The Effects of Polyphenol-Rich Black Elderberry on Oxidative Stress and Hepatic Cholesterol Metabolism
Abstract
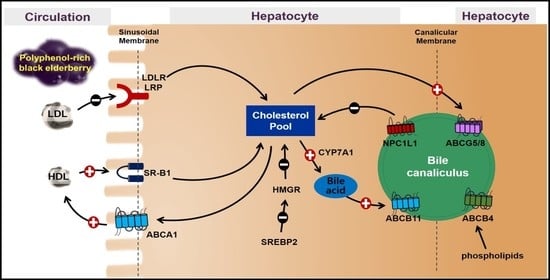
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Quantification of Total Phenolic, Flavonoid, and Anthocyanin Content
2.3. Total Antioxidant Capacity
2.4. Cell Culture and Treatment
2.5. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.6. Western Blot
2.7. Statistical Analysis
3. Results
3.1. Composition and Total Antioxidant Capacity of BEE
3.2. Effects of BEE on the Genes Involved in Cholesterol Biosynthesis and Absorption
3.3. Effects of BEE on the Genes Involved in Biliary Cholesterol Efflux
3.4. Effects of BEE on the Genes Involved in Fatty Acid Metabolism
3.5. Effects of BEE on the Alteration of HDACs and SIRTs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics—2021 Update: A Report From the American Heart Association. Circulation 2021, 143, e254–e743. [Google Scholar] [CrossRef]
- Mommersteeg, P.M.; Denollet, J.; Spertus, J.A.; Pedersen, S.S. Health status as a risk factor in cardiovascular disease: A systematic review of current evidence. Am. Hear. J. 2009, 157, 208–218. [Google Scholar] [CrossRef]
- Nelson, R.H. Hyperlipidemia as a Risk Factor for Cardiovascular Disease. Prim. Care Clin. Off. Pr. 2013, 40, 195–211. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.; Yang, H.; Song, B.-L. Mechanisms and regulation of cholesterol homeostasis. Nat. Rev. Mol. Cell Biol. 2020, 21, 225–245. [Google Scholar] [CrossRef]
- Groen, A.K.; Bloks, V.W.; Verkade, H.J.; Kuipers, F. Cross-talk between liver and intestine in control of cholesterol and energy homeostasis. Mol. Asp. Med. 2014, 37, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Millar, J.S.; Cuchel, M. Cholesterol metabolism in humans: A review of methods and comparison of results. Curr. Opin. Lipidol. 2018, 29, 1–9. [Google Scholar] [CrossRef]
- Wang, D.Q.H.; Portincasa, P.; Tso, P. Transintestinal cholesterol excretion: A secondary, nonbiliary pathway contributing to reverse cholesterol transport. Hepatology 2017, 66, 1337–1340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Temel, R.-E.; Brown, J.-M. A new framework for reverse cholesterol transport: Non-biliary contributions to reverse cholesterol transport. World J. Gastroenterol. 2010, 16, 5946–5952. [Google Scholar] [CrossRef]
- Temel, R.E.; Brown, J.M. A new model of reverse cholesterol transport: enTICEing strategies to stimulate intestinal cholesterol excretion. Trends Pharmacol. Sci. 2015, 36, 440–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grefhorst, A.; Verkade, H.J.; Groen, A.K. The TICE Pathway: Mechanisms and Lipid-Lowering Therapies. Methodist DeBakey Cardiovasc. J. 2019, 15, 70–76. [Google Scholar] [CrossRef]
- Mizranita, V.; Pratisto, E.H. Statin-associated ocular disorders: The FDA and ADRAC data. Int. J. Clin. Pharm. 2015, 37, 844–850. [Google Scholar] [CrossRef]
- Simonson, W. Update on statin drugs for lipid disorders. Geriatr. Nurs. 2018, 39, 350–351. [Google Scholar] [CrossRef]
- Ábel, T.; Fehér, J. Statin treatment and muscle disorders. Orvosi Hetil. 2009, 150, 261–263. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, T.; Morganti, R.; Marchioli, R.; De Caterina, R. Cholesterol Lowering and Stroke: No Longer Room for Pleiotropic Effects of Statins–Confirmation from PCSK9 Inhibitor Studies. Am. J. Med. 2020, 133, 95–99.e6. [Google Scholar] [CrossRef]
- Sgro, C.; Escousse, A. Side effects of fibrates (except liver and muscle). Therapie 1991, 46, 351–354. [Google Scholar]
- Mendonça, R.D.; Carvalho, N.C.; Martin-Moreno, J.M.; Pimenta, A.M.; Lopes, A.C.S.; Gea, A.; Martinez-Gonzalez, M.; Bes-Rastrollo, M. Total polyphenol intake, polyphenol subtypes and incidence of cardiovascular disease: The SUN cohort study. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Rienks, J.; Barbaresko, J.; Nöthlings, U. Association of Polyphenol Biomarkers with Cardiovascular Disease and Mortality Risk: A Systematic Review and Meta-Analysis of Observational Studies. Nutrients 2017, 9, 415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quiñones, M.; Miguel, M.; Aleixandre, A. Beneficial effects of polyphenols on cardiovascular disease. Pharmacol. Res. 2013, 68, 125–131. [Google Scholar] [CrossRef]
- Ito, F. Polyphenols can Potentially Prevent Atherosclerosis and Cardiovascular Disease by Modulating Macrophage Cholesterol Metabolism. Curr. Mol. Pharmacol. 2020, 14, 175–190. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, K. Polyphenols Regulate Endothelial Functions and Reduce the Risk of Cardiovascular Disease. Curr. Pharm. Des. 2019, 25, 2443–2458. [Google Scholar] [CrossRef]
- Tangney, C.C.; Rasmussen, H.E. Polyphenols, Inflammation, and Cardiovascular Disease. Curr. Atheroscler. Rep. 2013, 15, 1–10. [Google Scholar] [CrossRef]
- Quiñones, M.; Miguel, M.; Aleixandre, A. The polyphenols, naturally occurring compounds with beneficial effects on cardiovascular disease. Nutr. Hosp. 2012, 27, 76–89. [Google Scholar] [CrossRef]
- Barak, V.; Halperin, T.; Kalickman, I. The effect of Sambucol, a black elderberry-based, natural product, on the production of human cytokines: I. Inflammatory cytokines. Eur. Cytokine Netw. 2001, 12, 290–296. [Google Scholar]
- Młynarczyk, K.; Walkowiak-Tomczak, D.; Łysiak, G.P. Bioactive properties of Sambucus nigra L. as a functional ingredient for food and pharmaceutical industry. J. Funct. Foods 2018, 40, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Młynarczyk, K.; Walkowiak-Tomczak, D.; Staniek, H.; Kidoń, M.; Łysiak, G.P. The Content of Selected Minerals, Bioactive Compounds, and the Antioxidant Properties of the Flowers and Fruit of Selected Cultivars and Wildly Growing Plants of Sambucus nigra L. Molecules 2020, 25, 876. [Google Scholar] [CrossRef] [Green Version]
- Domínguez, R.; Zhang, L.; Rocchetti, G.; Lucini, L.; Pateiro, M.; Munekata, P.E.S.; Lorenzo, J.M. Elderberry (Sambucus nigra L.) as potential source of antioxidants. Characterization, optimization of extraction parameters and bioactive properties. Food Chem. 2020, 330, 127266. [Google Scholar] [CrossRef] [PubMed]
- Zielińska-Wasielica, J.; Olejnik, A.; Kowalska, K.; Olkowicz, M.; Dembczyński, R. Elderberry (Sambucus nigra L.) Fruit Extract Alleviates Oxidative Stress, Insulin Resistance, and Inflammation in Hypertrophied 3T3-L1 Adipocytes and Activated RAW 264.7 Macrophages. Foods 2019, 8, 326. [Google Scholar] [CrossRef] [Green Version]
- David, L.; Moldovan, B.; Vulcu, A.; Olenic, L.; Perde-Schrepler, M.; Fischer-Fodor, E.; Florea, A.; Crisan, M.; Chiorean, I.; Clichici, S.; et al. Green synthesis, characterization and anti-inflammatory activity of silver nanoparticles using European black elderberry fruits extract. Colloids Surf. B Biointerfaces 2014, 122, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Farrell, N.; Norris, G.; Gil Lee, S.; Chun, O.K.; Blesso, C.N. Anthocyanin-rich black elderberry extract improves markers of HDL function and reduces aortic cholesterol in hyperlipidemic mice. Food Funct. 2015, 6, 1278–1287. [Google Scholar] [CrossRef]
- Olivas-Aguirre, F.J.; Rodrigo-García, J.; Martínez-Ruiz, N.D.R.; Cárdenas-Robles, A.I.; Mendoza-Díaz, S.O.; Alvarez-Parrilla, E.; Gonzalez-Aguilar, G.A.; De la Rosa, L.A.; Ramos-Jimenez, A.; Wall-Medrano, A. Cyanidin-3-O-glucoside: Physical-chemistry, foodomics and health effects. Molecules 2016, 21, 1264. [Google Scholar] [CrossRef] [Green Version]
- Strugała, P.; Loi, S.; Bażanów, B.; Kuropka, P.; Kucharska, A.Z.; Włoch, A.; Gabrielska, J. A Comprehensive Study on the Biological Activity of Elderberry Extract and Cyanidin 3-O-Glucoside and Their Interactions with Membranes and Human Serum Albumin. Molecules 2018, 23, 2566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farrell, N.J.; Norris, G.H.; Ryan, J.; Porter, C.M.; Jiang, C.; Blesso, C.N. Black elderberry extract attenuates inflammation and metabolic dysfunction in diet-induced obese mice. Br. J. Nutr. 2015, 114, 1123–1131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeon, S.; Kim, M.; Kim, B. Polyphenol-Rich Black Elderberry Extract Stimulates Transintestinal Cholesterol Excretion. Appl. Sci. 2021, 11, 2790. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Acylated anthocyanins from edible sources and their applications in food systems. Biochem. Eng. J. 2003, 14, 217–225. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Q.; Wang, E.; Ma, L.; Zhai, P. Dietary resveratrol increases the expression of hepatic 7α-hydroxylase and ameliorates hypercholesterolemia in high-fat fed C57BL/6J mice. Lipids Health Dis. 2012, 11, 56. [Google Scholar] [CrossRef] [Green Version]
- Mashour, N.H.; Lin, G.I.; Frishman, W.H. Herbal Medicine for the Treatment of Cardiovascular Disease. Arch. Intern. Med. 1998, 158, 2225–2234. [Google Scholar] [CrossRef]
- Li, L.; Zhou, X.; Li, N.; Sun, M.; Lv, J.; Xu, Z. Herbal drugs against cardiovascular disease: Traditional medicine and modern development. Drug Discov. Today 2015, 20, 1074–1086. [Google Scholar] [CrossRef] [PubMed]
- Lange, Y.; Steck, T.L. Cholesterol homeostasis and the escape tendency (activity) of plasma membrane cholesterol. Prog. Lipid Res. 2008, 47, 319–332. [Google Scholar] [CrossRef] [Green Version]
- Millar, C.L.; Norris, G.H.; Jiang, C.; Kry, J.; Vitols, A.; Garcia, C.; Park, Y.; Lee, J.; Blesso, C.N. Long-Term Supplementation of Black Elderberries Promotes Hyperlipidemia, but Reduces Liver Inflammation and Improves HDL Function and Atherosclerotic Plaque Stability in Apolipoprotein E-Knockout Mice. Mol. Nutr. Food Res. 2018, 62, e1800404. [Google Scholar] [CrossRef] [PubMed]
- Sato, R. Sterol metabolism and SREBP activation. Arch. Biochem. Biophys. 2010, 501, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Madison, B.B. Srebp2: A master regulator of sterol and fatty acid synthesis. J. Lipid Res. 2016, 57, 333–335. [Google Scholar] [CrossRef] [Green Version]
- Brown, M.S.; Goldstein, J.L. Sterol regulatory element binding proteins (SREBPs): Controllers of lipid synthesis and cellular uptake. Nutr. Rev. 1998, 56, S1–S3. [Google Scholar] [CrossRef] [PubMed]
- Van De Sluis, B.; Wijers, M.; Herz, J. News on the molecular regulation and function of hepatic low-density lipoprotein receptor and LDLR-related protein 1. Curr. Opin. Lipidol. 2017, 28, 241–247. [Google Scholar] [CrossRef]
- Wong, T.Y.; Lin, S.-M.; Leung, L.K. The Flavone Luteolin Suppresses SREBP-2 Expression and Post-Translational Activation in Hepatic Cells. PLoS ONE 2015, 10, e0135637. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Lee, S.Y.; Kim, B.; Seo, W.D.; Jia, Y.; Wu, C.; Jun, H.-J.; Lee, S.-J. Barley sprout extract containing policosanols and polyphenols regulate AMPK, SREBP2 and ACAT2 activity and cholesterol and glucose metabolism in vitro and in vivo. Food Res. Int. 2015, 72, 174–183. [Google Scholar] [CrossRef]
- Tu, L.; Sun, H.; Tang, M.; Zhao, J.; Zhang, Z.; Sun, X.; He, S. Red raspberry extract (Rubus idaeus L shrub) intake ameliorates hyperlipidemia in HFD-induced mice through PPAR signaling pathway. Food Chem. Toxicol. 2019, 133, 110796. [Google Scholar] [CrossRef] [PubMed]
- Linton, M.F.; Tao, H.; Linton, E.F.; Yancey, P.G. SR-BI: A Multifunctional Receptor in Cholesterol Homeostasis and Atherosclerosis. Trends Endocrinol. Metab. 2017, 28, 461–472. [Google Scholar] [CrossRef]
- Shen, W.-J.; Azhar, S.; Kraemer, F. SR-B1: A Unique Multifunctional Receptor for Cholesterol Influx and Efflux. Annu. Rev. Physiol. 2018, 80, 95–116. [Google Scholar] [CrossRef] [PubMed]
- Kozarsky, K.F.; Donahee, M.H.; Rigotti, A.; Iqbal, S.N.; Edelman, E.R.; Krieger, M. Overexpression of the HDL receptor SR-BI alters plasma HDL and bile cholesterol levels. Nat. Cell Biol. 1997, 387, 414–417. [Google Scholar] [CrossRef]
- Basso, F.; Freeman, L.; Knapper, C.L.; Remaley, A.; Stonik, J.; Neufeld, E.B.; Tansey, T.; Amar, M.J.A.; Fruchart-Najib, J.; Duverger, N.; et al. Role of the hepatic ABCA1 transporter in modulating intrahepatic cholesterol and plasma HDL cholesterol concentrations. J. Lipid Res. 2003, 44, 296–302. [Google Scholar] [CrossRef] [Green Version]
- Field, F.J.; Watt, K.; Mathur, S.N. Origins of intestinal ABCA1-mediated HDL-cholesterol. J. Lipid Res. 2008, 49, 2605–2619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, K.; Jiang, T.; Zhao, G.-J. Quercetin induces the selective uptake of HDL-cholesterol via promoting SR-BI expression and the activation of the PPARγ/LXRα pathway. Food Funct. 2018, 9, 624–635. [Google Scholar] [CrossRef] [PubMed]
- Yü, Y.; Si, Y.; Song, G.; Luo, T.; Wang, J.; Qin, S. Ethanolic Extract of Propolis Promotes Reverse Cholesterol Transport and the Expression of ATP-Binding Cassette Transporter A1 and G1 in Mice. Lipids 2011, 46, 805–811. [Google Scholar] [CrossRef]
- Lee, N.-H.; Choi, S.-S.; Kim, B.-B.; Kim, S.-Y.; Kang, B.-S.; Lee, S.-J.; Park, H.-J. Effect of alcohol-free red wine concentrates on cholesterol homeostasis: An in vitro and in vivo study. Process. Biochem. 2013, 48, 1964–1971. [Google Scholar] [CrossRef]
- Jia, L.; Betters, J.L.; Yu, L. Niemann-Pick C1-Like 1 (NPC1L1) Protein in Intestinal and Hepatic Cholesterol Transport. Annu. Rev. Physiol. 2011, 73, 239–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Calvo, M.; Lisnock, J.; Bull, H.G.; Hawes, B.E.; Burnett, D.; Braun, M.P.; Crona, J.H.; Davis, H.R.; Dean, D.C.; Detmers, P.A.; et al. The target of ezetimibe is Niemann-Pick C1-Like 1 (NPC1L1). Proc. Natl. Acad. Sci. USA 2005, 102, 8132–8137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, T.-Y.; Chang, C. Ezetimibe Blocks Internalization of the NPC1L1/Cholesterol Complex. Cell Metab. 2008, 7, 469–471. [Google Scholar] [CrossRef] [Green Version]
- Kidambi, S.; Patel, S.B. Cholesterol and non-cholesterol sterol transporters: ABCG5, ABCG8 and NPC1L1: A review. Xenobiotica 2008, 38, 1119–1139. [Google Scholar] [CrossRef]
- Dikkers, A.; Tietge, U.-J. Biliary cholesterol secretion: More than a simple ABC. World J. Gastroenterol. 2010, 16, 5936–5945. [Google Scholar] [CrossRef] [PubMed]
- Ressaissi, A.; Attia, N.; Pacheco, R.; Falé, P.L.; Serralheiro, M.L.M. Cholesterol transporter proteins in HepG2 cells can be modulated by phenolic compounds present in Opuntia ficus-indica aqueous solutions. J. Funct. Foods 2020, 64, 103674. [Google Scholar] [CrossRef]
- Ontawong, A.; Pasachan, T.; Trisuwan, K.; Soodvilai, S.; Duangjai, A.; Pongchaidecha, A.; Amornlerdpison, D.; Srimaroeng, C. Coffea arabica pulp aqueous extract attenuates oxidative stress and hepatic lipid accumulation in HepG2 cells. J. Herb. Med. 2021, 29, 100465. [Google Scholar] [CrossRef]
- Chiang, J.Y. Regulation of bile acid synthesis: Pathways, nuclear receptors, and mechanisms. J. Hepatol. 2004, 40, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Zhang, Y.; Zhang, S.; Zhai, J.; Gao, H.; Tao, L.; Song, Y. Dysregulation of BSEP and MRP2 May Play an Important Role in Isoniazid-Induced Liver Injury via the SIRT1/FXR Pathway in Rats and HepG2 Cells. Biol. Pharm. Bull. 2018, 41, 1211–1218. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.; Wen, M.; Sun, L.; Li, H.; Liu, Y.; Li, R.; Wu, F.; Yang, R.; Lin, Y. Mechanistic insights into geniposide regulation of bile salt export pump (BSEP) expression. RSC Adv. 2018, 8, 37117–37128. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, H. Bile Salt Export Pump (BSEP/ABCB11): Trafficking and Sorting Disturbances. Curr. Mol. Pharmacol. 2013, 6, 95–103. [Google Scholar] [CrossRef]
- Chambers, K.F.; Day, P.E.; Aboufarrag, H.T.; Kroon, P.A. Polyphenol Effects on Cholesterol Metabolism via Bile Acid Biosynthesis, CYP7A1: A Review. Nutrients 2019, 11, 2588. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Xia, M.; Gao, S.; Li, D.; Zhang, Y.; Jin, T.; Ling, W. Cyanidin-3-O-β-glucoside upregulates hepatic cholesterol 7α-hydroxylase expression and reduces hypercholesterolemia in mice. Mol. Nutr. Food Res. 2012, 56, 610–621. [Google Scholar] [CrossRef]
- Hoppel, C. The role of carnitine in normal and altered fatty acid metabolism. Am. J. Kidney Dis. 2003, 41, S4–S12. [Google Scholar] [CrossRef]
- Christensen, E.; Woldseth, B.; Hagve, T.-A.; Poll-The, B.T.; Wanders, R.J.A.; Sprecher, H.; Stokke, O.; Christophersen, B.O. Peroxisomal β-oxidation of Polyunsaturated Long Chain Fatty Acids in Human Fibroblasts. The Polyunsaturated and the Saturated Long Chain Fatty Acids are Retroconverted by the Same Acyl-CoA Oxidase. Scand. J. Clin. Lab. Investig. 1993, 53, 61–74. [Google Scholar] [CrossRef]
- Infante, J.P.; Tschanz, C.L.; Shaw, N.; Michaud, A.L.; Lawrence, P.; Brenna, J. Straight-Chain Acyl-CoA Oxidase Knockout Mouse Accumulates Extremely Long Chain Fatty Acids from α-Linolenic Acid: Evidence for Runaway Carousel-Type Enzyme Kinetics in Peroxisomal β-Oxidation Diseases. Mol. Genet. Metab. 2002, 75, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Reiser, K.; Davis, M.A.; Hynes, M.J. AoxA is a major peroxisomal long chain fatty acyl-CoA oxidase required for β-oxidation in A. nidulans. Curr. Genet. 2010, 56, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Radler, U.; Stangl, H.; Lechner, S.; Lienbacher, G.; Krepp, R.; Zeller, E.; Brachinger, M.; Eller-Berndl, D.; Fischer, A.; Anzur, C.; et al. A Combination of (ω–3) Polyunsaturated Fatty Acids, Polyphenols and L-Carnitine Reduces the Plasma Lipid Levels and Increases the Expression of Genes Involved in Fatty Acid Oxidation in Human Peripheral Blood Mononuclear Cells and HepG2 Cells. Ann. Nutr. Metab. 2011, 58, 133–140. [Google Scholar] [CrossRef]
- Meaney, S. Epigenetic regulation of cholesterol homeostasis. Front. Genet. 2014, 5, 311. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Kumar, S.; Vikram, A.; Hoffman, T.A.; Naqvi, A.; Lewarchik, C.M.; Kim, Y.-R.; Irani, K. Histone and DNA Methylation–Mediated Epigenetic Downregulation of Endothelial Kruppel-Like Factor 2 by Low-Density Lipoprotein Cholesterol. Arter. Thromb. Vasc. Biol. 2013, 33, 1936–1942. [Google Scholar] [CrossRef] [Green Version]
- Malhotra, P.; Soni, V.; Kumar, A.; Anbazhagan, A.N.; Dudeja, A.; Saksena, S.; Gill, R.K.; Dudeja, P.K.; Alrefai, W.A. Epigenetic Modulation of Intestinal Cholesterol Transporter Niemann-Pick C1-like 1 (NPC1L1) Gene Expression by DNA Methylation. J. Biol. Chem. 2014, 289, 23132–23140. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Total Phenolics (mg GAE/g) | Total Flavonoid (mg QE/g) | Total Anthocyanin (mg CGE/g) | Total Antioxidant Capacity | |||
|---|---|---|---|---|---|---|
| DPPH (mM VCE/g) | ABTS (mM VCE/g) | FRAP (mM FeSO4/g) | ||||
| BEE | 222.8 ± 15.1 | 522.9 ± 43.9 | 242.9 ± 7.7 | 199.2 ± 21.2 | 369.1 ± 30.6 | 3.0 ± 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, S.; Lee, S.; Choi, Y.; Kim, B. The Effects of Polyphenol-Rich Black Elderberry on Oxidative Stress and Hepatic Cholesterol Metabolism. Appl. Sci. 2021, 11, 10018. https://0-doi-org.brum.beds.ac.uk/10.3390/app112110018
Jeon S, Lee S, Choi Y, Kim B. The Effects of Polyphenol-Rich Black Elderberry on Oxidative Stress and Hepatic Cholesterol Metabolism. Applied Sciences. 2021; 11(21):10018. https://0-doi-org.brum.beds.ac.uk/10.3390/app112110018
Chicago/Turabian StyleJeon, Sohyeon, Sanggil Lee, Yeoni Choi, and Bohkyung Kim. 2021. "The Effects of Polyphenol-Rich Black Elderberry on Oxidative Stress and Hepatic Cholesterol Metabolism" Applied Sciences 11, no. 21: 10018. https://0-doi-org.brum.beds.ac.uk/10.3390/app112110018