Monitoring In Vitro and In Vivo Aroma Release of Espresso Coffees with Proton-Transfer-Reaction Time-of-Flight Mass Spectrometry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Coffee Samples
2.2. PTR-ToF-MS Measurements
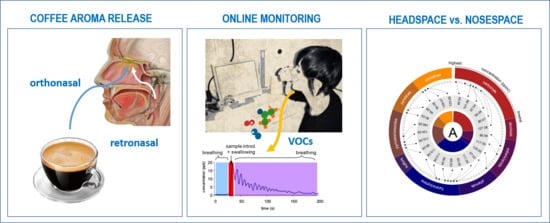
2.3. Nosespace (NS) Analysis
2.4. Headspace (HS) Analysis
2.5. Data Analysis
2.5.1. PTR-ToF-MS Spectra Processing
2.5.2. NS Data Treatment
2.5.3. HS Data Treatment
2.5.4. Software
3. Results and Discussion
3.1. In Vitro Aroma Release
3.2. In Vivo Aroma Release
3.3. Headspace vs. Nosespace
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Linforth, R.; Martin, F.; Carey, M.; Davidson, J.; Taylor, A.J. Retronasal Transport of Aroma Compounds. J. Agric. Food Chem. 2002, 50, 1111–1117. [Google Scholar] [CrossRef] [PubMed]
- Linforth, R.; Taylor, A. The Process of Flavour Release. In Flavour in Food; Elsevier: Amsterdam, The Netherlands, 2006; pp. 287–307. ISBN 978-1-85573-960-4. [Google Scholar]
- Yeretzian, C.; Pollien, P.; Lindinger, C.; Ali, S. Individualization of Flavor Preferences: Toward a Consumer-Centric and Individualized Aroma Science. Compr. Rev. Food Sci. Food Saf. 2004, 3, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Gierczynski, I.; Guichard, E.; Laboure, H. Aroma Perception in Dairy Products: The Roles of Texture, Aroma Release and Consumer Physiology. A Review. Flavour Fragr. J. 2011, 26, 141–152. [Google Scholar] [CrossRef]
- Guichard, E. Interactions between Flavor Compounds and Food Ingredients and Their Influence on Flavor Perception. Food Rev. Int. 2002, 18, 49–70. [Google Scholar] [CrossRef]
- Taylor, A.J.; Beauchamp, J.D.; Langford, V.S. Non-Destructive and High-Throughput—APCI-MS, PTR-MS and SIFT-MS as Methods of Choice for Exploring Flavor Release. In Dynamic Flavor: Capturing Aroma Using Real-Time Mass Spectrometry; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2021; Volume 1402, pp. 1–16. [Google Scholar]
- Graus, M.; Yeretzian, C.; Jordan, A.; Lindinger, W. In-Mouth Aroma: Breath-by-Breath Analysis of NosesSpace by PTR-MS While Drinking Coffee. In Proceedings of the XIIIth Symposium on Atomic, Cluster and Surface Physics, Kitzbühel, Austria, 17–23 February 2002. [Google Scholar]
- Barron, D.; Pineau, N.; Matthey-Doret, W.; Ali, S.; Sudre, J.; Germain, J.C.; Kolodziejczyk, E.; Pollien, P.; Labbe, D.; Jarisch, C.; et al. Impact of Crema on the Aroma Release and the In-Mouth Sensory Perception of Espresso Coffee. Food Funct. 2012, 3, 923–930. [Google Scholar] [CrossRef]
- Romano, A.; Cappellin, L.; Ting, V.; Aprea, E.; Navarini, L.; Gasperi, F.; Biasioli, F. Nosespace Analysis by PTR-ToF-MS for the Characterization of Food and Tasters: The Case Study of Coffee. Int. J. Mass Spectrom. 2014, 365–366, 20–27. [Google Scholar] [CrossRef]
- Charles, M.; Romano, A.; Yener, S.; Barnabà, M.; Navarini, L.; Märk, T.D.; Biasoli, F.; Gasperi, F. Understanding Flavour Perception of Espresso Coffee by the Combination of a Dynamic Sensory Method and In-Vivo Nosespace Analysis. Food Res. Int. 2015, 69, 9–20. [Google Scholar] [CrossRef]
- Colzi, I.; Taiti, C.; Marone, E.; Magnelli, S.; Gonnelli, C.; Mancuso, S. Covering the Different Steps of the Coffee Processing: Can Headspace VOC Emissions Be Exploited to Successfully Distinguish between Arabica and Robusta? Food Chem. 2017, 237, 257–263. [Google Scholar] [CrossRef]
- Yener, S.; Romano, A.; Cappellin, L.; Märk, T.D.; Del Pulgar, J.S.; Gasperi, F.; Navarinic, L.; Biasioli, F. PTR-ToF-MS Characterisation of Roasted Coffees (C. Arabica) from Different Geographic Origins. J. Mass Spectrom. 2014, 49, 929–935. [Google Scholar] [CrossRef]
- Yener, S.; Romano, A.; Cappellin, L.; Granitto, P.M.; Aprea, E.; Navarini, L.; Märk, T.D.; Gasperi, F.; Biasioli, F. Tracing Coffee Origin by Direct Injection Headspace Analysis with PTR/SRI-MS. Food Res. Int. 2015, 69, 235–243. [Google Scholar] [CrossRef]
- Lopes, G.R.; Petronilho, S.; Ferreira, A.S.; Pinto, M.; Passos, C.P.; Coelho, E.; Rodrigues, C.; Figueira, C.; Rocha, S.M.; Coimbra, M.A. Insights on Single-Dose Espresso Coffee Capsules’ Volatile Profile: From Ground Powder Volatiles to Prediction of Espresso Brew Aroma Properties. Foods 2021, 10, 2508. [Google Scholar] [CrossRef]
- Cappellin, L.; Biasioli, F.; Granitto, P.M.; Schuhfried, E.; Soukoulis, C.; Costa, F.; Märk, T.D.; Gasperi, F. On Data Analysis in PTR-TOF-MS: From Raw Spectra to Data Mining. Sens. Actuators B Chem. 2011, 155, 183–190. [Google Scholar] [CrossRef]
- Cappellin, L.; Biasioli, F.; Fabris, A.; Schuhfried, E.; Soukoulis, C.; Märk, T.D.; Gasperi, F. Improved Mass Accuracy in PTR-TOF-MS: Another Step towards Better Compound Identification in PTR-MS. Int. J. Mass Spectrom. 2010, 290, 60–63. [Google Scholar] [CrossRef]
- Cappellin, L.; Biasioli, F.; Schuhfried, E.; Soukoulis, C.; Märk, T.D.; Gasperi, F. Extending the Dynamic Range of Proton Transfer Reaction Time-of-Flight Mass Spectrometers by a Novel Dead Time Correction: Extending the Dynamic Range of PTR-TOF-MS. Rapid Commun. Mass Spectrom. 2011, 25, 179–183. [Google Scholar] [CrossRef]
- Lindinger, W.; Hansel, A.; Jordan, A. On-Line Monitoring of Volatile Organic Compounds at Pptv Levels by Means of Proton-Transfer-Reaction Mass Spectrometry (PTR-MS) Medical Applications, Food Control and Environmental Research. Int. J. Mass Spectrom. Ion Process. 1998, 173, 191–241. [Google Scholar] [CrossRef]
- Chong, I.-G.; Jun, C.-H. Performance of Some Variable Selection Methods When Multicollinearity Is Present. Chemom. Intell. Lab. Syst. 2005, 78, 103–112. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Schenker, S.; Heinemann, C.; Huber, M.; Pompizzi, R.; Perren, R.; Escher, R. Impact of Roasting Conditions on the Formation of Aroma Compounds in Coffee Beans. J. Food Sci. 2002, 67, 60–66. [Google Scholar] [CrossRef]
- Del Terra, L.; Lonzarich, V.; Asquini, E.; Navarini, L.; Graziosi, G.; Suggi Liverani, F.; Pallavicini, A. Functional Characterization of Three Coffea Arabica L. Monoterpene Synthases: Insights into the Enzymatic Machinery of Coffee Aroma. Phytochemistry 2013, 89, 6–14. [Google Scholar] [CrossRef]
- Flament, I.; Bessière-Thomas, Y. Coffee Flavor Chemistry; Wiley: Chichester, UK; New York, NY, USA, 2002; ISBN 978-0-471-72038-6. [Google Scholar]
- Buettner, A.; Beer, A.; Hannig, C.; Settles, M.; Schieberle, P. Physiological and Analytical Studies on Flavor Perception Dynamics as Induced by the Eating and Swallowing Process. Food Qual. Prefer. 2002, 13, 497–504. [Google Scholar] [CrossRef]
- Muñoz-González, C.; Brule, M.; Martin, C.; Feron, G.; Canon, F. Molecular Mechanisms of Aroma Persistence: From Noncovalent Interactions between Aroma Compounds and the Oral Mucosa to Metabolization of Aroma Compounds by Saliva and Oral Cells. Food Chem. 2022, 373, 131467. [Google Scholar] [CrossRef]
- Ijichi, C.; Wakabayashi, H.; Sugiyama, S.; Ihara, Y.; Nogi, Y.; Nagashima, A.; Ihara, S.; Niimura, Y.; Shimizu, Y.; Kondo, K.; et al. Metabolism of Odorant Molecules in Human Nasal/Oral Cavity Affects the Odorant Perception. Chem. Senses 2019, 44, 465–481. [Google Scholar] [CrossRef]



| Original | Headspace | Nosespace | |||||
| Predicted | Predicted | ||||||
| Coffee A | Coffee B | Coffee E | Coffee A | Coffee B | Coffee E | ||
| Coffee A | 11 | 0 | 1 | 14 | 0 | 1 | |
| Coffee B | 0 | 11 | 0 | 0 | 14 | 1 | |
| Coffee E | 0 | 0 | 11 | 0 | 0 | 15 | |
| Measured Mass m/z (Th) | Sum Formula | Tentative Identification | Coffee A (ppbV) | Coffee B (ppbV) | Coffee E (ppbV) | p-Value |
|---|---|---|---|---|---|---|
| 33.033 | CH5O+ | Methanol | 6157 ± 1651 a | 10,263 ± 3610 b | 11,102 ± 2342 b | <10−3 |
| 46.038 | C[13]CH5O+ | 13C isotope acetaldehyde | 262 ± 79 a | 385 ± 107 b | 478 ± 110 b | <10−3 |
| 47.050 | C2H7O+ | Ethanol | 32 ± 8 a | 51 ± 14 b | 52 ± 9 b | <10−3 |
| 71.087 | C5H11+ | Terpene fragment | 15 ± 5 a | 31 ± 9 b | 37 ± 9 b | <10−3 |
| 84.088 | C5[13]CH11+ | 13C isotope fragment (diverse origin) | 1.8 ± 1.1 a | 4.6 ±1.2 b | 5.8 ± 0.8 b | <10−3 |
| 85.103 | C6H13+ | Methyl-butene/aldehyde fragment | 4.5 ± 2.4 a | 4.8 ± 3.7 a | 13.9 ± 6.1 b | <10−3 |
| 95.007 | C2H7S2+ | Dimethyl-disulfide | 132 ± 42 a | 215 ± 48 b | 235 ± 43 b | <10−3 |
| 97.028 | C5H5O2+ | Furfural | 2925 ± 998 a | 2461 ± 850 a | 5280 ± 1111 b | <10−3 |
| 101.060 | C5H9O2+ | Pentanedione/methyl-tetrahydrofuranone | 988 ± 324 a | 1504 ± 524 b | 1619 ± 407 b | <10−3 |
| 111.044 | C6H7O2+ | Acetyl-furan/methyl-furfural | 1641 ± 554 a | 2556 ± 827 b | 2579 ± 491 b | <10−3 |
| 127.150 | N.a. | N.a. | 0.6 ± 0.3 a | 0.8 ± 0.4 a | 2.3 ± 0.8 b | <10−3 |
| 135.117 | C10H15+ | Terpene fragment | 1.7 ± 0.6 a | 2.8 ± 0.8 b | 5.4 ± 1.2 c | <10−3 |
| 137.134 | C10H17+ | Various monoterpenes | 4.9 ± 1.6 a | 7.6 ± 2.5 a | 22.3 ± 6.3 b | <10−3 |
| 153.131 | C10H17O+ | Decadienal | 3.1 ± 1.0 a | 7.2 ± 1.6 b | 11.4 ± 2.1 c | <10−3 |
| 157.124 | C9H17O2+ | Hydroxy-nonenal | 0.7 ± 0.2 a | 1.8± 0.5 b | 1.9 ± 0.3 b | <10−3 |
| Measured Mass m/z (Th) | Sum Formula | Tentative Identification | Parameter * | Coffee A | Coffee B | Coffee E | p-Value | |||
|---|---|---|---|---|---|---|---|---|---|---|
| 68.056 | C4H6N+ | Pyrrole | Slope | 0.008 ± | 0.003 a | 0.013 ± | 0.002 a | 0.010 ± | 0.002 a | 0.031 |
| Area | 119.32 ± | 23.58 a | 198.17 ± | 63.67 a | 153.5 ± | 59.45 a | 0.149 | |||
| 79.049 | C6H7+ | Benzene/benzaldehyde fragment | Area | 64.04 ± | 30.26a | 122.81 ± | 55.95 a | 68.65 ± | 21.50 a | 0.037 |
| 80.050 | C5H6N+ | Pyridine | Area | 526.84 ± | 175.12 a | 1529.2 ± | 747.68 b | 680.9 ± | 345.33 a | <10−3 |
| Median | 2.32 ± | 0.96 a | 6.39 ± | 4.16 a | 3.02 ± | 1.58 a | 0.049 | |||
| 82.070 | C5H8N+ | Methyl-pyrrole | Slope | 0 ± | 0 a | 0.013 ± | 0.003 c | 0.009 ± | 0.003 b | <10−3 |
| Area | 0 ± | 0 a | 82.65 ± | 36.43 b | 63.30 ± | 26.30 b | <10−3 | |||
| Median | 0 ± | 0 a | 0.22 ± | 0.10 b | 0.19 ± | 0.07 b | <10−3 | |||
| Final | 0 ± | 0 a | 0.15 ± | 0.10 b | 0.18 ± | 0.09 b | <10−3 | |||
| Maximum | 0 ± | 0 a | 8.66 ± | 5.83 b | 7.36 ± | 5.94 b | 0.004 | |||
| 98.074 | C5H8ON+ | Dimethyl-oxazole | Median | 0 ± | 0 a | 0.06 ± | 0.01 b | 0 ± | 0 a | <10−3 |
| Area | 0 ± | 0 a | 14.44 ± | 4.68 b | 0 ± | 0 a | <10−3 | |||
| Slope | 0 ± | 0 a | 0.006 ± | 0.003 b | 0 ± | 0 a | <10−3 | |||
| Final | 0 ± | 0 a | 0.05 ± | 0.02 b | 0 ± | 0 a | <10−3 | |||
| Maximum | 0 ± | 0 a | 0.68 ± | 0.39 b | 0 ± | 0 a | <10−3 | |||
| 99.082 | C6H11O+ | Hexenal/methyl-pentenone | Area | 0 ± | 0 a | 54.32 ± | 20.15 b | 50.14 ± | 15.70 b | <10−3 |
| Final | 0 ± | 0 a | 0.23 ± | 0.06 b | 0.24 ± | 0.10 b | <10−3 | |||
| Median | 0 ± | 0 a | 0.30 ± | 0.11 b | 0.29 ± | 0.10 b | <10−3 | |||
| Slope | 0 ± | 0 a | 0.006 ± | 0.002 b | 0.005 ± | 0.002 b | <10−3 | |||
| Maximum | 0 ± | 0 a | 1.96 ± | 1.36 b | 1.70 ± | 1.22 b | 0.002 | |||
| 125.065 | C7H9O2+ | guaiacol/methyl-benzenediol/furyl acetone | Area | 37.73 ± | 8.39 a | 59.62 ± | 21.73 a | 43.73 ± | 12.48 a | 0.198 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romano, A.; Cappellin, L.; Bogialli, S.; Pastore, P.; Navarini, L.; Biasioli, F. Monitoring In Vitro and In Vivo Aroma Release of Espresso Coffees with Proton-Transfer-Reaction Time-of-Flight Mass Spectrometry. Appl. Sci. 2022, 12, 10272. https://0-doi-org.brum.beds.ac.uk/10.3390/app122010272
Romano A, Cappellin L, Bogialli S, Pastore P, Navarini L, Biasioli F. Monitoring In Vitro and In Vivo Aroma Release of Espresso Coffees with Proton-Transfer-Reaction Time-of-Flight Mass Spectrometry. Applied Sciences. 2022; 12(20):10272. https://0-doi-org.brum.beds.ac.uk/10.3390/app122010272
Chicago/Turabian StyleRomano, Andrea, Luca Cappellin, Sara Bogialli, Paolo Pastore, Luciano Navarini, and Franco Biasioli. 2022. "Monitoring In Vitro and In Vivo Aroma Release of Espresso Coffees with Proton-Transfer-Reaction Time-of-Flight Mass Spectrometry" Applied Sciences 12, no. 20: 10272. https://0-doi-org.brum.beds.ac.uk/10.3390/app122010272