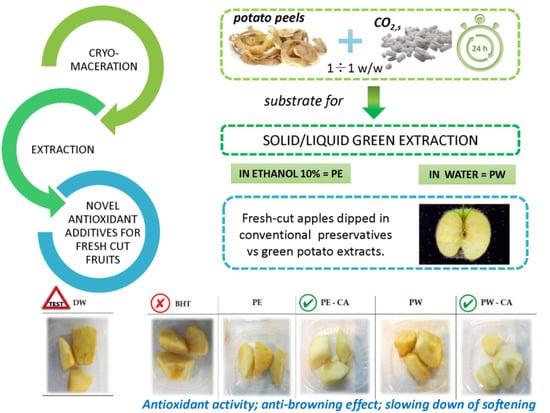
Potato Peels as a Source of Novel Green Extracts Suitable as Antioxidant Additives for Fresh-Cut Fruits
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Potato Peel Extraction
2.2. Potato Peel Extracts Characterization
2.2.1. Total Phenols Content
2.2.2. Antioxidant Capacity of the Extracts Determined by ABTS and FRAP Assays
2.3. Fresh-Cut Apple Processing and Sample Preparation
2.4. Assessment of Shelflife Analysis
2.5. Statistical Analysis
3. Results
3.1. Extraction Yield and Chemical Characterization of the Potato Peel Extracts
3.2. Effect of Dipping on Browning
3.3. Effect of Dipping on TSS
3.4. Effect of Dipping on Firmness
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Varela, P.; Salvador, A.; Fiszman, S.M. The use of calcium chloride in minimally processed apples: A sensory approach. Eur. Food Res. Technol. 2007, 224, 461–467. [Google Scholar] [CrossRef]
- Tappi, S.; Tylewicz, U.; Romani, S.; Dalla Rosa, M.; Rizzi, F.; Rocculi, P. Study on the quality and stability of minimally processed apples impregnated with green tea polyphenols during storage. Innov. Food Sci. Emerg. Technol. 2017, 39, 148–155. [Google Scholar] [CrossRef]
- Trivellini, A.; Lucchesini, M.; Maggini, R.; Mosadegha, H.; Villamarin, T.S.S.; Vernieri, P.; Mensuali-Sodi, A.; Pardossi, A. Lamiaceae phenols as multifaceted compounds: Bioactivity, industrial prospects and role of positive-stress. Ind. Crops Prod. 2016, 83, 241–254. [Google Scholar] [CrossRef]
- Bansal, V.; Rahman, M.S.; Siddiqui, M.W. Minimally processed foods: Overview. A review. In Minimally Processed Foods, 1st ed.; Siddiqui, M.W., Rahman, M.S., Eds.; Springer International Publishing: Basel, Switzerland, 2015; pp. 1–15. [Google Scholar]
- Paiva-Martins, F.; Correia, R.; Felix, S.; Ferreira, P.; Gordon, M.H. Effects of enrichment of refined olive oil with phenolic compounds from olive leaves. J. Agric. Food Chem. 2007, 55, 4139–4143. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Hu, W.; He, Y.; Jiang, A.; Zhang, R. Effect of citric acid combined with UV-C on the quality of fresh-cut apples. Postharvest Biol. Technol. 2016, 111, 126–131. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Vardanega, R.; Prado, J.M.; Meireles, M.A.A. Adding value to agri-food residues by means of supercritical technology. J. Supercrit. Fluids 2015, 96, 217–227. [Google Scholar] [CrossRef]
- Rodríguez Amado, I.; Franco, D.; Sánchez, M.; Zapata, C.; Vázquez, J.A. Optimization of antioxidant extraction from Solanum tuberosum potato peel waste by surface response methodology. Food Chem. 2014, 165, 290–299. [Google Scholar] [CrossRef]
- Strati, I.F.; Oreopoulou, V. Recovery of carotenoids from tomato processing by-products—A review. Food Res. Int. 2014, 65, 311–321. [Google Scholar] [CrossRef]
- Akyol, H.; Riciputi, Y.; Capanoglu, E.; Caboni, M.F.; Verardo, V. Phenolic Compounds in the Potato and Its Byproducts: An Overview. Int. J. Mol. Sci. 2016, 17, 835. [Google Scholar] [CrossRef]
- Schieber, A.; Aranda Saldaña, M.D. Potato Peels: A Source of Nutritionally and Pharmacologically Interesting Compounds—A Review. Food 2009, 3, 23–29. [Google Scholar]
- Pinela, J.; Prieto, M.A.; Barreiro, M.F.; Carvalho, A.M.; Oliveira, M.B.P.P.; Curran, T.P.; Ferreira, I.C.F.R. Valorisation of tomato wastes for development of nutrient-rich antioxidant ingredients: A sustainable approach towards the needs of the today’s society. Innov. Food Sci. Emerg. Technol. 2017, 41, 160–171. [Google Scholar] [CrossRef]
- Durante, M.; Lenucci, M.S.; Mita, G. Supercritical Carbon Dioxide Extraction of Carotenoids from Pumpkin (Cucurbita spp.): A Review. Int. J. Mol. Sci. 2014, 15, 6725–6740. [Google Scholar] [CrossRef] [PubMed]
- Venturi, F.; Sanmartin, C.; Taglieri, I.; Andrich, G.; Zinnai, A. A simplified method to estimate Sc-CO2 extraction of bioactive compounds from different matrices: Chili pepper vs. tomato by-products. Appl. Sci. 2017, 7, 361. [Google Scholar] [CrossRef]
- Sánchez-Camargo, A.P.; García-Cañas, V.; Herrero, M.; Cifuentes, A.; Ibáñez, E. Comparative study of green sub- and supercritical processes to obtain carnosic acid and carnosol-enriched rosemary extracts with in vitro anti-proliferative activity on colon cancer cells. Int. J. Mol. Sci. 2016, 17, 2046. [Google Scholar] [CrossRef]
- Robles-Ramírez, M.C.; Monterrubio-López, R.; Mora-Escobedo, R.; Beltrán-Orozco, M.D. Evaluation of extracts from potato and tomato wastes as natural antioxidant additives. Archivos Latinoamericanos de Nutrición Órgano Oficial de la Sociedad Latinoamericana de Nutrición 2016, 66, 66–73. [Google Scholar]
- Fritsch, C.; Staebler, A.; Happel, A.; Cubero Márquez, M.A.; Aguiló-Aguayo, I.; Abadias, M.; Gallur, M.; Cigognini, I.M.; Montanari, A.; López, M.J.; et al. Processing, Valorization and Application of Bio-Waste Derived Compounds from Potato, Tomato, Olive and Cereals: A Review. Sustainability 2017, 9, 1492. [Google Scholar] [CrossRef]
- Zinnai, A.; Venturi, F.; Andrich, G. Time evolution of phenols extractions from Sangiovese grapes with and without the addition of solid carbon dioxide. Agrochimica 2011, 55, 193–202. [Google Scholar]
- Zinnai, A.; Venturi, F.; Sanmartin, C.; Taglieri, I.; Andrich, G. The utilization of solid carbon dioxide in the extraction of extra-virgin olive oil. Agro-Food Ind. Hi Technol. 2015, 26, 24–26. [Google Scholar]
- Zinnai, A.; Venturi, F.; Quartacci, M.F.; Sanmartin, C.; Favati, F.; Andrich, G. Solid carbon dioxide to promote the extraction of extra-virgin olive oil. Grasas Y Aceites 2016, 67, e121. [Google Scholar] [CrossRef] [Green Version]
- Nari, A.; Taglieri, I.; Pistelli, L.; Ascrizzi, R.; Andrich, G.; Zinnai, A. The effect of ripening degree and irrigation regimes of fruits on the quality of extra-virgin olive oil extracted with or without the addition of carbonic snow. Agrochimica 2018, 62, 79–91. [Google Scholar]
- Venturi, F.; Andrich, G.; Sanmartin, C.; Taglieri, I.; Serni, E.; Zinnai, A. Winemaking of Sangiovese grapes with and without the addition of different oenological tannins in order to increase the colour intensity of Chianti wine. Agrochimica 2015, 59, 261–271. [Google Scholar]
- Horwitz, W.; Latimer, G.W., Jr. AOAC International Official Methods of Analysis, 21st ed.; Latimer, G.W., Ed.; AOAC International: Rockville, MD, USA, 2019. [Google Scholar]
- Liu, X.; Yang, Q.; Lu, Y.; Li, Y.; Li, T.; Zhou, B.; Qiao, L. Effect of purslane (Portulaca oleracea L.) extract on anti-browning of fresh cut potato slices during storage. Food Chem. 2019, 283, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Sanmartin, C.; Venturi, F.; Macaluso, M.; Nari, A.; Quartacci, M.F.; Sgherri, C.; Flamini, G.; Taglieri, I.; Ascrizzi, R.; Andrich, G.; et al. Preliminary results about the use of argon and carbon dioxide in the extra virgin olive oil (EVOO) storage to extend oil shelf life: Chemical and sensorial point of view. Eur. J. Lipid. Sci. Technol. 2018, 120, 1800156. [Google Scholar] [CrossRef]
- Dávila-Aviña, J.; Solis, L.; Rojas-Verde, G.; Salas, N. Sustainability and challenges of minimally processed foods. In: Washing, Peeling and Cutting of Fresh-Cut Fruits and Vegetables. Food Eng. Ser. 2015. [Google Scholar] [CrossRef]
- Son, S.M.; Moon, K.D.; Lee, C.Y. Inhibitory effects of various antibrowning agents on apple slices. Food Chem. 2001, 73, 23–30. [Google Scholar] [CrossRef]
- Delaquis, P.J.; Stewart, S.; Toivonen, P.M.A.; Moyls, A. Effect of warm, chlorinated water on the microbial flora of shredded iceberg lettuce. Food Res. Int. 1999, 32, 7–14. [Google Scholar] [CrossRef]
- Nicoli, M.C. Shelf Life Assessment of Food; Barbosa-Cánovas, G.V., Ed.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2012. [Google Scholar]
- Buera, M.P.; Lozano, R.D.; Petriella, C. Definition of colour in the non-enzymatic browning process. Die Farbe 1985, 32–33, 318–322. [Google Scholar]
- Albishi, T.; John, J.A.; Al-Khalifa, A.S.; Shahidi, F. Phenolic content and antioxidant activities of selected potato varieties and their processing by-products. J. Funct. Foods 2013, 5, 590–600. [Google Scholar] [CrossRef]
- Jin, C.Y.; Liu, H.; Xu, D.; Zeng, F.K.; Zhao, Y.C.; Zhang, H.; Liu, G. Glycoalkaloids and phenolic compounds in three commercial potato cultivars grown in Hebei, China. Food Sci. Hum. Wellness 2018, 7, 156–162. [Google Scholar] [CrossRef]
- Choi, S.H.; Kozukue, N.; Kim, H.J.; Friedman, M. Analysis of protein amino acids, non-protein amino acids and metabolites, dietary protein, glucose, fructose, sucrose, phenolic, and flavonoid content and antioxidative properties of potato tubers, peels, and cortexes (pulps). J. Food Compos. Anal. 2016, 50, 77–87. [Google Scholar] [CrossRef]
- Ascrizzi, R.; Taglieri, I.; Sgherri, C.; Flamini, G.; Macaluso, M.; Sanmartin, C.; Venturi, F.; Quartacci, M.F.; Pistelli, L.; Zinnai, A. Nutraceutical Oils Produced by Olives and Citrus Peel of Tuscany Varieties as Sources of Functional Ingredients. Molecules 2019, 24, 65. [Google Scholar] [CrossRef] [PubMed]
- Andrich, G.; Stevanin, E.; Zinnai, A.; Venturi, F.; Fiorentini, R. Extraction Kinetics of Natural Antioxidants from Potato Industry by-Products; ISASF: Versailles, France, 2003; pp. 159–163. [Google Scholar]
- Friedman, M.; Kozukue, N.; Kim, H.J.; Choi, S.H.; Mizuno, M. Glycoalkaloid, phenolic, and flavonoid content and antioxidative activities of conventional nonorganic and organic potato peel powders from commercial gold, red, and Russet potatoes. J. Food Compos. Anal. 2017, 62, 69–75. [Google Scholar] [CrossRef]
- Friedman, M. Potato glycoalkaloids and metabolites: Roles in the plant and in the diet. J. Agric. Food Chem. 2006, 54, 8655–8681. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.; Levin, C.E. Glycoalkaloids and calystegine alkaloids in potatoes. In Advances in Potato Chemistry and Technology, 2nd ed.; Singh, J., Kaur, L., Eds.; Elsevier: Oxford, UK, 2016; pp. 167–194. [Google Scholar]
- Mensinga, T.T.; Sips, A.J.A.M.; Rompelberg, C.J.M.; van Twillert, K.; Meulenbelt, J.; van den Top, H.J.; van Egmond, H.P. Potato glycoalkaloids and adverse effects on humans: An ascending dose study. Regul. Toxicol. Pharmacol. 2005, 41, 66–72. [Google Scholar] [CrossRef]
- Vinson, J.A.; Demkosky, C.A.; Navarre, D.A.; Smyda, M.A. High-antioxidant potatoes: Acute in vivo antioxidant source and hypotensive agent in humans after supplementation to hypertensive subjects. J. Agric. Food Chem. 2012, 60, 6749–6754. [Google Scholar] [CrossRef] [PubMed]
- Franco, D.; Pateiro, M.; Rodríguez Amado, I.; López Pedrouso, M.; Zapata, C.; Vázquez, J.A.; Lorenzo, J.M. Antioxidant ability of potato (Solanum tuberosum) peel extracts to inhibit soybean oil oxidation. Eur. J. Lipid Sci. Technol. 2016, 118, 1891–1902. [Google Scholar] [CrossRef]
- Ben Jeddou, K.; Bouaziz, F.; Zouari-Ellouzi, S.; Chaari, F.; Ellouz-Chaabouni, S.; Ellouz-Ghorbel, R.; Nouri-Ellouz, O. Improvement of texture and sensory properties of cakes by addition of potato peel powder with high level of dietary fiber and protein. Food Chem. 2017, 217, 668–677. [Google Scholar] [CrossRef]
- Toivonen, P.M.; Brummell, D.A. Biochemical bases of appearance and texture changes in fresh-cut fruit and vegetables. Postharvest Biol. Technol. 2008, 48, 1–14. [Google Scholar] [CrossRef]
- Artés, F.; Gómez, P.A.; Artés-Hernández, F. Physical, physiological and microbial deterioration of minimally fresh processed fruits and vegetables. Revista de Agaroquimica y Tecnologia de Alimentos 2007, 13, 177–188. [Google Scholar] [CrossRef]
- Martinez, M.; Whitaker, J.R. The biochemistry and control of enzymatic browning. Trends Food Sci. Technol. 1995, 6, 195–200. [Google Scholar] [CrossRef]
- Amiot, M.J.; Tacchini, M.; Aubert, S.Y.; Oleszek, W. Influence of Cultivar, Maturity Stage, and Storage Conditions on Phenolic Composition and Enzymic Browning of Pear Fruits. J. Agric. Food Chem. 1995, 43, 1132–1137. [Google Scholar] [CrossRef]
- Saltveit, M.E. Wound induced changes in phenolic metabolism and tissue browning are altered by heat shock. Postharvest Biol. Technol. 2000, 21, 61–69. [Google Scholar] [CrossRef]
- Amiot, M.J.; Tacchini, M.; Aubert, S.; Nicholas, J. Phenolic composition and browning susceptibility of various apple cultivars at maturity. J. Food Sci. 1992, 57, 958–962. [Google Scholar] [CrossRef]
- Hakkim, F.L.; Essa, M.M.; Arivazhagan, G.; Guizani, N.; Hyuk, S. Evaluation of food protective property of five natural products using fresh-cut apple alice model. Pakistan J. Biol. Sci. 2012, 15, 10–18. [Google Scholar] [CrossRef]
- Gurbuz, G.; Lee, C.Y. Color of minimally processed potatoes as affected by modified atmosphere packaging and antibrowning agents. J. Food Sci. 1997, 62, 572–575. [Google Scholar]
- Musacchi, S.; Serra, S. Apple fruit quality: Overview on pre-harvest factors. Sci. Hortic. 2018, 234, 409–430. [Google Scholar] [CrossRef]
- Yahia, E.M. Apple Flavor. In Horticultural Reviews; Janick, J., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1994; Chapter 6; Volume 16, pp. 197–234. [Google Scholar]
- Augusto, A.; Simões, T.; Pedrosa, R.; Silva, S.F.J. Evaluation of seaweed extracts functionality as post-harvest treatment for minimally processed Fuji apples. IFSET 2016, 33, 589–595. [Google Scholar] [CrossRef]
- Ling, L.; Li, Q.P.; Wang, B.G.; Cao, J.K.; Jiang, W.B. Inhibition of core browning in yali pear fruit by post-harvest treatment with ascorbic acid. J. Hortic. Sci. Biotechnol. 2007, 82, 397402. [Google Scholar]
- Singh, J.; Mirza, A. Influence of ascorbic acid application on quality and storage life of fruits. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 4319–4328. [Google Scholar] [CrossRef]
- Rux, G.; Caleb, O.J.; Fröhling, A.; Herppich, W.B.; Mahajan, P.V. Respiration and Storage Quality of Fresh-Cut Apple Slices Immersed in Sugar Syrup and Orange Juice. Food Bioprocess. Technol. 2017, 10, 2081–2091. [Google Scholar] [CrossRef]
- Rocculi, P.; Dalla Rosa, M.; Romani, S. Evaluation of physico-chemical parameters of MP apples packed in non-conventional modified atmosphere. Food Res. Int. 2004, 37, 329–335. [Google Scholar] [CrossRef]
- Olivas, G.I.; Mattinson, D.S.; Barbosa-Canovas, G.V. Alginate coatings of minimally processed ‘Gala’ apples. Postharvest Biol. Technol. 2007, 1, 89–96. [Google Scholar] [CrossRef]
- Fontes, L.C.B.; Sarmento, S.B.S.; Spoto, M.H.F.; Dias, C.T.S. Conservação de maçã minimamente processada com o uso de películas comestíveis. Ciência Tecnol. Aliment. 2008, 28, 1–9. [Google Scholar] [CrossRef]
- Fagundes, C.; Carciofi, B.A.M.; Monteiro, A.R. Estimate of respiration rate and physicochemical changes of fresh-cut apples stored under different temperatures. Food Sci. Technol. 2013, 33, 60–67. [Google Scholar] [CrossRef] [Green Version]
- Van Buggenhout, S.; Sila, D.N.; Duvetter, T.; Van Loey, A.; Hendrickx, M. Pectins in processed fruits and vegetables: Part III—Texture engineering. Compr. Rev. Food Sci. Food Saf. 2009, 8, 105–117. [Google Scholar] [CrossRef]
- Nayak, A.; Bhushan, B. An overview of the recent trends on the waste valorization techniques for food wastes. J. Environ. Manag. 2019, 233, 352–370. [Google Scholar] [CrossRef] [PubMed]




| Code | Composition of Solutions | pH of Solutions |
|---|---|---|
| DW | distilled water, control | 7.00 |
| BHT * | 1% butylated hydroxytoluene | 7.75 |
| CA * | 1.0% citric acid | 2.01 |
| AA * | 0.5% ascorbic acid | 2.66 |
| AA-CA * | 1.5% ascorbic acid + citric acid | 2.00 |
| PE ** | water solution of E10 1:1 | 6.10 |
| PE-CA ** | PE +1% citric acid | 2.18 |
| PW ** | water solution of W 1:1 | 6.10 |
| PW-CA ** | PW +1% citric acid | 2.17 |
| W | E10 | |
|---|---|---|
| Phenolic content (gallic acid mg/g dry weight) | 2.92 ± 0.41 | 3.95 ± 0.02 * |
| Antioxidant capacity (μmol TEAC/mL) | 0.17 ± 0.02 | 0.21 ± 0.01 * |
| Ferric reducing antioxidant power (μmolFe2+/mL) | 0.19 ± 0.004 | 0.28 ± 0.007 ** |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venturi, F.; Bartolini, S.; Sanmartin, C.; Orlando, M.; Taglieri, I.; Macaluso, M.; Lucchesini, M.; Trivellini, A.; Zinnai, A.; Mensuali, A. Potato Peels as a Source of Novel Green Extracts Suitable as Antioxidant Additives for Fresh-Cut Fruits. Appl. Sci. 2019, 9, 2431. https://0-doi-org.brum.beds.ac.uk/10.3390/app9122431
Venturi F, Bartolini S, Sanmartin C, Orlando M, Taglieri I, Macaluso M, Lucchesini M, Trivellini A, Zinnai A, Mensuali A. Potato Peels as a Source of Novel Green Extracts Suitable as Antioxidant Additives for Fresh-Cut Fruits. Applied Sciences. 2019; 9(12):2431. https://0-doi-org.brum.beds.ac.uk/10.3390/app9122431
Chicago/Turabian StyleVenturi, Francesca, Susanna Bartolini, Chiara Sanmartin, Matteo Orlando, Isabella Taglieri, Monica Macaluso, Mariella Lucchesini, Alice Trivellini, Angela Zinnai, and Anna Mensuali. 2019. "Potato Peels as a Source of Novel Green Extracts Suitable as Antioxidant Additives for Fresh-Cut Fruits" Applied Sciences 9, no. 12: 2431. https://0-doi-org.brum.beds.ac.uk/10.3390/app9122431