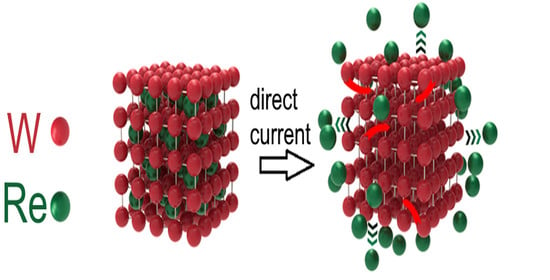
A Porous Tungsten Substrate for Catalytic Reduction of Hydrogen by Dealloying of a Tungsten–Rhenium Alloy in an Aqueous Solution of Hydrochloric Acid
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Pu, Z.; Zhao, J.; Amiinu, I.S.; Li, W.; Wang, M.; He, D.; Mu, S. A universal synthesis strategy for P-rich noble metal diphosphide-based electrocatalysts for the hydrogen evolution reaction. Energy Environ. Sci. 2019, 12, 952–957. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Liu, Z.; Zhang, D. Novel 3-D urchin-like Ni–Co–W porous nanostructure as efficient bifunctional superhydrophilic electrocatalyst for both hydrogen and oxygen evolution reactions. Int. J. Hydrogen Energy 2020, 45, 17504–17516. [Google Scholar] [CrossRef]
- Kotkondawar, A.V.; Mangrulkar, P.; Wanjari, S.; Maddigapu, P.R.; Rayalu, S. Photothermal hydrogen production from oxidative hydrolysis of electrochemically synthesized nano-sized zinc. Int. J. Hydrogen Energy 2019, 44, 6514–6524. [Google Scholar] [CrossRef]
- Schmidt, O.; Gambhir, A.; Staffell, I.; Hawkes, A.; Nelson, J.; Few, S. Future cost and performance of water electrolysis: An expert elicitation study. Int. J. Hydrogen Energy 2017, 42, 30470–30492. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, X.; Zhang, B.; Yang, H.; Liang, C. Electrocatalytic properties of porous Ni-Co-WC composite electrode toward hydrogen evolution reaction in acid medium. Int. J. Hydrogen Energy 2019, 44, 19771–19781. [Google Scholar] [CrossRef]
- Xu, Y.; Xiao, X.; Ye, Z.; Zhao, S.; Shen, R.; He, C.; Zhang, J.; Li, Y.; Chen, X. Cage-Confinement Pyrolysis Route to Ultrasmall Tungsten Carbide Nanoparticles for Efficient Electrocatalytic Hydrogen Evolution. J. Am. Chem. Soc. 2017, 139, 5285–5288. [Google Scholar] [CrossRef] [PubMed]
- Nady, H.; Negem, M. Ni–Cu nano-crystalline alloys for efficient electrochemical hydrogen production in acid water. RSC Adv. 2016, 6, 51111. [Google Scholar] [CrossRef]
- Ngamlerdpokin, K.; Tantavichet, N. Electrodeposition of nickel–copper alloys to use as a cathode for hydrogen evolution in an alkaline media. Int. J. Hydrogen Energy 2014, 39, 2505–2515. [Google Scholar] [CrossRef]
- Chang, Z.; Zhu, L.; Zhao, J.; Chen, P.; Chen, D.; Gao, H. NiMo/Cu-nanosheets/Ni-foam composite as a high performance electrocatalyst for hydrogen evolution over a wide pH range. Int. J. Hydrogen Energy 2020, 46, 3493–3503. [Google Scholar] [CrossRef]
- Lotfi, N.; Shahrabi, T.; Yaghoubinezhad, Y.; Barati Darband, G.H. Electrodeposition of cedar leaf-like graphene Oxide@Ni–Cu@Ni foam electrode as a highly efficient and ultra-stable catalyst for hydrogen evolution reaction. Electrochim. Acta 2019, 326, 134949. [Google Scholar] [CrossRef]
- Sun, Q.; Zhou, M.; Shen, Y.; Wang, L.; Ma, Y.; Li, Y.; Bo, X.; Wang, Z.; Zhao, C. Hierarchical nanoporous Ni(Cu) alloy anchored on amorphous NiFeP as efficient bifunctional electrocatalysts for hydrogen evolution and hydrazine oxidation. J. Catal. 2019, 373, 180–189. [Google Scholar] [CrossRef]
- Nady, H.; Negem, M. Electroplated Zn–Ni nanocrystalline alloys as an efficient electrocatalyst cathode for the generation of hydrogen fuel in acid medium. Int. J. Hydrogen Energy 2018, 43, 4942–4950. [Google Scholar] [CrossRef]
- Kuznetsov, V.V.; Gamburg, Y.D.; Zhulikov, V.V.; Batalov, R.S.; Filatova, E.A. Re–Ni cathodes obtained by electrodeposition as a promising electrode material for hydrogen evolution reaction in alkaline solutions. Electrochim. Acta 2019, 317, 358–366. [Google Scholar] [CrossRef]
- Youn, J.; Jeong, S.; Kang, H.; Kovendhan, M.; Park, C.; Jeon, K. Effect of carbon coating on Cu electrodes for hydrogen production by water splitting. Int. J. Hydrogen Energy 2019, 44, 20641–20648. [Google Scholar] [CrossRef]
- Martins, P.; Lopes, P.; Ticianelli, E.A.; Stamenkovic, V.R.; Markovic, N.M.; Strmcnik, D. Hydrogen evolution reaction on copper: Promoting water dissociation by tuning the surface oxophilicity. Electrochem. Commun. 2019, 100, 30–33. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, T.; Liu, T.; Lv, K.; Wu, Z.; Wang, D. Tungsten phosphide (WP) nanoparticles with tunable crystallinity, W vacancies, and electronic structures for hydrogen production. Electrochim. Acta 2019, 323, 134798. [Google Scholar] [CrossRef]
- Wu, L.; Pu, Z.; Tu, Z.; Amiinu, I.S.; Liu, S.; Wang, P.; Mu, S. Integrated design and construction of WP/W nanorod array electrodes toward efficient hydrogen evolution reaction. Chem. Eng. J. 2017, 327, 705–712. [Google Scholar] [CrossRef]
- Nikitina, E.V.; Karfidov, E.A. Corrosion of construction materials of separator in molten carbonates of alkali metal. Int. J. Hydrogen Energy 2021, 46, 16925–16931. [Google Scholar] [CrossRef]
- Chernyshev, A.A.; Darintseva, A.B.; Ostanina, T.N.; Panashchenko, I.A.; Orlova, A.A.; Novikov, A.E.; Artamonov, A.S. Electrocrystallization of metals on a rotating drum-cathode. Int. J. Hydrogen Energy 2021, 46, 16848–16856. [Google Scholar] [CrossRef]
- Abd El-Hafez, G.M.; Mahmoud, N.H.; Walcarius, A.; Fekry, A.M. Evaluation of the electrocatalytic properties of Tungsten electrode towards hydrogen evolution reaction in acidic solutions. Int. J. Hydrogen Energy 2019, 44, 16487–16496. [Google Scholar] [CrossRef]
- Badawy, W.A.; Abd El-Hafez, G.M.; Nady, H. Electrochemical performance of tungsten electrode as cathode for hydrogen evolution in alkaline solutions. Int. J. Hydrogen Energy 2015, 40, 6276–6282. [Google Scholar] [CrossRef]
- Chen, L.; Fan, J.L.; Gong, H.R. Atomistic simulation of mechanical properties of tungsten-hydrogen system and hydrogen diffusion in tungsten. Solid State Commun. 2020, 306, 113772. [Google Scholar] [CrossRef]
- Zhang, J.; Li, C.M. Nanoporous metals: Fabrication strategies and advanced electrochemical applications in catalysis, sensing and energy systems. Chem. Soc. Rev. 2012, 41, 7016–7031. [Google Scholar] [CrossRef] [PubMed]
- Tappan, B.C.; Steiner, S.A.; Luther, E.P. Nanoporous Metal Foams. Angew. Chem. Int. Ed. 2010, 49, 4544–4565. [Google Scholar] [CrossRef]
- Walcarius, A. Mesoporous materials and electrochemistry. Chem. Soc. Rev. 2013, 42, 4098–4140. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Z. On the electrochemical dealloying of Al-based alloys in a NaCl aqueous solution. Phys. Chem. Chem. Phys. 2010, 12, 1453–1472. [Google Scholar] [CrossRef]
- Graf, M.; Roschning, B.; Weissmüller, J. Nanoporous Gold by Alloy Corrosion: Method-Structure-Property Relationships. J. Electrochem. Soc. 2017, 164, C194. [Google Scholar] [CrossRef]
- Hakamada, M.; Mabuchi, M. Preparation of Nanoporous Palladium by Dealloying: Anodic Polarization Behaviors of Pd-M (M=Fe, Co, Ni) Alloys. Mater. Trans. 2009, 50, 431–435. [Google Scholar] [CrossRef] [Green Version]
- Nikitina, E.V.; Karfidov, E.A.; Kazakovtseva, N.A. Anodic selective dissolution of copper alloys in chloride and carbonate melts. J. Alloy Compd. 2020, 845, 156238. [Google Scholar] [CrossRef]
- Ye, L.; Ouyang, Z.; Chen, Y.; Liu, S. Recovery of rhenium from tungsten-rhenium wire by alkali fusion in KOH-K2CO3 binary molten salt. Int. J. Refract. Met. Hard Mater. 2020, 87, 105148. [Google Scholar] [CrossRef]
- Kuznetsova, O.G.; Levin, A.M.; Sevostyanov, M.A.; Bolshih, A.O. The improvement of rhenium recovery technology from W-Re alloys. J. Phys. Conf. Ser. 2018, 1134, 012032. [Google Scholar] [CrossRef] [Green Version]
- Entezari-Zarandi, A.; Azizi, D.; Nikolaychuk, P.A.; Larachi, F.; Pasquier, L.C. Selective Recovery of Molybdenum over Rhenium from Molybdenite Flue Dust Leaching Solution Using PC88A Extractant. Metals 2020, 10, 1423. [Google Scholar] [CrossRef]
- Andreev, Y.Y. Electrochemistry of Metals and Alloys; High Education and Science: Moskow, Russia, 2016; 320p. [Google Scholar]






| Component | Solution 1 | Solution 2 | Solution 3 | Solution 4 |
|---|---|---|---|---|
| HCl, g L−1 | 200 | 200 | 350 | 350 |
| KCl, g L−1 | 0 | 340 | 0 | 340 |
| Figure 5A | Component | Spectrum 1 | Spectrum 2 | Spectrum 3 | Spectrum 4 |
| W, wt.% | 88.25 | 88.75 | 86.22 | 86.31 | |
| Re, wt.% | 11.75 | 11.25 | 13.78 | 13.69 | |
| Figure 5B | Component | Spectrum 1 | Spectrum 2 | Spectrum 3 | Spectrum 4 |
| W, wt.% | 90.66 | 90.73 | 88.63 | 88.36 | |
| Re, wt.% | 9.34 | 9.27 | 11.37 | 11.64 |
| Time, s | 3000 | 6000 | 30,000 |
|---|---|---|---|
| ZA(W) | 1.08 | 1.29 | 2.35 |
| ZA(Re) | 0.92 | 0.77 | 0.41 |
| UL, sm/s | 1.4 × 10−9 | 2.9 × 10−9 | 7.2 × 10−8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chernyshev, A.A.; Nikitina, E.V. A Porous Tungsten Substrate for Catalytic Reduction of Hydrogen by Dealloying of a Tungsten–Rhenium Alloy in an Aqueous Solution of Hydrochloric Acid. Appl. Sci. 2022, 12, 1029. https://0-doi-org.brum.beds.ac.uk/10.3390/app12031029
Chernyshev AA, Nikitina EV. A Porous Tungsten Substrate for Catalytic Reduction of Hydrogen by Dealloying of a Tungsten–Rhenium Alloy in an Aqueous Solution of Hydrochloric Acid. Applied Sciences. 2022; 12(3):1029. https://0-doi-org.brum.beds.ac.uk/10.3390/app12031029
Chicago/Turabian StyleChernyshev, Aleksander A., and Evgenia V. Nikitina. 2022. "A Porous Tungsten Substrate for Catalytic Reduction of Hydrogen by Dealloying of a Tungsten–Rhenium Alloy in an Aqueous Solution of Hydrochloric Acid" Applied Sciences 12, no. 3: 1029. https://0-doi-org.brum.beds.ac.uk/10.3390/app12031029