT was associated with significant E. faecalis inhibition at the second visit. |
| de Mirandaand Colombo (2018) [55] | Non- treated molars diagnosed with pulp necrosis and radiographic apical periodontitis | MB (25 μg/mL) | Diode laser (660 nm; 100 mW) | The PS intracanal application; 5 min + 5 min irradiation | Both aPDT and conventional therapies promoted an increase in periapical healing over time, but aPDT resulted in better healing at 6-month follow-up compared to conventional endodontic treatment alone. |
| Barciela B. et al. (2019) [56] | Non-treated single-rooted teeth diagnosed with necrotic pulp and apical periodontitis | MB (0.5 mg/mL) | Diode laser (660 nm; 320 J/cm2) | The PS intracanal application; 5 min + 90 s irradiation | The post-operative pain between aPDT and conventional endodontic treatment was similar. |
| Coelho M.S. et al. (2019) [57] | Non-treated single-rooted teeth diagnosed with necrotic pulp | MB (1.56 μM/mL) | CO2 or ND:Yag (660 nm; 100 mW; 600 J/cm2) | The PS intracanal application; 2 min + 3 min irradiation | aPDT was efficient in reducing post-operative pain in single-visit root canal treatment of teeth with necrotic pulps. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfirdous, R.A.; Garcia, I.M.; Balhaddad, A.A.; Collares, F.M.; Martinho, F.C.; Melo, M.A.S. Advancing Photodynamic Therapy for Endodontic Disinfection with Nanoparticles: Present Evidence and Upcoming Approaches. Appl. Sci. 2021, 11, 4759. https://0-doi-org.brum.beds.ac.uk/10.3390/app11114759
Alfirdous RA, Garcia IM, Balhaddad AA, Collares FM, Martinho FC, Melo MAS. Advancing Photodynamic Therapy for Endodontic Disinfection with Nanoparticles: Present Evidence and Upcoming Approaches. Applied Sciences. 2021; 11(11):4759. https://0-doi-org.brum.beds.ac.uk/10.3390/app11114759
Chicago/Turabian StyleAlfirdous, Rayyan A., Isadora M. Garcia, Abdulrahman A. Balhaddad, Fabrício M. Collares, Frederico C. Martinho, and Mary Anne S. Melo. 2021. "Advancing Photodynamic Therapy for Endodontic Disinfection with Nanoparticles: Present Evidence and Upcoming Approaches" Applied Sciences 11, no. 11: 4759. https://0-doi-org.brum.beds.ac.uk/10.3390/app11114759