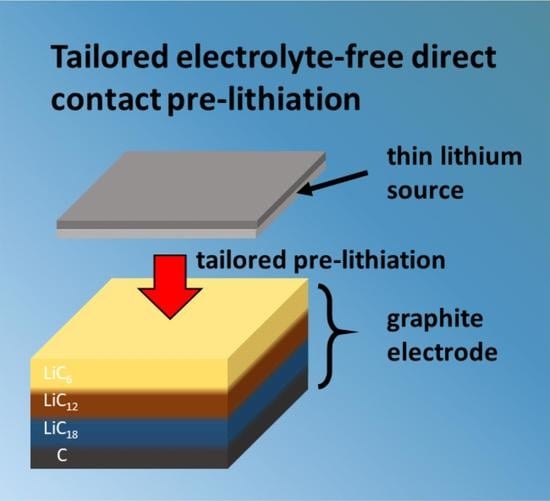
Tailored Pre-Lithiation Using Melt-Deposited Lithium Thin Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Lithium Melt Deposition
2.2. Electrolyte-Free Direct Contact Pre-Lithiation
2.3. Electrochemical Characterization
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Placke, T.; Kloepsch, R.; Dühnen, S.; Winter, M. Lithium ion, lithium metal, and alternative rechargeable battery technologies: The odyssey for high energy density. J. Solid State Electrochem. 2017, 21, 1939–1964. [Google Scholar] [CrossRef]
- Cheng, X.-B.; Zhang, R.; Zhao, C.-Z.; Zhang, Q. Toward Safe Lithium Metal Anode in Rechargeable Batteries: A Review. Chem. Rev. 2017, 117, 10403–10473. [Google Scholar] [CrossRef] [PubMed]
- An, S.J.; Li, J.; Daniel, C.; Mohanty, D.; Nagpure, S.; Wood, D.L., III. The state of understanding of the lithium-ion-battery graphite solid electrolyte interphase (SEI) and its relationship to formation cycling. Carbon 2016, 105, 52–76. [Google Scholar] [CrossRef] [Green Version]
- Patil, A.; Patil, V.; Shin, D.W.; Choi, J.W.; Paik, D.S.; Yoon, S.J. Issue and challenges facing rechargeable thin film lithium batteries. Mater. Res. Bull. 2008, 43, 1913–1942. [Google Scholar] [CrossRef]
- Ashuri, M.; He, Q.; Shaw, L.L. Silicon as a potential anode material for Li-ion batteries: Where size, geometry and structure matter. Nanoscale 2016, 8, 74–103. [Google Scholar] [CrossRef]
- Zhao, J.; Lu, Z.; Wang, H.; Liu, W.; Lee, H.W.; Yan, K.; Zhuo, D.; Lin, D.; Liu, N.; Cui, Y. Artificial solid electrolyte interphase-protected Li x Si nanoparticles: An efficient and stable prelithiation reagent for lithium-ion batteries. J. Am. Chem. Soc. 2015, 137, 8372–8375. [Google Scholar] [CrossRef]
- ADAC. Tesla Model X 100D (01/17–03/19): Technische Daten, Bilder, Preise|ADAC 2021. Available online: https://www.adac.de/rund-ums-fahrzeug/autokatalog/marken-modelle/tesla/model-x/1generation/268176/ (accessed on 22 June 2021).
- Zhang, S.; Andreas, N.S.; Li, R.; Zhang, N.; Sun, C.; Lu, D.; Gao, T.; Chen, L.; Fan, X. Mitigating irreversible capacity loss for higher-energy lithium batteries. Energy Storage Mater. 2022, 48, 44–73. [Google Scholar] [CrossRef]
- Abouimrane, A.; Cui, Y.; Chen, Z.; Belharouak, I.; Yahia, H.B.; Wu, H.; Assary, R.; Curtiss, L.A.; Amine, K. Enabling high energy density Li-ion batteries through Li2O activation. Nano Energy 2016, 27, 196–201. [Google Scholar] [CrossRef] [Green Version]
- Bie, Y.; Yang, J.; Wang, J.; Zhou, J.; Nuli, Y. Li2O2 as a cathode additive for the initial anode irreversibility compensation in lithium-ion batteries. Chem. Commun. 2017, 53, 8324–8327. [Google Scholar] [CrossRef]
- Kim, J.; Kang, H.; Hwang, K.; Yoon, S. Thermal decomposition study on Li2O2 for Li2NiO2 synthesis as a sacrificing positive additive of lithium-ion batteries. Molecules 2019, 24, 4624. [Google Scholar] [CrossRef]
- Jote, B.A.; Shitaw, K.N.; Weret, M.A.; Yang, S.C.; Huang, C.J.; Wang, C.H.; Weng, Y.T.; Wu, S.H.; Su, W.N.; Hwang, B.J. Lithium nitrate as a surplus lithium source for anode-free cell with Ni-rich (NMC811) cathode. J. Power Sources 2022, 532, 231303. [Google Scholar] [CrossRef]
- Park, H.; Yoon, T.; Kim, Y.U.; Ryu, J.H.; Oh, S.M. Li2NiO2 as a sacrificing positive additive for lithium-ion batteries. Electrochim. Acta 2013, 108, 591–595. [Google Scholar] [CrossRef]
- Shanmukaraj, D.; Grugeon, S.; Laruelle, S.; Douglade, G.; Tarascon, J.M.; Armand, M. Sacrificial salts: Compensating the initial charge irreversibility in lithium batteries. Electrochem. Commun. 2010, 12, 1344–1347. [Google Scholar] [CrossRef]
- Aravindan, V.; Arun, N.; Shubha, N.; Sundaramurthy, J.; Madhavi, S. Overlithiated Li1+xNi0.5Mn1.5O4 in all one dimensional architecture with conversion type α-Fe2O3: A new approach to eliminate irreversible capacity loss. Electrochim. Acta 2016, 215, 647–651. [Google Scholar] [CrossRef]
- Aravindan, V.; Nan, S.; Keppeler, M.; Madhavi, S. Pre-lithiated LixMn2O4: A new approach to mitigate the irreversible capacity loss in negative electrodes for Li-ion battery. Electrochim. Acta 2016, 208, 225–230. [Google Scholar] [CrossRef]
- Gabrielli, G.; Marinaro, M.; Mancini, M.; Axmann, P.; Wohlfahrt-Mehrens, M. A new approach for compensating the irreversible capacity loss of high-energy Si/C|LiNi0.5Mn1.5O4 lithium-ion batteries. J. Power Sources 2017, 351, 35–44. [Google Scholar] [CrossRef]
- Kasnatscheew, J.; Evertz, M.; Streipert, B.; Wagner, R.; Klöpsch, R.; Vortmann, B.; Hahn, H.; Nowak, S.; Amereller, M.; Gentschev, A.C.; et al. The truth about the 1st cycle Coulombic efficiency of LiNi1/3Co1/3Mn1/3O2 (NCM) cathodes. Phys. Chem. Chem. Phys. PCCP 2016, 18, 3956–3965. [Google Scholar] [CrossRef]
- Kasnatscheew, J.; Evertz, M.; Streipert, B.; Wagner, R.; Nowak, S.; Laskovic, I.C.; Winter, M. Improving cycle life of layered lithium transition metal oxide (LiMO2) based positive electrodes for Li ion batteries by smart selection of the electrochemical charge conditions. J. Power Sources 2017, 359, 458–467. [Google Scholar] [CrossRef]
- Peramunage, D.; Abraham, K.M. Preparation and electrochemical characterization of overlithiated spinel LiMn2O4. J. Electrochem. Soc. 1998, 145, 1131. [Google Scholar] [CrossRef]
- Tarascon, J.M.; Guyomard, D. Li Metal-Free Rechargeable Batteries Based on Li1+xMn2O4 Cathodes (0 ≤ x ≤ 1) and Carbon Anodes. J. Electrochem. Soc. 1991, 138, 2864. [Google Scholar] [CrossRef]
- Holtstiege, F.; Bärmann, P.; Nölle, R.; Winter, M.; Placke, T. Pre-lithiation strategies for rechargeable energy storage technologies: Concepts, promises and challenges. Batteries 2018, 4, 4. [Google Scholar] [CrossRef] [Green Version]
- Jin, L.; Shen, C.; Shellikeri, A.; Wu, Q.; Zheng, J.; Andrei, P.; Zhang, J.G.; Zheng, J.P. Progress and perspectives on pre-lithiation technologies for lithium ion capacitors. Energy Environ. Sci. 2020, 13, 2341–2362. [Google Scholar] [CrossRef]
- Scott, M.G.; Whitehead, A.H.; Owen, J.R. Chemical formation of a solid electrolyte interface on the carbon electrode of a Li-ion cell. J. Electrochem. Soc. 1998, 145, 1506. [Google Scholar] [CrossRef]
- Tabuchi, T.; Yasuda, H.; Yamachi, M. Li-doping process for LixSiO-negative active material synthesized by chemical method for lithium-ion cells. J. Power Sources 2005, 146, 507–509. [Google Scholar] [CrossRef]
- Tabuchi, T.; Yasuda, H.; Yamachi, M. Mechanism of Li-doping into Li4Ti5O12 negative active material for Li-ion cells by new chemical method. J. Power Sources 2006, 162, 813–817. [Google Scholar] [CrossRef]
- Takezawa, H.; Ito, S.; Yoshizawa, H.; Abe, T. Electrochemical Properties of a SiOx Film Anode Pre-lithiated by Evaporation of Metallic Li in Li-ion Batteries. Chem. Lett. 2017, 46, 1365–1367. [Google Scholar] [CrossRef]
- Adhitama, E.; Dias Brandao, F.; Dienwiebel, I.; Bela, M.M.; Javed, A.; Haneke, L.; Stan, M.C.; Winter, M.; Gomez-Martin, A.; Placke, T. Pre-Lithiation of Silicon Anodes by Thermal Evaporation of Lithium for Boosting the Energy Density of Lithium Ion Cells. Adv. Funct. Mater. 2022, 32, 2201455. [Google Scholar] [CrossRef]
- Kim, H.J.; Choi, S.; Lee, S.J.; Seo, M.W.; Lee, J.G.; Deniz, E.; Lee, Y.J.; Kim, E.K.; Choi, J.W. Controlled prelithiation of silicon monoxide for high performance lithium-ion rechargeable full cells. Nano Lett. 2016, 16, 282–288. [Google Scholar] [CrossRef]
- de la Llave, E.; Borgel, V.; Park, K.J.; Hwang, J.Y.; Sun, Y.K.; Hartmann, P.; Chesneau, F.F.; Aurbach, D. Comparison between Na-ion and Li-ion cells: Understanding the critical role of the cathodes stability and the anodes pretreatment on the cells behavior. ACS Appl. Mater. Interfaces 2016, 8, 1867–1875. [Google Scholar] [CrossRef]
- Nayak, P.K.; Penki, T.R.; Markovsky, B.; Aurbach, D. Electrochemical performance of Li-and Mn-rich cathodes in full cells with prelithiated graphite negative electrodes. ACS Energy Lett. 2017, 2, 544–548. [Google Scholar] [CrossRef]
- Sun, Y.; Tang, J.; Qin, F.; Yuan, J.; Zhang, K.; Li, J.; Zhu, D.M.; Qin, L.C. Hybrid lithium-ion capacitors with asymmetric graphene electrodes. J. Mater. Chem. A 2017, 5, 13601–13609. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, Z.; Lu, P.; Jiang, M.; Shi, F.; Song, X.; Zheng, Z.; Zhou, X.; Fu, Y.; Abdelbast, G.; et al. Toward practical application of functional conductive polymer binder for a high-energy lithium-ion battery design. Nano Lett. 2014, 14, 6704–6710. [Google Scholar] [CrossRef] [Green Version]
- Forney, M.W.; Ganter, M.J.; Staub, J.W.; Ridgley, R.D.; Landi, B.J. Prelithiation of silicon–carbon nanotube anodes for lithium ion batteries by stabilized lithium metal powder (SLMP). Nano Lett. 2013, 13, 4158–4163. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Fu, Y.; Zhang, Z.; Yuan, S.; Amine, K.; Battaglia, V.; Liu, G. Application of stabilized lithium metal powder (SLMP®) in graphite anode—A high efficient prelithiation method for lithium-ion batteries. J. Power Sources 2014, 260, 57–61. [Google Scholar] [CrossRef] [Green Version]
- Seong, I.W.; Kim, K.T.; Yoon, W.Y. Electrochemical behavior of a lithium-pre-doped carbon-coated silicon monoxide anode cell. J. Power Sources 2009, 189, 511–514. [Google Scholar] [CrossRef]
- Pan, Q.; Zuo, P.; Mu, T.; Du, C.; Cheng, X.; Ma, Y.; Gao, Y.; Yin, G. Improved electrochemical performance of micro-sized SiO-based composite anode by prelithiation of stabilized lithium metal powder. J. Power Sources 2017, 347, 170–177. [Google Scholar] [CrossRef]
- Li, Y.; Fitch, B. Effective enhancement of lithium-ion battery performance using SLMP. Electrochem. Commun. 2011, 13, 664–667. [Google Scholar] [CrossRef]
- Tahir, M.S.; Weinberger, M.; Balasubramanian, P.; Diemant, T.; Behm, R.J.; Lindén, M.; Wohlfahrt-Mehrens, M. Silicon carboxylate derived silicon oxycarbides as anodes for lithium ion batteries. J. Mater. Chem. A 2017, 5, 10190–10199. [Google Scholar] [CrossRef]
- Jarvis, C.R.; Lain, M.J.; Yakovleva, M.V.; Gao, Y. A prelithiated carbon anode for lithium-ion battery applications. J. Power Sources 2006, 162, 800–802. [Google Scholar] [CrossRef]
- Wang, L.; Fu, Y.; Battaglia, V.S.; Liu, G. SBR–PVDF based binder for the application of SLMP in graphite anodes. RSC Adv. 2013, 3, 15022–15027. [Google Scholar] [CrossRef]
- Ai, G.; Wang, Z.; Zhao, H.; Mao, W.; Fu, Y.; Yi, R.; Gao, Y.; Battaglia, V.; Wang, D.; Lopatin, S.; et al. Scalable process for application of stabilized lithium metal powder in Li-ion batteries. J. Power Sources 2016, 309, 33–41. [Google Scholar] [CrossRef] [Green Version]
- Mazouzi, D.; Karkar, Z.; Hernandez, C.R.; Manero, P.J.; Guyomard, D.; Roué, L.; Lestriez, B. Critical roles of binders and formulation at multiscales of silicon-based composite electrodes. J. Power Sources 2015, 280, 533–549. [Google Scholar] [CrossRef]
- Shellikeri, A.; Watson, V.G.; Adams, D.L.; Kalu, E.E.; Read, J.A.; Jow, T.R.; Zheng, J.P. Pre-lithiation of carbon anodes using different lithium-sources. ECS Trans. 2017, 77, 293. [Google Scholar] [CrossRef]
- Liu, N.; Hu, L.; McDowell, M.T.; Jackson, A.; Cui, Y. Prelithiated silicon nanowires as an anode for lithium ion batteries. ACS Nano 2011, 5, 6487–6493. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; He, X.; Ren, J.; Li, J.; Jiang, C.; Wan, C. Hard carbon/lithium composite anode materials for Li-ion batteries. Electrochim. Acta 2007, 52, 4312–4316. [Google Scholar] [CrossRef]
- Fei, L.; Yoo, S.H.; Villamayor, R.A.R.; Williams, B.P.; Gong, S.Y.; Park, S.; Shin, K.; Joo, Y.L. Graphene oxide involved air-controlled electrospray for uniform, fast, instantly dry, and binder-free electrode fabrication. Appl. Mater. Interfaces 2017, 9, 9738–9746. [Google Scholar] [CrossRef]
- Wang, Y.; Xing, G.; Han, Z.J.; Shi, Y.; Wong, J.I.; Huang, Z.X.; Ostrikov, K.K.; Yang, H.Y. Pre-lithiation of onion-like carbon/MoS 2 nano-urchin anodes for high-performance rechargeable lithium ion batteries. Nanoscale 2014, 6, 8884–8890. [Google Scholar] [CrossRef]
- Stumper, B.; Mayr, A.; Reinhart, G. Application of Thin Lithium Foil for Direct Contact Prelithiation of Anodes within Lithium-Ion Battery Production. Procedia CIRP 2020, 93, 156–161. [Google Scholar] [CrossRef]
- Laliberté, R.; Sirois, P.; Gagnon, R.; Bathium Canada Inc. Lamination Process and Apparatus for Alkali Metals or Alloys Thereof. U.S. Patent 7,513,136, 7 April 2009. [Google Scholar]
- Vanleeuw, D.; Sapundjiev, D.; Sibbens, G.; Oberstedt, S.; Salvador Castiñeira, P. Physical vapour deposition of metallic lithium. J. Radioanal. Nucl. Chem. 2014, 299, 1113–1120. [Google Scholar] [CrossRef]
- Schönherr, K.; Schumm, B.; Hippauf, F.; Lissy, R.; Althues, H.; Leyens, C.; Kaskel, S. Liquid lithium metal processing into ultrathin metal anodes for solid state batteries. Chem. Eng. J. Adv. 2022, 9, 100218. [Google Scholar] [CrossRef]
- Kaskel, S.; Althues, H.; Schumm, B.; Dresel, N.; Schoenherr, K. Method for Producing a Substrate, Which Is Coated with an Alkali Metal by Means of a Promoter Layer, and a Coated Substrate. U.S. Patent 16/613,134, 26 March 2020. [Google Scholar]
- Holleman, A.F.; Wiberg, E.; Wiberg, N. Lehrbuch der Anorganischen Chemie; De Gruyter: Berlin, Germany, 2007. [Google Scholar]
- Krat, S.A.; Popkov, A.S.; Gasparyan, Y.M.; Pisarev, A.A.; Fiflis, P.; Szott, M.; Christenson, M.; Kalathiparambil, K.; Ruzic, D.N. Wetting properties of liquid lithium on lithium compounds. Fusion Eng. Des. 2017, 117, 199–203. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Zhang, Z.; Lan, M.; Yang, S.; Cheng, J.; Cai, J.; Shen, J.; Zhu, Y.; Zhang, K.; Zhang, W. Lithiophilic Cu-CuO-Ni hybrid structure: Advanced current collectors toward stable lithium metal anodes. Adv. Mater. 2018, 30, 1705830. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Lin, D.; Zhao, J.; Lu, Z.; Liu, Y.; Liu, C.; Lu, Y.; Wang, H.; Yan, K.; Tao, X.; et al. Composite lithium metal anode by melt infusion of lithium into a 3D conducting scaffold with lithiophilic coating. Proc. Natl. Acad. Sci. USA 2016, 113, 2862–2867. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wang, H.; Xie, J.; Yang, A.; Pei, A.; Wu, C.L.; Shi, F.; Liu, Y.; Lin, D.; Gong, Y.; et al. Fundamental study on the wetting property of liquid lithium. Energy Stor. Mater. 2018, 14, 345–350. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, W.; Ming, H.; Cao, G.; Fan, L.Z.; Zhang, H. Chemical energy release driven lithiophilic layer on 1 m2 commercial brass mesh toward highly stable lithium metal batteries. Nano Lett. 2019, 19, 1832–1837. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.H.; Yue, J.; Dong, W.; Zuo, T.T.; Li, J.Y.; Liu, X.; Zhang, X.D.; Liu, L.; Shi, J.L.; Yin, Y.X.; et al. Tuning wettability of molten lithium via a chemical strategy for lithium metal anodes. Nat. Commun. 2019, 10, 4930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stuth, T. Verfahren zur Herstellung von Nickelband. German Patent DE102010010536, 3 May 2010. [Google Scholar]
- Kaskel, S.; Althues, H.; Schumm, B.; Abendroth, T.; Schönherr, K. Methods for the Lithiation of Electrodes of Lithium-Based Electrical Energy Storage Elements and Electrical Energy Storage Element Produced by the Method. European Patent EP3758105A1, 16 June 2020. [Google Scholar]
- Shellikeri, A.; Watson, V.; Adams, D.; Kalu, E.E.; Read, J.A.; Jow, T.R.; Zheng, J.S.; Zheng, J.P. Investigation of pre-lithiation in graphite and hard-carbon anodes using different lithium source structures. J. Electrochem. Soc. 2017, 164, A3914–A3924. [Google Scholar] [CrossRef]
- Jost, W. Diffusion in Solids, Liquids, Gases; Academic Press: New York, NY, USA, 1969. [Google Scholar]



| Web Speed [mm min−1] | Bath Temperature [°C] | Lithium Loading [mg cm−2] |
|---|---|---|
| 50 | 210 | 0.087 |
| 100 | 210 | 0.201 |
| 200 | 210 | 0.369 |
| 320 | 210 | 0.657 |
| Experiments | Pressure [MPa] | Temperature [°C] | Time [min] |
|---|---|---|---|
| Pressure variation | 5–40 | 150 | 15 |
| Temperature variation 1 | 20 | 20–150 | 15 |
| Temperature variation 2 | 40 | 20–180 | 15 |
| Time variation | 20 | 150 | 0–120 |
| Desired Degree of Pre-Lithiation [%] | Required Lithium [mg cm−2] | Electrochemically Useable Lithium [mg cm−2] | Achieved Degree of Pre-Lithiation [%] |
|---|---|---|---|
| 7 | 0.063 | 0.067 | 7.5 |
| 15 | 0.135 | 0.104 | 11.6 |
| 30 | 0.270 | 0.267 | 29.9 |
| 50 | 0.450 | 0.393 | 44.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schönherr, K.; Pöthe, M.; Schumm, B.; Althues, H.; Leyens, C.; Kaskel, S. Tailored Pre-Lithiation Using Melt-Deposited Lithium Thin Films. Batteries 2023, 9, 53. https://0-doi-org.brum.beds.ac.uk/10.3390/batteries9010053
Schönherr K, Pöthe M, Schumm B, Althues H, Leyens C, Kaskel S. Tailored Pre-Lithiation Using Melt-Deposited Lithium Thin Films. Batteries. 2023; 9(1):53. https://0-doi-org.brum.beds.ac.uk/10.3390/batteries9010053
Chicago/Turabian StyleSchönherr, Kay, Markus Pöthe, Benjamin Schumm, Holger Althues, Christoph Leyens, and Stefan Kaskel. 2023. "Tailored Pre-Lithiation Using Melt-Deposited Lithium Thin Films" Batteries 9, no. 1: 53. https://0-doi-org.brum.beds.ac.uk/10.3390/batteries9010053