Pickering Emulsions Containing Cellulose Microfibers Produced by Mechanical Treatments as Stabilizer in the Food Industry
Abstract
:Featured Application
Abstract
1. Introduction
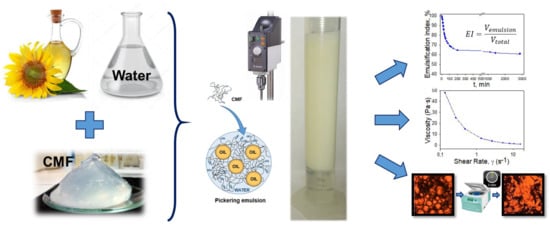
2. Materials and Methods
3. Results and Discussion
3.1. CMF Characterization
3.2. Stability over Time
3.3. Rheological Measurements
3.4. Stability toward Centrifugation
3.5. Application of Pickering Emulsions as an Alternative to Trans and Saturated Fats in the Food Industry
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Garti, N.; Binyamin, H.; Aserin, A. Stabilization of water-in-oil emulsions by submicrocrystalline α-form fat particles. J. Am. Oil Chem. Soc. 1998, 75, 1825–1831. [Google Scholar] [CrossRef]
- Lupi, F.R.; Gabriele, D.; De Cindio, B.; Sánchez, M.C.; Gallegos, C. A rheological analysis of structured water-in-olive oil emulsions. J. Food Eng. 2011, 107, 296–303. [Google Scholar] [CrossRef]
- Tarimala, S.; Dai, L.L. Structure of microparticles in solid-stabilized emulsions. Langmuir 2004, 20, 3492–3494. [Google Scholar] [CrossRef] [PubMed]
- Barkhordari, M.R.; Fathi, M. Production and characterization of chitin nanocrystals from prawn shell and their application for stabilization of Pickering emulsions. Food Hydrocoll. 2018, 82, 338–345. [Google Scholar] [CrossRef]
- Zhou, J.; Qiao, X.; Binks, B.P.; Sun, K.; Bai, M.; Li, Y.; Liu, Y. Magnetic Pickering emulsions stabilized by Fe3O4 nanoparticles. Langmuir 2011, 27, 3308–3316. [Google Scholar] [CrossRef]
- Rayner, M.; Timgren, A.; Sjöö, M.; Dejmek, P. Quinoa starch granules: A candidate for stabilising food-grade Pickering emulsions. J. Sci. Food Agric. 2012, 92, 1841–1847. [Google Scholar] [CrossRef] [PubMed]
- Saari, H.; Heravifar, K.; Rayner, M.; Wahlgren, M.; Sjöö, M. Preparation and characterization of starch particles for use in pickering emulsions. Cereal Chem. 2016, 93, 116–124. [Google Scholar] [CrossRef]
- Mathapa, B.G.; Paunov, V.N. Cyclodextrin stabilised emulsions and cyclodextrinosomes. Phys. Chem. Chem. Phys. 2016, 15, 17903–17914. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, H.; Li, Y.; Huang, Q. Fabrication of milled cellulose particles-stabilized Pickering emulsions. Food Hydrocoll. 2018, 77, 427–435. [Google Scholar] [CrossRef]
- Cunha, A.G.; Mougel, J.B.; Cathala, B.; Berglund, L.A.; Capron, I. Preparation of double Pickering emulsions stabilized by chemically tailored nanocelluloses. Langmuir 2014, 30, 9327–9335. [Google Scholar] [CrossRef]
- Zhai, X.C.; Lin, D.H.; Liu, D.J.; Yang, X.B. Emulsions stabilized by nanofibers from bacterial cellulose: New potential food-grade Pickering emulsions. Food Res. Int. 2018, 103, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Mezdour, S.; Lepine, A.; Erazo-Majewicz, P.; Ducept, F.; Michon, C. Oil/water surface rheological properties of hydroxypropyl cellulose (HPC) alone and mixed with lecithin: Contribution to emulsion stability. Colloid Surf. A 2008, 331, 76–83. [Google Scholar] [CrossRef]
- Mikulcová, V.; Bordes, R.; Kašpárková, V. On the preparation and antibacterial activity of emulsions stabilized with nanocellulose particles. Food Hydrocoll. 2016, 61, 780–792. [Google Scholar] [CrossRef]
- Gestranius, M.; Stenius, P.; Kontturi, E.; Sjöblom, J.; Tammelin, T. Phase behaviour and droplet size of oil-in-water Pickering emulsions stabilised with plant-derived nanocellulosic materials. Colloid Surf. A 2017, 519, 60–70. [Google Scholar] [CrossRef]
- Kalashnikova, I.; Bizot, H.; Cathala, B.; Capron, I. New Pickering emulsions stabilized by bacterial cellulose nanocrystals. Langmuir 2011, 27, 7471–7479. [Google Scholar] [CrossRef] [PubMed]
- Varanasi, S.; Henzel, L.; Prathapan, R.; Batchelor, W.; Tabor, R.; Garnier, G. Pickering emulsions electrostatically stabilized by Cellulose nanocrystals. Front. Chem. 2018, 6, 409. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Xu, R.; Shen, W.; Xie, M.; Abid, M.; Jabbar, S.; Wang, P.; Zeng, X.; Wu, T. Stabilizing oil-in-water emulsion with amorphous cellulose. Food Hydrocoll. 2015, 43, 275–282. [Google Scholar] [CrossRef]
- Mikulcová, V.; Bordes, R.; Minařík, A.; Kašpárková, V. Pickering oil-in-water emulsions stabilized by carboxylated cellulose nanocrystals–Effect of the pH. Food Hydrocoll. 2018, 80, 60–67. [Google Scholar] [CrossRef]
- Zoppe, J.O.; Venditti, R.A.; Rojas, O.J. Pickering emulsions stabilized by cellulose nanocrystals grafted with thermo-responsive polymer brushes. J. Colloid Interface Sci. 2012, 369, 202–209. [Google Scholar] [CrossRef]
- Bajpai, P. Production of Nanocellulose. In Pulp and Paper Industry: Nanotechnology in Forest Industry, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2016; Chapter 4; pp. 41–67. [Google Scholar]
- Nechyporchuk, O.; Belgacem, M.N.; Bras, J. Production of cellulose nanofibrils: A review of recent advances. Ind. Crop. Prod. 2016, 93, 2–25. [Google Scholar] [CrossRef]
- Berglund, L. Cellulose-based nanocomposites. In Natural Fibers, Biopolymers and Biocomposites, 1st ed.; Mohanty, A.K., Misra, M., Drzal, L.T., Eds.; CRC Press: Boca Raton, FL, USA, 2005; Chapter 26; pp. 807–832. [Google Scholar]
- Blanco, A.; Monte, M.C.; Campano, C.; Balea, A.; Merayo, N.; Negro, C. Nanocellulose for Industrial Use: Cellulose Nanofibers (CNF), Cellulose Nanocrystals (CNC), and Bacterial Cellulose (BC). In Handbook of Nanomaterials for Industrial Applications, 1st ed.; Hussain, C.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; Chapter 5; pp. 74–126. [Google Scholar]
- Gómez, C.; Serpa, A.; Velásquez-Cock, J.; Gañán, P.; Castro, C.; Vélez, L.; Zuluaga, R. Vegetable nanocellulose in food science: A review. Food Hydrocoll. 2016, 57, 178–186. [Google Scholar] [CrossRef]
- Kim, J.H.; Shim, B.S.; Kim, H.S.; Lee, Y.J.; Min, S.K.; Jang, D.; Abas, Z.; Kim, J. Review of nanocellulose for sustainable future materials. Int. J. Precis. Eng. Manuf. Green Technol. 2015, 2, 197–213. [Google Scholar] [CrossRef]
- Chen, Q.H.; Zheng, J.; Xu, Y.T.; Yin, S.W.; Liu, F.; Tang, C.H. Surface modification improves fabrication of Pickering high internal phase emulsions stabilized by cellulose nanocrystals. Food Hydrocoll. 2018, 75, 125–130. [Google Scholar] [CrossRef]
- Bai, L.; Xiang, W.; Huan, S.; Rojas, O.J. Formulation and stabilization of concentrated edible oil-in-water emulsions based on electrostatic complexes of a food-grade cationic surfactant (ethyl lauroyl arginate) and cellulose nanocrystals. Biomacromolecules 2018, 19, 1674–1685. [Google Scholar] [CrossRef] [PubMed]
- Strom, G.; Ohgren, C.; Ankerfors, M. Nanocellulose as an Additive in Foodstuff. Innventia Rep. 2013, 403, 1–25. [Google Scholar]
- Turbak, A.F.; Snyder, F.W.; Sandberg, K.R. Suspensions Containing Microfibrillated Cellulose. U.S. Patent No. 4,378,381, 29 March 1983. [Google Scholar]
- Robson, A. Tackling obesity: Can food processing be a solution rather than a problem? Agro-Food Ind. Hi Tech 2012, 23 (Suppl. 2), 10–11. [Google Scholar]
- Winuprasith, T.; Suphantharika, M. Properties and stability of oil-in-water emulsions stabilized by microfibrillated cellulose from mangosteen rind. Food Hydrocoll. 2015, 43, 690–699. [Google Scholar] [CrossRef]
- Bai, L.; Huan, S.; Xiang, W.; Rojas, O.J. Pickering emulsions by combining cellulose nanofibrils and nanocrystals: Phase behavior and depletion stabilization. Green Chem. 2018, 20, 1571–1582. [Google Scholar] [CrossRef]
- Brody, T. Lipids, In Nutritional Biochemistry, 2nd ed.; Academic Press: San Diego, CA, USA, 1999; Chapter 6; pp. 311–378. [Google Scholar]
- Klonoff, D.C. Replacements for trans fats—Will there be an oil shortage? J. Diabetes Sci. Technol. 2007, 1, 415–422. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). Summary of conclusions and dietary recommendations on total fat and fatty acids. In Fats and Fatty Acids in Human Nutrition. Report of an Expert Consultation; FAO: Rome, Italy, 2010; Chapter 2; pp. 9–19. [Google Scholar]
- Negro, C.; Garcia-Ochoa, F.; Tanguy, P.; Ferreira, G.; Thibault, J.; Yamamoto, S.; Gani, R. Barcelona Declaration–10th World Congress of Chemical Engineering, 1–5 October 2017. Chem. Eng. Res. Des. 2018, 129, 34. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). Processing, manufacturing, uses and labelling of fats in the food supply. In Fats and Fatty Acids in Human Nutrition. Report of an Expert Consultation; FAO: Rome, Italy, 2010; Chapter 14; pp. 153–158. [Google Scholar]
- Mensink, R.P.; Sanders, T.A.; Baer, D.J.; Hayes, K.C.; Howles, P.N.; Marangoni, A. The increasing use of interesterified lipids in the food supply and their effects on health parameters. Adv. Nutr. 2016, 7, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Balea, A.; Merayo, N.; Fuente, E.; Delgado-Aguilar, M.; Mutje, P.; Blanco, A.; Negro, C. Valorization of corn stalk by the production of cellulose nanofibers to improve recycled paper properties. BioResources 2016, 11, 3416–3431. [Google Scholar] [CrossRef]
- Habibi, Y.; Chanzy, H.; Vignon, M.R. TEMPO-mediated surface oxidation of cellulose whiskers. Cellulose 2006, 13, 679–687. [Google Scholar] [CrossRef] [Green Version]
- González, I.; Alcalà, M.; Chinga-Carrasco, G.; Vilaseca, F.; Boufi, S.; Mutjé, P. From paper to nanopaper: Evolution of mechanical and physical properties. Cellulose 2014, 21, 2599–2609. [Google Scholar] [CrossRef]
- Henriksson, M.; Berglund, L.A.; Isaksson, P.; Lindström, T.; Nishino, T. Cellulose nanopaper structures of high toughness. Biomacromolecules 2008, 9, 1579–1585. [Google Scholar] [CrossRef] [PubMed]
- ISO standard ISO 5351. Pulps—Determination of Limiting Viscosity Number in Cupri-Ethylenediamine (CED) Solution; ISO: Geneva, Switzerland, 2010. [Google Scholar]
- Wahlgren, M.; Bergenståhl, B.; Nilsson, L.; Rayner, M. Formulation of emulsions. In Engineering Aspects of Food Emulsification and Homogenization, 1st ed.; Rayner, M., Dejmek, P., Eds.; CRC Press: Boca Raton, FL, USA, 2015; Chapter 3; pp. 51–100. [Google Scholar]
- Balea, A.; Blanco, A.; Monte, M.C.; Merayo, N.; Negro, C. Effect of bleached eucalyptus and pine cellulose nanofibers on the physico-mechanical properties of cartonboard. BioResources 2016, 11, 8123–8138. [Google Scholar] [CrossRef]
- Delgado-Aguilar, M.; González, I.; Pèlach, M.A.; De La Fuente, E.; Negro, C.; Mutjé, P. Improvement of deinked old newspaper/old magazine pulp suspensions by means of nanofibrillated cellulose addition. Cellulose 2015, 22, 789–802. [Google Scholar] [CrossRef]
- Fujisawa, S.; Togawa, E.; Kuroda, K. Nanocellulose-stabilized Pickering emulsions and their applications. Sci. Technol. Adv. Mater. 2017, 18, 959–971. [Google Scholar] [CrossRef] [Green Version]
- Glasser, W.G.; Atalla, R.H.; Blackwell, J.; Brown, R.M.; Burchard, W.; French, A.D.; Nishiyama, Y. About the structure of cellulose: Debating the Lindman hypothesis. Cellulose 2012, 19, 589–598. [Google Scholar] [CrossRef]
- Horozov, T.S.; Binks, B.P. Particle-Stabilized Emulsions: A Bilayer or a Bridging Monolayer? Angew. Chem. 2006, 118, 787–790. [Google Scholar] [CrossRef]
- Xhanari, K.; Syverud, K.; Chinga-Carrasco, G.; Paso, K.; Stenius, P. Structure of nanofibrillated cellulose layers at the o/w interface. J. Colloid Interf. Sci. 2011, 356, 58–62. [Google Scholar] [CrossRef] [PubMed]








| Parameter | Units | Value |
|---|---|---|
| Consistency | (%) | 3.67 ± 0.02 |
| Carboxylic groups | (mmol COOH/g) | 0.1 ± 0.03 |
| Nanofibrillation degree | (%) | 6.1 ± 0.5 |
| Transmittance 800 nm | (%) | 3.8 ± 0.2 |
| Cationic demand | (µeq/g) | 19.0 ± 1.0 |
| Polymerization degree | (monomeric units) | 399 ± 11 |
| CMF Dose (%) | µa Max (Pa·s) | OCEP (%) | EI (%) | O/W Ratio |
|---|---|---|---|---|
| 0.25 | 5.1 | 73.8 | 54.2 | 40/60 |
| 0.50 | 6.3 | 65.8 | 60.8 | 40/60 |
| 0.75 | 9.1 | 62.9 | 79.5 | 50/50 |
| 1.00 | 8.2 | 67.8 | 88.5 | 60/40 |
| Food Product | T1 | T2 | E1 | E2 |
|---|---|---|---|---|
| Emulsion ingredients | Butter | Sunflower oil (+ water) | Pickering emulsionsusing CMF as stabilizer | |
| O/W, %/% | 82/18 | 82/18 | 79.5 1/20.5 | 62.9 1/37.1 |
| CMF, wt% | 0 | 0 | 0.50/(0.83 2) | 0.75/(0.94 2) |
| Emulsion, g | 21.5 | |||
| Flour, g | 30.4 | |||
| Brown sugar, g | 13.0 | |||
| Energy (kcal) | 544.6 | 542.8 | 533.9 | 478.6 |
| Total Fat (g) | 31.0 | 31.0 | 30.1 | 23.9 |
| SFA (g) | 20.9 | 4.1 | 4.0 | 3.2 |
| MUFA (g) | 7.9 | 7.4 | 7.2 | 5.7 |
| PUFA (g) | 1.5 | 19.6 | 19.0 | 15.1 |
| TFA (g) | 0.7 | - | - | - |
| Carbohydrates (g) | 60.8 | 60.2 | 60.2 | 60.2 |
| Proteins (g) | 5.6 | 5.4 | 5.4 | 5.4 |
| Dietary fiber (g) | 0 | 0 | 0.31 | 0.34 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanchez-Salvador, J.L.; Balea, A.; Monte, M.C.; Blanco, A.; Negro, C. Pickering Emulsions Containing Cellulose Microfibers Produced by Mechanical Treatments as Stabilizer in the Food Industry. Appl. Sci. 2019, 9, 359. https://0-doi-org.brum.beds.ac.uk/10.3390/app9020359
Sanchez-Salvador JL, Balea A, Monte MC, Blanco A, Negro C. Pickering Emulsions Containing Cellulose Microfibers Produced by Mechanical Treatments as Stabilizer in the Food Industry. Applied Sciences. 2019; 9(2):359. https://0-doi-org.brum.beds.ac.uk/10.3390/app9020359
Chicago/Turabian StyleSanchez-Salvador, Jose Luis, Ana Balea, M. Concepcion Monte, Angeles Blanco, and Carlos Negro. 2019. "Pickering Emulsions Containing Cellulose Microfibers Produced by Mechanical Treatments as Stabilizer in the Food Industry" Applied Sciences 9, no. 2: 359. https://0-doi-org.brum.beds.ac.uk/10.3390/app9020359