Removal of Algae, and Taste and Odor Compounds by a Combination of Plant-Mineral Composite (PMC) Coagulant with UV-AOPs: Laboratory and Pilot Scale Studies
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Experimental Procedure
2.2.1. Lab-Scale Experiment
2.2.2. Pilot-Scale Experiment
2.3. Analysis
3. Results and Discussion
3.1. PMC for Algae and Organic Matters Removal, Lab-Scale
3.2. Zeta Potential of PMC
3.3. pH and Alkalinity
3.4. PMC for Geosmin and 2-MIB Removal
3.5. UV/H2O2 and UV/Cl2 for T&O Removal, Lab-Scale
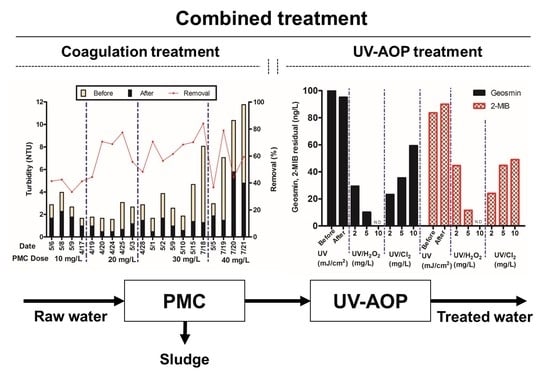
3.6. Coagulation Test in Pilot Scale
3.7. UV/H2O2 and UV/Cl2 Process in Pilot Scale
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cook, D.; Newcombe, G. Can we predict the removal of MIB and geosmin with PAC by using water quality parameters? Water Sci. Technol. Water Supply 2004, 4, 221–226. [Google Scholar] [CrossRef]
- Jiang, J.Q.; Kim, C. Comparison of algal removal by coagulation with clays and Al-based coagulants. Sep. Sci. Technol. 2008, 43, 1677–1686. [Google Scholar] [CrossRef]
- Pirbazari, M.; Ravindran, V.; Badriyha, B.N.; Craig, S.; McGuire, M.J. GAC adsorber design protocol for the removal of off-flavors. Water Res. 1993, 27, 1153–1166. [Google Scholar] [CrossRef]
- Kang, S.J.; Lim, S.I.; Lee, B.H. The removal of red tide organisms by using microscreen and ozone. J. Korea Technol. Soc. Water Waste Water Treat. 2001, 9, 11–17. [Google Scholar]
- Zamyadi, A.; Henderson, R.; Stuetz, R.; Hofmann, R.; Ho, L.; Newcombe, G. Fate of geosmin and 2-methylisoborneol in full-scale water treatment plants. Water Res. 2015, 83, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Knappe, D.R.; Belk, R.C.; Briley, D.S.; Gandy, S.R.; Rastogi, N.; Rike, A.H.; Glasgow, H.; Hannon, E.; Frazier, W.D.; Kohl, P.; et al. Algae Detection and Removal Strategies for Drinking Water Treatment Plants; AWWA Research Foundation: Denver, CO, USA, 2004. [Google Scholar]
- Zhang, G.; Wang, B.; Zhang, P.; Wang, L.; Wang, H. Removal of algae by sonication-coagulation. J. Environ. Sci. Health Part A 2006, 41, 1379–1390. [Google Scholar] [CrossRef] [PubMed]
- Betatache, H.; Aouabed, A.; Drouiche, N.; Lounici, H. Conditioning of sewage sludge by prickly pear cactus (Opuntia ficus Indica) juice. Ecol. Eng. 2014, 70, 465–469. [Google Scholar] [CrossRef]
- Jodi, M.; Birnin-Yauri, U.; Yahaya, Y.; Sokoto, M. The use of some plants in water purification. Glob. Adv. Res. J. Chem. Mater. Sci. 2012, 4, 071–075. [Google Scholar]
- Kim, B.H.; Lee, J.H.; Kim, K.H.; Yu, Y.H.; Hwang, S.J. Algal growth inhibition activity of domestic plants and minerals using simple extraction method. Korean J. Ecol. Environ. 2010, 43, 221–231. [Google Scholar]
- Kim, B.H.; Lee, J.H.; Park, C.H.; Kwon, D.Y.; Park, H.J.; Mun, B.C.; Mun, B.J.; Choi, I.C.; Kim, N.Y.; Min, H.N. Effects of plant-mineral composites (PMC) on the water quality, plankton community and microcystin-LR in eutrophic waters. Korean J. Ecol. Environ. 2011, 44, 347–357. [Google Scholar]
- Rosenfeldt, E.J.; Melcher, B.; Linden, K.G. UV and UV/H2 O2 treatment of methylisoborneol (MIB) and geosmin in water. J. Water Supply Res. Technol.-AQUA 2005, 54, 423–434. [Google Scholar] [CrossRef]
- Comninellis, C.; Kapalka, A.; Malato, S.; Parsons, S.A.; Poulios, I.; Mantzavinos, D. Advanced oxidation processes for water treatment: Advances and trends for R&D. J. Chem. Technol. Biotechnol. 2008, 83, 769–776. [Google Scholar]
- Das, P.; Lei, W.; Aziz, S.S.; Obbard, J.P. Enhanced algae growth in both phototrophic and mixotrophic culture under blue light. Bioresour. Technol. 2011, 102, 3883–3887. [Google Scholar] [CrossRef] [PubMed]
- Bolton, J.R.; Linden, K.G. Linden, Standardization of methods for fluence (UV dose) determination in bench-scale UV experiments. J. Environ. Eng. 2003, 129, 209–215. [Google Scholar] [CrossRef]
- Kwon, M.; Kim, S.; Yoon, Y.; Jung, Y.; Hwang, T.M.; Kang, J.W. Prediction of the removal efficiency of pharmaceuticals by a rapid spectrophotometric method using Rhodamine B in the UV/H2 O2 process. Chem. Eng. J. 2014, 236, 438–447. [Google Scholar] [CrossRef]
- Kwon, M.; Kim, S.; Yoon, Y.; Jung, Y.; Hwang, T.M.; Lee, J.; Kang, J.W. Comparative evaluation of ibuprofen removal by UV/H2 O2 and UV/S2 O82—Processes for wastewater treatment. Chem. Eng. J. 2015, 269, 379–390. [Google Scholar] [CrossRef]
- Liu, S.; Tang, L.; Wu, M.; Fu, H.; Xu, J.; Chen, W.; Ma, F. Parameters influencing elimination of geosmin and 2-methylisoborneol by K2FeO4. Sep. Purif. Technol. 2017, 182, 128–133. [Google Scholar] [CrossRef]
- Kwon, M.; Yoon, Y.; Kim, S.; Jung, Y.; Hwang, T.M.; Kang, J.W. Removal of sulfamethoxazole, ibuprofen and nitrobenzene by UV and UV/chlorine processes: A comparative evaluation of 275 nm LED-UV and 254 nm LP-UV. Sci. Total Environ. 2018, 637, 1351–1357. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Kwon, M.; Ahn, Y.; Jung, Y.; Nam, S.N.; Choi, I.H.; Kang, J.W. Characteristics of intracellular algogenic organic matter and its reactivity with hydroxyl radicals. Water Res. 2018, 144, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Andrews, S.A.; Stefan, M.I.; Bolton, J.R. Optimal methods for quenching H2O2 residuals prior to UFC testing. Water Res. 2003, 37, 3697–3703. [Google Scholar] [CrossRef]
- Wu, X.; Ge, X.; Wang, D.; Tang, H. Distinct coagulation mechanism and model between alum and high Al 13-PACl. Coll. Surf. A Physicochem. Eng. Asp. 2007, 305, 89–96. [Google Scholar] [CrossRef]
- Edzwald, J.K.; Kaminski, G.S. A practical method for water plants to select coagulant dosing. J. N. Engl. Water Works Assoc. 2009, 123, 15–31. [Google Scholar]
- Matilainen, A.; Lindqvist, N.; Tuhkanen, T. Comparison of the effiency of aluminium and ferric sulphate in the removal of natural organic matter during drinking water treatment process. Environ. Technol. 2005, 26, 867–876. [Google Scholar] [CrossRef] [PubMed]
- Ghernaout, D.; Ghernaout, B.; Kellil, A. Natural organic matter removal and enhanced coagulation as a link between coagulation and electrocoagulation. Desalin. Water Treat. 2009, 2, 203–222. [Google Scholar] [CrossRef]
- Vilgé-Ritter, A.; Masion, A.; Boulangé, T.; Rybacki, D.; Bottero, J.Y. Removal of natural organic matter by coagulation-flocculation: A pyrolysis-GC-MS study. Environ. Sci. Technol. 1999, 33, 3027–3032. [Google Scholar] [CrossRef]
- Matilainen, A.; Sillanpää, M. Removal of natural organic matter from drinking water by advanced oxidation processes. Chemosphere 2010, 80, 351–365. [Google Scholar] [CrossRef] [PubMed]
- Ndabigengesere, A.; Narasiah, K.S.; Talbot, B.G. Active agents and mechanism of coagulation of turbid waters using Moringa oleifera. Water Res. 1995, 29, 703–710. [Google Scholar] [CrossRef]
- Ives, K.J. Electrokinetic phenomena of planktonic algae. Proc. Soc. Water Treat. Exam. 1956, 5, 41–58. [Google Scholar]
- Ye, C.; Wang, D.; Shi, B.; Yu, J.; Qu, J.; Edwards, M.; Tang, H. Alkalinity effect of coagulation with polyaluminum chlorides: Role of electrostatic patch. Coll. Surf. A Physicochem. Eng. Asp. 2007, 294, 163–173. [Google Scholar] [CrossRef]
- Yan, M.; Wang, D.; Yu, J.; Ni, J.; Edwards, M.; Qu, J. Enhanced coagulation with polyaluminum chlorides: Role of pH/alkalinity and speciation. Chemosphere 2008, 71, 1665–1673. [Google Scholar] [CrossRef] [PubMed]
- Bruce, D.; Westerhoff, P.; Brawley-Chesworth, A. Removal of 2-methylisoborneol and geosmin in surface water treatment plants in Arizona. J. Water Supply Res. Technol.-AQUA 2002, 51, 183–198. [Google Scholar] [CrossRef]
- Luo, Y.; Guo, W.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci. Total Environ. 2014, 473, 619–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suffet, I.M.; Khiari, D.; Bruchet, A. The drinking water taste and odor wheel for the millennium: Beyond geosmin and 2-methylisoborneo. Water Sci. Technol. 1999, 40, 1–13. [Google Scholar] [CrossRef]
- Kim, T.K.; Moon, B.R.; Kim, T.; Kim, M.K.; Zoh, K.D. Degradation mechanisms of geosmin and 2-MIB during UV photolysis and UV/chlorine reactions. Chemosphere 2016, 162, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Liu, J.; Shang, C.; Fan, C. Degradation Investigation of Selected Taste and Odor Compounds by a UV/Chlorine Advanced Oxidation Process. Int. J. Environ. Res. Public Health 2018, 15, 284. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.; Hart, J.; Croll, B.; Gregory, R. Laboratory-scale investigations of algal toxin removal by water treatment. Water Environ. J. 2000, 14, 143–149. [Google Scholar] [CrossRef]
- Henderson, R.; Parsons, S.A.; Jefferson, B. The impact of algal properties and pre-oxidation on solid–liquid separation of algae. Water Res. 2008, 42, 1827–1845. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeldt, E.J.; Linden, K.G. The R OH, UV concept to characterize and the model UV/H2O2 process in natural waters. Environ.Sci. Technol. 2007, 41, 2548–2553. [Google Scholar] [CrossRef]
- Peter, A.; Von Gunten, U. Oxidation kinetics of selected taste and odor compounds during ozonation of drinking water. Environ. Sci. Technol. 2007, 41, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Watts, M.J.; Linden, K.G. Chlorine photolysis and subsequent OH radical production during UV treatment of chlorinated water. Water Res. 2007, 41, 2871–2878. [Google Scholar] [CrossRef] [PubMed]
- Graham, N.; Gang, F.; Fowler, G.; Watts, M. Characterisation and coagulation performance of a tannin-based cationic polymer: A preliminary assessment. Coll. Surf. A Physicochem. Eng. Asp. 2008, 327, 9–16. [Google Scholar] [CrossRef]
- Yan, M.; Wang, D.; You, S.; Qu, J.; Tang, H. Enhanced coagulation in a typical North-China water treatment plant. Water Res. 2006, 40, 3621–3627. [Google Scholar] [CrossRef] [PubMed]
- Choy, S.Y.; Prasad, K.N.; Wu, T.Y.; Raghunandan, M.E.; Ramanan, R.N. Performance of conventional starches as natural coagulants for turbidity removal. Ecol. Eng. 2016, 94, 352–364. [Google Scholar] [CrossRef]










| Parameters | Lab-Scale Test | First Pilot-Scale Test | Second Pilot-Scale Test |
|---|---|---|---|
| Turbidity (NTU) | 4.6 | 1.1 | 15.6 |
| DOC (mg/L) | 3.4 | 0.9 | 0.8 |
| UV254 (cm−1) | 0.034 | 0.015 | 0.027 |
| pH | 7.8 | 6.9 | 6.7 |
| chlorophyll-a (mg/m3) | 97.1 | 0.5 | 0.7 |
| Target chemicals | 134.6 ng/L geosmin 159.0 ng/L 2-methylisoborneol (2-MIB) | 100.0 ng/L geosmin 80.0 ng/L 2-MIB | 100.0 ng/L geosmin 80.0 ng/L 2-MIB |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abrha, Y.W.; Kye, H.; Kwon, M.; Lee, D.; Kim, K.; Jung, Y.; Ahn, Y.; Kang, J.-W. Removal of Algae, and Taste and Odor Compounds by a Combination of Plant-Mineral Composite (PMC) Coagulant with UV-AOPs: Laboratory and Pilot Scale Studies. Appl. Sci. 2018, 8, 1502. https://0-doi-org.brum.beds.ac.uk/10.3390/app8091502
Abrha YW, Kye H, Kwon M, Lee D, Kim K, Jung Y, Ahn Y, Kang J-W. Removal of Algae, and Taste and Odor Compounds by a Combination of Plant-Mineral Composite (PMC) Coagulant with UV-AOPs: Laboratory and Pilot Scale Studies. Applied Sciences. 2018; 8(9):1502. https://0-doi-org.brum.beds.ac.uk/10.3390/app8091502
Chicago/Turabian StyleAbrha, Yirga Weldu, Homin Kye, Minhwan Kwon, Doorae Lee, Kiho Kim, Youmi Jung, Yongtae Ahn, and Joon-Wun Kang. 2018. "Removal of Algae, and Taste and Odor Compounds by a Combination of Plant-Mineral Composite (PMC) Coagulant with UV-AOPs: Laboratory and Pilot Scale Studies" Applied Sciences 8, no. 9: 1502. https://0-doi-org.brum.beds.ac.uk/10.3390/app8091502