Electricity Evaluation and Emission Characteristics of Poultry Litter Co-Combustion Process
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
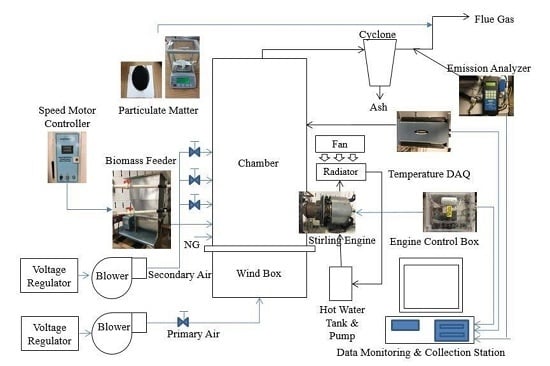
2.1. Apparatus and Experiment Setup
2.2. Materials
2.3. Experimental Conditions
3. Results and Discussion
3.1. Poultry Litter as an Energy Resource for Electrcity Production
3.2. Effect of Operating Conditions on Emissions
3.2.1. Effect of EA on Emissions
3.2.2. Effects of SA Ratio and SA Height on Emissions
3.2.3. Effect of MR on Emissions
3.3. PM Emission and Fly Ash Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lynch, D.; Henihan, A.M.; Bowen, B.; Lynch, D.; McDonnell, K.; Kwapinski, W.; Leahy, J.J. Utilisation of poultry litter as an energy feedstock. Biomass Bioenergy 2013, 49, 197–204. [Google Scholar] [CrossRef] [Green Version]
- Qian, X.; Lee, S.; Soto, A.-M.; Chen, G. Regression model to predict the higher heating value of poultry waste from proximate analysis. Resources 2018, 7, 39. [Google Scholar] [CrossRef]
- United States Department of Agriculture (USDA). National Agricultural Statistics Service Poultry Statistics Fact Sheet, Poultry Production Data. Available online: https://www.usda.gov/sites/default/files/documents/nass-poultry-stats-factsheet.pdf (accessed on 19 August 2019).
- Chastain, J.P.; Coloma-del Valle, A.; Moore, K.P. Using broiler litter as an energy source: Energy content and ash composition. Appl. Eng. Agric. 2012, 28, 513–522. [Google Scholar] [CrossRef]
- Dalólio, F.S.; da Silva, J.N.; de Oliveira, A.C.C.; Tinôco, I.D.F.F.; Barbosa, R.C.; de Oliveira Resende, M.; Albino, L.F.T.; Coelho, S.T. Poultry litter as biomass energy: A review and future perspectives. Renew. Sustain. Energy Rev. 2017, 76, 941–949. [Google Scholar] [CrossRef]
- Li, S.; Wu, A.; Deng, S.; Pan, W.P. Effect of co-combustion of chicken litter and coal on emissions in a laboratory-scale fluidized bed combustor. Fuel Process. Technol. 2008, 89, 7–12. [Google Scholar] [CrossRef]
- Kelleher, B.P.; Leahy, J.J.; Henihan, A.M.; O’dwyer, T.F.; Sutton, D.; Leahy, M.J. Advances in poultry litter disposal technology–a review. Bioresour. Technol. 2002, 83, 27–36. [Google Scholar] [CrossRef]
- Perera, P.; Bandara, W. Potential of Using Poultry Litter as a Feedstock for Energy Production; Louisiana Forest Product Development Center: Baton Rouge, LA, USA, 2010. [Google Scholar]
- Abelha, P.; Gulyurtlu, I.; Boavida, D.; Barros, J.S.; Cabrita, I.; Leahy, J.; Kelleher, B.; Leahy, M. Combustion of poultry litter in a fluidised bed combustor. Fuel 2003, 82, 687–692. [Google Scholar] [CrossRef]
- Manure-to-Energy Poultry Plant Celebrated in Maryland. Available online: https://www.farmprogress.com/manure/manure-energy-poultry-plant-celebrated-maryland (accessed on 19 August 2019).
- The Case for Built-Up Litter in US Broiler Complexes. Available online: https://poultryhealthtoday.com/case-built-litter-us-broiler-complexes/ (accessed on 19 August 2019).
- Zhang, X.; Myhrvold, N.P.; Caldeira, K. Key factors for assessing climate benefits of natural gas versus coal electricity generation. Environ. Res. Lett. 2014, 9, 114022. [Google Scholar] [CrossRef] [Green Version]
- Liang, F.Y.; Ryvak, M.; Sayeed, S.; Zhao, N. The role of natural gas as a primary fuel in the near future, including comparisons of acquisition, transmission and waste handling costs of as with competitive alternatives. Chem. Cent. J. 2012, 6, S4. [Google Scholar] [CrossRef]
- Zhu, S.; Lee, S.W. Co-combustion performance of poultry wastes and natural gas in the advanced Swirling Fluidized Bed Combustor (SFBC). Waste Manag. 2005, 25, 511–518. [Google Scholar] [CrossRef]
- Zhu, S.; Lee, S.; Hargrove, S.K.; Chen, G. Prediction of combustion efficiency of chicken litter using an artificial neural network approach. Fuel 2007, 86, 877–886. [Google Scholar] [CrossRef]
- Henihan, A.M.; Leahy, M.J.; Leahy, J.J.; Cummins, E.; Kelleher, B.P. Emissions modeling of fluidised bed co-combustion of poultry litter and peat. Bioresour. Technol. 2003, 87, 289–294. [Google Scholar] [CrossRef] [Green Version]
- Jia, L.; Anthony, E.J. Combustion of poultry-derived fuel in a coal-fired pilot-scale circulating fluidized bed combustor. Fuel Process. Technol. 2011, 92, 2138–2144. [Google Scholar] [CrossRef]
- Topal, H.; Amirabedin, E. Determination of some important emissions of poultry waste co-combustion. Sci. J. Riga Tech. Univ. Environ. Clim. Technol. 2012, 8, 12–17. [Google Scholar] [CrossRef] [Green Version]
- Armesto, L.; Bahillo, A.; Veijonen, K.; Cabanillas, A.; Otero, J. Combustion behaviour of rice husk in a bubbling fluidised bed. Biomass Bioenerg. 2002, 23, 171–179. [Google Scholar] [CrossRef]
- Varol, M.; Atimtay, A.T.; Olgun, H.; Atakül, H. Emission characteristics of co-combustion of a low calorie and high sulfur–lignite coal and woodchips in a circulating fluidized bed combustor: Part 1. Effect of excess air ratio. Fuel 2014, 117, 792–800. [Google Scholar] [CrossRef]
- Suksankraisorn, K.; Patumsawad, S.; Vallikul, P.; Fungtammasan, B.; Accary, A. Co-combustion of municipal solid waste and Thai lignite in a fluidized bed. Energ. Convers. Manag. 2004, 45, 947–962. [Google Scholar] [CrossRef]
- Chyang, C.S.; Wu, K.T.; Lin, C.S. Emission of nitrogen oxides in a vortexing fluidized bed combustor. Fuel 2007, 86, 234–243. [Google Scholar] [CrossRef]
- Duan, F.; Chyang, C.; Chin, Y.; Tso, J. Pollutant emission characteristics of rice husk combustion in a vortexing fluidized bed incinerator. J. Environ. Sci. 2013, 25, 335–339. [Google Scholar] [CrossRef]
- Eiamsa-Ard, S.; Kaewkohkiat, Y.; Thianpong, C.; Promvonge, P. Combustion behavior in a dual-staging vortex rice husk combustor with snail entry. Inter. Commun. Heat Mass 2008, 35, 1134–1140. [Google Scholar] [CrossRef]
- Madhiyanon, T.; Sathitruangsak, P.; Soponronnarit, S. Combustion characteristics of rice-husk in a short-combustion-chamber fluidized-bed combustor (SFBC). Appl. Eng. 2010, 30, 347–353. [Google Scholar] [CrossRef]
- Nussbaumer, T. Combustion and co-combustion of biomass: Fundamentals, technologies, and primary measures for emission reduction. Energ. Fuels 2003, 17, 1510–1521. [Google Scholar] [CrossRef]
- Fang, M.; Yang, L.; Chen, G.; Shi, Z.; Luo, Z.; Cen, K. Experimental study on rice husk combustion in a circulating fluidized bed. Fuel Process. Technol. 2004, 85, 1273–1282. [Google Scholar] [CrossRef]
- Kuprianov, V.I.; Kaewklum, R.; Chakritthakul, S. Effects of operating conditions and fuel properties on emission performance and combustion efficiency of a swirling fluidized-bed combustor fired with a biomass fuel. Energy 2011, 36, 2038–2048. [Google Scholar] [CrossRef]
- Piao, G.; Aono, S.; Kondoh, M.; Yamazaki, R.; Mori, S. Combustion test of refuse derived fuel in a fluidized bed. Waste Manag. 2000, 20, 443–447. [Google Scholar] [CrossRef]
- Sun, P.; Hui, S.E.; Gao, Z.; Zhou, Q.; Tan, H.; Zhao, Q.; Xu, T. Experimental investigation on the combustion and heat transfer characteristics of wide size biomass co-firing in 0.2 MW circulating fluidized bed. Appl. Eng. 2013, 52, 284–292. [Google Scholar] [CrossRef]
- Simões Amaral, S.; Andrade de Carvalho, J.; Martins Costa, M.; Pinheiro, C. Particulate matter emission factors for biomass combustion. Atmosphere 2016, 7, 141. [Google Scholar] [CrossRef]
- Billen, G.; Lassaletta, L.; Garnier, J. A vast range of opportunities for feeding the world in 2050: Trade-off between diet, N contamination and international trade. Environ. Res. Lett. 2015, 10, 025001. [Google Scholar] [CrossRef]







| Fuel Types | Major Findings | Ref. |
|---|---|---|
| Chicken litter + coal | Increasing of chicken litter mass fraction reduces SO2, but increases CO. | [6] |
| Poultry litter + peat | Secondary air (SA) in two stages reduces nitrogen oxide (NOx) (about 160–220 ppm) in freeboard and also reduces CO emission (about 1450–5820 ppm). | [9] |
| Chicken litter + peat | Concentrations (e.g., CO & volatile organic compound) decreased with the primary air (PA)/SA ratio is 0.4. SA decreases N to NOx from 30% to 15%. | [16] |
| Poultry-derived fuel (PDF) + coal | Co-combustion emitted about 20% higher NOx (in a range of 265–280 ppm) than 100% coal. | [17] |
| Poultry wastes + coal | Excess air (EA) had a remarkable effect on CO & CH4. SA reduces CO emissions, but NOx formation is still not very clear. | [18] |
| Fuel Components | As Received (wt %) | Dry Basis (wt %) |
|---|---|---|
| Proximate analysis (analysis method) | ||
| Moisture (D3302/D3173) | 21.20 | - |
| Volatile matter (D3175) | 50.40 | 63.96 |
| Fixed carbon (difference, calculated) | 9.44 | 11.98 |
| Ash (D3174) | 18.96 | 24.06 |
| Ultimate analysis (analysis method) | ||
| Carbon (C) | 26.88 | 34.11 |
| Hydrogen (H) | 4.44 | 5.64 |
| Oxygen (O) (difference, calculated) | 24.20 | 30.71 |
| Nitrogen (N) | 3.28 | 4.16 |
| Sulfur (S) | 1.04 | 1.32 |
| Heating value (Btu/lb) (D5865/5864) | 4856 (11.30 MJ/kg) | 6163 (14.34 MJ/kg) |
| Parameters | As Received (Raw) Basis (%) | Dry Basis (%) | Method Reference |
|---|---|---|---|
| Available phosphate (P2O5) | 8.8 | 9.0 | AOAC 993.31 |
| Total phosphate (P2O5) | 9.77 | 9.98 | AOAC 965.09/ICP |
| Soluble potash (K2O) | 4.5 | 4.6 | AOAC 969.04 |
| Total potash (K2O) | 5.58 | 5.70 | AOAC 965.09/ICP |
| Total nitrogen | 2 | 2 | Combustion |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qian, X.; Lee, S.; Chandrasekaran, R.; Yang, Y.; Caballes, M.; Alamu, O.; Chen, G. Electricity Evaluation and Emission Characteristics of Poultry Litter Co-Combustion Process. Appl. Sci. 2019, 9, 4116. https://0-doi-org.brum.beds.ac.uk/10.3390/app9194116
Qian X, Lee S, Chandrasekaran R, Yang Y, Caballes M, Alamu O, Chen G. Electricity Evaluation and Emission Characteristics of Poultry Litter Co-Combustion Process. Applied Sciences. 2019; 9(19):4116. https://0-doi-org.brum.beds.ac.uk/10.3390/app9194116
Chicago/Turabian StyleQian, Xuejun, Seong Lee, Raghul Chandrasekaran, Yulai Yang, Marc Caballes, Oludayo Alamu, and Guangming Chen. 2019. "Electricity Evaluation and Emission Characteristics of Poultry Litter Co-Combustion Process" Applied Sciences 9, no. 19: 4116. https://0-doi-org.brum.beds.ac.uk/10.3390/app9194116