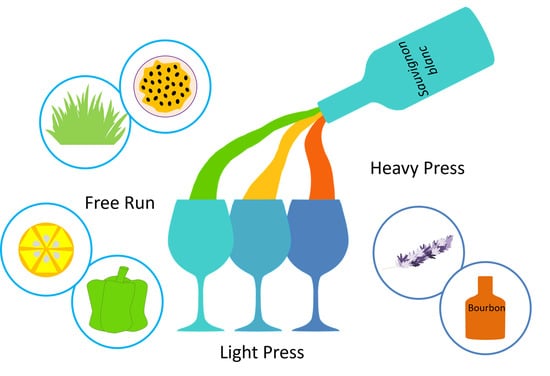
Aroma and Sensory Profiles of Sauvignon Blanc Wines from Commercially Produced Free Run and Pressed Juices
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Grape Processing and Winemaking
2.3. Analysis of Polyfucntional Mercaptans
2.4. Methoxypyrazine Analysis
2.5. Analysis of Other Volatiles
2.6. Sensory Analysis
2.7. Data Analysis
3. Results and Discussion
3.1. Post Pressing Juice Analysis
3.2. Basic Wine Parameters
3.3. Wine Aroma Analysis
3.4. Sensory Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tominaga, T.; Furrer, A.; Henry, R.; Dubourdieu, D. Identification of new volatile thiols in the aroma of Vitis. vinifera L. var. Sauvignon blanc wines. Flavour Fragr. J. 1998, 13, 159–162. [Google Scholar] [CrossRef]
- Allen, T.; Herbst-Johnstone, M.; Girault, M.; Butler, P.; Logan, G.; Jouanneau, S.; Nicolau, L.; Kilmartin, P. Influence of grape-harvesting steps on varietal thiol aromas in Sauvignon blanc wines. J. Agric. Food Chem. 2011, 59, 10641–10650. [Google Scholar] [CrossRef] [PubMed]
- Benkwitz, F.; Tominaga, T.; Kilmartin, P.A.; Lund, C.; Wohlers, M.; Nicolau, L. Identifying the chemical composition related to the distinct aroma characteristics of New Zealand Sauvignon blanc wines. Am. J. Enol. Vitic. 2012, 63, 62–72. [Google Scholar] [CrossRef]
- Patel, P.; Herbst-Johnstone, M.; Lee, S.; Gardner, R.; Weaver, R.; Nicolau, L.; Kilmartin, P. Influence of juice pressing conditions on polyphenols, antioxidants, and varietal aroma of Sauvignon blanc microferments. J. Agric. Food Chem. 2010, 58, 7280–7288. [Google Scholar] [CrossRef]
- Nikolantonaki, M.; Thibon, C.; Shinoda, K.; Teissedre, P.L.; Darriet, P. Levels and influence of flavan-3-ol must content on varietal aroma of young Sauvignon blanc wines: Effects of pressing conditions, grape origin and vintage. In Proceedings of the International Symposium of Oenology (OENO), Bordeaux, France, 15–17 June 2011. [Google Scholar]
- Roland, A.; Schneider, R.; Charrier, F.; Cavelier, F.; Rossignol, M.; Razungles, A. Distribution of varietal thiol precursors in the skin and the pulp of Melon B. and Sauvignon blanc grapes. Food Chem. 2011, 125, 139–144. [Google Scholar] [CrossRef]
- Makhotkina, O.; Herbst-Johnstone, M.; Logan, G.; du Toit, W.; Kilmartin, P.A. Influence of sulfur dioxide additions at harvest on polyphenols, C6-compounds, and varietal thiols in Sauvignon blanc. Am. J. Enol. Vitic. 2013, 64, 203–213. [Google Scholar] [CrossRef]
- Makhotkina, O.; Araujo, L.D.; Olejar, K.; Herbst-Johnstone, M.; Fedrizzi, B.; Kilmartin, P.A. Aroma impact of ascorbic acid and glutathione additions to sauvignon Blanc at harvest to supplement sulfur dioxide. Am. J. Enol. Vitic. 2014, 65, 388–393. [Google Scholar] [CrossRef]
- Coetzee, C.; Lisjak, K.; Nicolau, L.; Kilmartin, P.; du Toit, W.J. Oxygen and sulfur dioxide additions to Sauvignon blanc must: Effect on must and wine composition. Flavour Fragr. J. 2013, 28, 155–167. [Google Scholar] [CrossRef]
- Lyu, X.; Dias Araujo, L.; Quek, S.-Y.; Kilmartin, P.A. Effects of antioxidant and elemental sulfur additions at crushing on aroma profiles of Pinot Gris, Chardonnay and Sauvignon Blanc wines. Food Chem. 2021, 346, 128914. [Google Scholar] [CrossRef]
- Escudero, A.; Campo, E.; Ugliano, M. Analytical characterisation of the aroma of five premium red wines. Insights into the role of odor families and the concept of fruitiness of wines. J. Agric. Food Chem. 2007, 55, 4501–4510. [Google Scholar] [CrossRef]
- Moyano, L.; Zea, L.; Moreno, J.; Medina, M. Analytical study of aromatic series in sherry wines subjected to biological aging. J. Agric. Food Chem. 2002, 50, 7356–7361. [Google Scholar] [CrossRef] [PubMed]
- Selli, S.; Bagatar, B.; Sen, K.; Kelebek, H. Evaluation of differences in the aroma composition of free-run and pressed neutral grape juices obtained from emir (Vitis vinifera L.). Chem. Biodivers. 2011, 8, 1776–1782. [Google Scholar] [CrossRef] [PubMed]
- Augustyn, O.; Rapp, A.; Wyk, C. Some volatile aroma components of Vitis vinifera L. cv. Sauvignon blanc. S. Afr. J. Enol. Vitic. 1982, 3, 53–60. [Google Scholar] [CrossRef] [Green Version]
- Sefton, M.; Francis, I.; Williams, P. Free and bound volatile secondary metabolites of Vitis vinifera grape cv. Sauvignon blanc. J. Food Sci. 1994, 59, 142–147. [Google Scholar] [CrossRef]
- Marais, J.; van Wyk, C. Effect of grape maturity and juice temperature on terpene concentrations and wine quality of Vitis vinifera L. cv. Weisser, Riesling and Bukettraube. S. Afr. J. Enol. Vitic. 1986, 7, 26–35. [Google Scholar]
- Louw, L.; Tredoux, A.; Van Rensberg, P.; Kidd, M.; Naes, T.; Nieuwoudt, H. Fermentation-derived aroma compounds in varietal young wines from South Africa. S. Afr. J. Enol. Vitic. 2010, 31, 213–225. [Google Scholar] [CrossRef] [Green Version]
- Boulton, B.; Singleton, V.; Bisson, L.; Kunkee, R. Principals and Practices of Winemaking; Chapman & Hall: New York, NY, USA, 1999. [Google Scholar]
- Hebditch, K.R.; Nicolau, L.; Brimble, M.A. Synthesis of isotopically labelled thiol volatiles and cysteine conjugates for quantification of Sauvignon Blanc wine. J. Label. Compd. Radiopharm. 2007, 50, 237–243. [Google Scholar] [CrossRef]
- Maggu, M.; Winz, R.; Kilmartin, P.; Trought, M.; Nicolau, L. Effect of skin contact and pressure on the composition of Sauvignon blanc must. J. Agric. Food Chem. 2007, 55, 10281–10288. [Google Scholar] [CrossRef]
- Parr, W.V.; Green, J.A.; White, K.G.; Sherlock, R.R. The distinctive flavour of New Zealand Sauvignon blanc: Sensory characterisation by wine professionals. Food Qual. Prefer. 2007, 18, 849–861. [Google Scholar] [CrossRef]
- Parish, K.J.; Herbst-Johnstone, M.; Bouda, F.; Klaere, S.; Fedrizzi, B. Pre-fermentation fining effects on the aroma chemistry of Marlborough Sauvignon blanc press fractions. Food Chem. 2016, 208, 326–335. [Google Scholar] [CrossRef]
- Pinu, F.R.; Edwards, P.J.B.; Jouanneau, S.; Kilmartin, P.A.; Gardner, R.C.; Villas-Boas, S.G. Sauvignon blanc metabolomics: Grape juice metabolites affecting the development of varietal thiols and other aroma compounds in wines. Metabolomics 2014, 10, 556–573. [Google Scholar] [CrossRef]
- Lund, C.M.; Thompson, M.K.; Benkwitz, F.; Wohler, M.W.; Triggs, C.M.; Gardner, R.; Heymann, H.; Nicolau, L. New Zealand Sauvignon blanc distinct flavor characteristics: Sensory, chemical, and consumer Aspects. Am. J. Enol. Vitic. 2009, 60, 1–12. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2014. [Google Scholar]
- Oksanen, J.; Guillaume Blanchet, F.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; Henry, M.; Stevens, H.; et al. Vegan: Community Ecology Package; R package version 2.0-10. 2013. Available online: https://www.researchgate.net/publication/258996451_Vegan_Community_Ecology_Package_R_Package_Version_20-10 (accessed on 10 January 2021).
- Jolliffe, I. Principal Component Analysis; Springer: New York, NY, USA, 1986. [Google Scholar]
- Mardia, K.V.; Kent, J.T.; Bibby, J. Multivariate Analysis; Academic Press: London, UK, 1979. [Google Scholar]
- Zoecklein, B.W.; Fugelsang, K.C.; Gump, B.H.; Nury, F.S. Wine Analysis and Production; Aspen Publishers Inc.: Gaithersburg, MD, USA, 1999. [Google Scholar]
- Jouanneau, S.; Weaver, R.; Nicolau, L.; Herbst-Johnstone, M.; Benkwitz, F.; Kilmartin, P. Subregional survey of aroma compounds in Marlborough Sauvignon blanc wines. Aust. J. Grape Wine Res. 2012, 18, 329–343. [Google Scholar] [CrossRef]
- Marais, J. Sauvignon blanc cultivar aroma—A review. S. Afr. J. Enol. Vitic. 1994, 15, 41–45. [Google Scholar] [CrossRef] [Green Version]
- Parish, K.J.; Herbst-Johnstone, M.; Bouda, F.; Klaere, S.; Fedrizzi, B. Sauvignon Blanc aroma and sensory profile modulation from high fining rates. Aust. J. Grape Wine Res. 2017, 23, 359–367. [Google Scholar] [CrossRef]
- Tarko, T.; Duda-Chodak, A.; Sroka, P.; Siuta, M. The Impact of Oxygen at Various Stages of Vinification on the Chemical Composition and the Antioxidant and Sensory Properties of White and Red Wines. Int. J. Food Sci. 2020, 2020, 7902974. [Google Scholar] [CrossRef] [Green Version]
- Edwards, C.G.; Beelman, R.B.; Bartley, C.E.; McConnell, A.L. Production of Decanoic Acid and Other Volatile Compounds and the Growth of Yeast and Malolactic Bacteria During Vinification. Am. J. Enol. Vitic. 1990, 41, 48–56. [Google Scholar]
- Liu, P.T.; Yu, K.J.; Li, Y.T.; Duan, C.Q.; Yan, G.L. The content of linoleic acid in grape must influences the aromatic effect of branched-chain amino acids addition on red wine. Food Res. Int. 2018, 114, 214–222. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. (Eds.) Handbook of Enology; John Wiley & Sons, LTD: West Sussex, UK, 2001; Volume 2. [Google Scholar]
- Chatonnet, P.; Dubourdie, D.; Boidron, J.-n.; Pons, M. The origin of ethylphenols in wines. J. Sci. Food Agric. 1992, 60, 165–178. [Google Scholar] [CrossRef]
- Pineau, B.; Barbe, J.; van Leeuwen, C.; Dubourdieu, D. Which impact for beta-damascenone on red wine aromas? J. Agric. Food Chem. 2007, 55, 4103–4108. [Google Scholar] [CrossRef]
- Antalick, G.; Perello, M.-C.; de Revel, G. Development, validation and application of a specific method for the quantitative determination of wine esters by headspace-solid-phase microextraction-gas chromatography–mass spectrometry. Food Chem. 2010, 121, 1236–1245. [Google Scholar] [CrossRef]
- Campo, E.; Ferreira, V. Prediction of the wine sensory properties related to grape variety form dynaimc-headspace gas chromatography-olfactometry data. J. Agric. Food Chem. 2005, 53, 5682–5690. [Google Scholar] [CrossRef] [PubMed]
- Benkwitz, F.; Nicolau, L.; Lund, C.; Beresford, M.; Wohlers, M.; Kilmartin, P.A. Evaluation of key odorants in sauvignon blanc wines using three different methodologies. J. Agric. Food Chem. 2012, 60, 6293–6302. [Google Scholar] [CrossRef] [PubMed]


| Wairau Valley (A) | Awatere Valley (B) | |||||
|---|---|---|---|---|---|---|
| FR | LP | HP | FR | LP | HP | |
| Juice Temperature (°C) | 7.4 | 10.3 | 11.4 | 20.9 | 20.3 | 20.6 |
| Soluble Solids (°Brix) | 21.5 | 22.0 | 21.6 | 23.0 | 23.0 | 23.1 |
| pH | 3.19 | 3.79 | 4.05 | 3.37 | 3.69 | 3.89 |
| Total Acidity (g tartaric acid/L) | 10.4 | 7.5 | 8.2 | 12.1 | 9.5 | 9.4 |
| Free SO2 (mg/L) | 21 | 1 | 0 | 14 | 1 | 0 |
| Total SO2 (mg/L) | 42 | 8 | 7 | 41 | 8 | 10 |
| Solids (%) | 5.0 | 0.7 | 0.2 | 5.0 | 3.5 | 0.8 |
| Wairau Valley (A) | Awatere Valley (B) | ||||||
|---|---|---|---|---|---|---|---|
| Compound | Concentration Units | FR | LP | HP | FR | LP | HP |
| IBMP | (ng/L) | 4.9 ± 0.1 a | 4.7 ± 0.2 a | 5.1 ± 0.3 a | 6.2 ± 0.2 a | 3.7 ± 0.2 b | 4.0 ± 0.3 b |
| 3MH | (ng/L) | 971 ± 55 a | 128 ± 0.4 b | 123 ± 11 b | 2368 ± 115 a | 246 ± 12 b | 162 ± 5.3 b |
| 3MHA | (ng/L) | 290 ± 47 a | 149 ± 41 b | 188 ± 46 a,b | 705 ± 87 a | 136 ± 26 b | 93 ± 8 b |
| cis/trans-Rose oxide | (μg/L) | 0.247 ± 0.010 a | 0.394 ± 0.009 b | 0.597 ± 0.019 c | 0.296 ± 0.005 a | 0.532 ± 0.014 b | 0.809 ± 0.015 c |
| Linalool | (μg/L) | 4.1 ± 0.1 a | 4.0 ± 0.2 a | 4.4 ± 0.2 a | 6.9 ± 0.4 a | 7.9 ± 0.3 b | 9.5 ± 0.3 c |
| α-Terpineol | (μg/L) | 4.4 ± 0.5 a | 2.6 ± 0.3 b | 5.7 ± 0.2 c | 4.3 ± 0.1 a | 3.3 ± 0.1 b | 6.6 ± 0.2 c |
| β-Citronellol | (μg/L) | 5.3 ± 0.4 a | 6.7 ± 0.2 b | 8.7 ± 0.3 c | 5.8 ± 0.1 a | 7.6 ± 0.2 b | 10.9 ± 0.3 c |
| Nerol | (μg/L) | 3.4 ± 0.2 a | 3.9 ± 0.2 a | 6.4 ± 0.2 b | 4.2 ± 0.2 a | 4.1 ± 0.2 a | 6.9 ± 0.2 b |
| β-Damascenone | (μg/L) | 4.4 ± 0.4 a | 2.4 ± 0.1 b | 2.1 ± 0.1 b | 3.0 ± 0.1 a | 1.7 ± 0.1 b | 1.4 ± 0.1 c |
| β-Ionone | (μg/L) | 0.281 ± 0.002 a | 0.242 ± 0.002 b | 0.218 ± 0.005 c | 0.301 ± 0.009 a | 0.241 ± 0.010 b | 0.247 ± 0.010 b |
| Hexanol | (μg/L) | 1199 ± 74 a | 3346 ± 154 b | 10,195 ± 420 c | 380 ± 8 a | 3261 ± 55 b | 6658 ± 150 c |
| trans-3-Hexen-1-ol | (μg/L) | 1.7 ± 0.1 a | 40.4 ± 0.3 b | 112.0 ± 0.5 c | 5.8 ± 0.4 a | 41.6 ± 2.9 b | 93.8 ± 3.6 c |
| cis-3-Hexen-1-ol | (μg/L) | ND a | ND a | 2790 ± 189 b | ND a | ND a | 1559 ± 94 b |
| Ethyl isobutyrate | (μg/L) | 12.9 ± 0.3 a | 5.4 ± 0.9 b | 4.2 ± 0.4 b | 8.4 ± 0.6 a | 4.7 ± 0.6 b | 3.7 ± 0.3 b |
| Ethyl butanoate | (μg/L) | 554 ± 1 a | 734 ± 46 b | 669 ± 34 b | 608 ± 7 a | 527 ± 11 b | 535 ± 28 b |
| Ethyl isovalerate | (μg/L) | 1.79 ± 0.08 a | 2.36 ± 0.03 b | 1.94 ± 0.08 a | 1.96 ± 0.04 a | 2.29 ± 0.43 a | 1.94 ± 0.23 a |
| Ethyl hexanoate | (μg/L) | 1814 ± 46 a | 2648 ± 92 b | 2768 ± 74 b | 1916 ± 57 a | 1827 ± 88 a | 1860 ± 68 a |
| Ethyl octanoate | (ug/L) | 761 ± 43 a | 1120 ± 25 b | 1259 ± 225 c | 1455 ± 31 a | 1646 ± 62 b | 1700 ± 40 b |
| Ethyl decanoate | (μg/L) | 261 ± 35 a | 494 ± 19 b | 622 ± 15 c | 524 ± 44 a | 770 ± 38 b | 746 ± 32 b |
| Ethyl dodecanoate | (μg/L) | 47 ± 12 a | 56 ± 7 a,b | 71 ± 6 b | 44 ± 5 a | 54 ± 7 a | 51 ± 10 a |
| Isobutyl acetate | (μg/L) | 13.3 ± 0.9 a | 17.2 ± 0.4 b | 15.2 ± 0.9 c | 15.1 ± 0.2 a | 12.2 ± 1.9 a | 14.2 ± 0.6 a |
| Isoamyl acetate | (μg/L) | 6608 ± 1539 a | 13,532 ± 804 b | 10,092 ± 688 c | 13,292 ± 680 a | 8544 ± 345 b | 7563 ± 463 b |
| Hexyl acetate | (μg/L) | 627 ± 29 a | 2261 ± 115 b | 4430 ± 20 c | 487 ± 20 a | 1356 ± 95 b | 1823 ± 60 c |
| cis-3-Hexenyl acetate | (μg/L) | 50 ± 2 a | 184 ± 5 b | 311 ± 8 c | 35 ± 3 a | 84 ± 4 b | 147 ± 7 c |
| β-Phenylethyl acetate | (μg/L) | 412 ± 75 a | 1173 ± 7 b | 802 ± 48 c | 1122 ± 55 a | 820 ± 19 b | 534 ± 11 c |
| Methyl octanoate | (μg/L) | 0.681 ± 0.002 a | 1.157 ± 0.021 b | 1.704 ± 0.014 c | 0.895 ± 0.010 a | 1.744 ± 0.038 b | 2.440 ± 0.041 c |
| Ethyl-(L)-lactate | (μg/L) | 6266 ± 323 a | 5354 ± 156 b | 6080 ± 155 a | 7617 ± 59 a | 5718 ± 817 b | 5344 ± 457 b |
| Diethyl succinate | (μg/L) | 219 ± 22 a | 102 ± 5 b | 87 ± 3 b | 105 ± 5 a | 97 ± 7 a,b | 91 ± 3 b |
| Isobutyric acid | (mg/L) | 0.66 ± 0.02 a | 0.95 ± 0.06 b | 1.37 ± 0.03 c | 1.08 ± 0.03 a | 1.75 ± 0.08 b | 2.11 ± 0.06 c |
| Hexanoic acid | (mg/L) | 6.9 ± 0.3 a | 7.7 ± 0.3 b | 8.4 ± 0.3 b | 6.8 ± 0.3 a | 6.4 ± 0.2 a | 6.7 ± 0.3 a |
| Octanoic acid | (mg/L) | 11.9 ± 0.7 a | 12.3 ± 1.2 a | 12.4 ± 0.6 a | 9.5 ± 0.5 a | 8.2 ± 0.3 b | 9.1 ± 0.6 a,b |
| Decanoic acid | (mg/L) | 2.7 ± 0.2 a | 0.8 ± 0.6 b | 2.3 ± 0.3 a | 1.0 ± 0.2 a | 0.3 ± 0.2 b | 0.5 ± 0.2 b |
| Isobutanol | (μg/L) | 16,972 ± 1474 a | 22,601 ± 1214 b | 20,169 ± 1751 a,b | 25,484 ± 848 a | 23,043 ± 396 a | 23,783 ± 1545 a |
| Butan-1-ol | (μg/L) | 1155 ± 92 a | 990 ± 28 a | 806 ± 79 b | 1340 ± 68 a | 803 ± 24 b | 789 ± 40 b |
| Isoamyl alcohol | (μg/L) | 154,756 ± 9212 a | 181,938 ± 8111 b | 152,970 ± 7739 a | 194,812 ± 2275 a | 160,303 ± 4842 b | 160,940 ± 5409 b |
| Methionol | (μg/L) | 1831 ± 16 a | 1033 ± 44 b | 1356 ± 276 b | 1471 ± 363 a | 995 ± 114 a | 928 ± 49 a |
| Benzyl alcohol | (μg/L) | 52 ± 4 a | 125 ± 2 a | 540 ± 51 b | 80 ± 2 a | 195 ± 15 b | 507 ± 2 c |
| Phenylethyl alcohol | (μg/L) | 26,421 ± 1053 a | 40,779 ± 1077 b | 34,805 ± 1880 c | 37,583 ± 840 a | 37,064 ± 570 a | 33,271 ± 306 b |
| Benzaldehyde | (μg/L) | 0.41 ± 0.02 a | 12.50 ± 0.23 b | 20.33 ± 2.05 c | 0.64 ± 0.06 a | 19.03 ± 1.63 b | 47.17 ± 1.95 c |
| Ethyl cinnamate | (μg/L) | 0.169 ± 0.001 a | 0.397 ± 0.011 b | 0.692 ± 0.040 c | 0.290 ± 0.016 a | 0.445 ± 0.007 b | 0.577 ± 0.016 c |
| Ethyl dihydrocinnamate | (μg/L) | 0.418 ± 0.018 a | 0.657 ± 0.003 b | 0.668 ± 0.014 b | 0.704 ± 0.005 a | 0.858 ± 0.021 b | 0.884 ± 0.024 b |
| 4-Vinylphenol | (μg/L) | 388 ± 1 a | 296 ± 37 b | 102 ± 6 c | 1,186 ± 59 a | 206 ± 23 b | 96 ± 8 c |
| 4-Vinylguaiacol | (μg/L) | 143 ± 3 a | 141 ± 15 a | 181 ± 2 b | 270 ± 3 a | 179 ± 14 b | 132 ± 1 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parish-Virtue, K.; Herbst-Johnstone, M.; Bouda, F.; Fedrizzi, B.; Deed, R.C.; Kilmartin, P.A. Aroma and Sensory Profiles of Sauvignon Blanc Wines from Commercially Produced Free Run and Pressed Juices. Beverages 2021, 7, 29. https://0-doi-org.brum.beds.ac.uk/10.3390/beverages7020029
Parish-Virtue K, Herbst-Johnstone M, Bouda F, Fedrizzi B, Deed RC, Kilmartin PA. Aroma and Sensory Profiles of Sauvignon Blanc Wines from Commercially Produced Free Run and Pressed Juices. Beverages. 2021; 7(2):29. https://0-doi-org.brum.beds.ac.uk/10.3390/beverages7020029
Chicago/Turabian StyleParish-Virtue, Katie, Mandy Herbst-Johnstone, Flo Bouda, Bruno Fedrizzi, Rebecca C. Deed, and Paul A. Kilmartin. 2021. "Aroma and Sensory Profiles of Sauvignon Blanc Wines from Commercially Produced Free Run and Pressed Juices" Beverages 7, no. 2: 29. https://0-doi-org.brum.beds.ac.uk/10.3390/beverages7020029