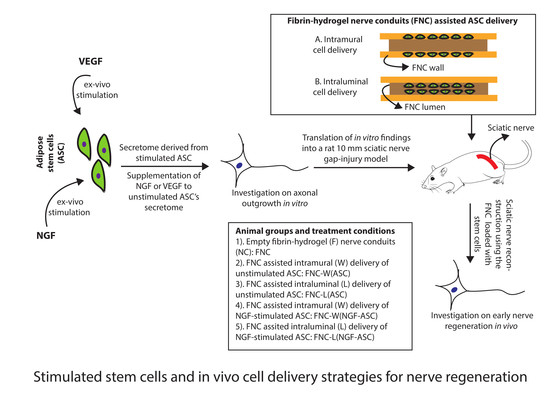
Ex-Vivo Stimulation of Adipose Stem Cells by Growth Factors and Fibrin-Hydrogel Assisted Delivery Strategies for Treating Nerve Gap-Injuries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation and Culture of Adipose Stem Cells (ASC)
2.2. ASC Characterization
2.3. Isolation of Chicken Embryonic Dorsal Root Ganglions (DRG)
2.4. ASC Stimulation and Secretome Harvest
2.5. Experimental Design In Vitro
2.6. Immunocytochemistry of DRG Cultures
2.7. Quantitative Measurements of Axonal Outgrowth
2.8. Stimulated ASC for Animal Studies
2.9. Fibrin-Hydrogel Nerve Conduits (FNC)
2.10. Intramural Delivery of ASC
2.11. Intraluminal Delivery of ASC
2.12. Surgical Procedure and Experimental Groups In Vivo
2.13. Tissue Processing and Immunohistochemistry
2.14. Histological Analysis
2.15. Statistical Analysis
3. Results
3.1. Characterization of Isolated ASC
3.2. Stem Cell Derived Secretome and Axonal Growth In Vitro
3.3. Stimulated Stem Cells and Delivery Route Impacted Early Nerve Regeneration
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lundborg, G. A 25-year perspective of peripheral nerve surgery: Evolving neuroscientific concepts and clinical significance. J. Hand Surg. Am. 2000, 25, 391–414. [Google Scholar] [CrossRef] [PubMed]
- Wiberg, M.; Terenghi, G. Will it be possible to produce peripheral nerves? Surg. Technol. Int. 2003, 11, 303–310. [Google Scholar] [PubMed]
- Webber, C.; Zochodne, D. The nerve regenerative microenvironment: Early behavior and partnership of axons and Schwann cells. Exp. Neurol. 2010, 223, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Allodi, I.; Udina, E.; Navarro, X. Specificity of peripheral nerve regeneration: Interactions at the axon level. Prog. Neurobiol. 2012, 98, 16–37. [Google Scholar] [CrossRef] [PubMed]
- Widgerow, A.D.; Salibian, A.A.; Lalezari, S.; Evans, G.R. Neuromodulatory nerve regeneration: Adipose tissue-derived stem cells and neurotrophic mediation in peripheral nerve regeneration. J. Neurosci. Res. 2013, 91, 1517–1524. [Google Scholar] [CrossRef] [PubMed]
- Nakagami, H.; Maeda, K.; Morishita, R.; Iguchi, S.; Nishikawa, T.; Takami, Y.; Kikuchi, Y.; Saito, Y.; Tamai, K.; Ogihara, T.; et al. Novel autologous cell therapy in ischemic limb disease through growth factor secretion by cultured adipose tissue-derived stromal cells. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2542–2547. [Google Scholar] [CrossRef]
- di Summa, P.G.; Kalbermatten, D.F.; Pralong, E.; Raffoul, W.; Kingham, P.J.; Terenghi, G. Long-term in vivo regeneration of peripheral nerves through bioengineered nerve grafts. Neuroscience 2011, 181, 278–291. [Google Scholar] [CrossRef]
- Dezawa, M.; Takahashi, I.; Esaki, M.; Takano, M.; Sawada, H. Sciatic nerve regeneration in rats induced by transplantation of in vitro differentiated bone-marrow stromal cells. Eur. J. Neurosci. 2001, 14, 1771–1776. [Google Scholar] [CrossRef]
- Tohill, M.; Mantovani, C.; Wiberg, M.; Terenghi, G. Rat bone marrow mesenchymal stem cells express glial markers and stimulate nerve regeneration. Neurosci. Lett. 2004, 362, 200–203. [Google Scholar] [CrossRef]
- Keilhoff, G.; Goihl, A.; Langnase, K.; Fansa, H.; Wolf, G. Transdifferentiation of mesenchymal stem cells into Schwann cell-like myelinating cells. Eur. J. Cell Biol. 2006, 85, 11–24. [Google Scholar] [CrossRef]
- Caddick, J.; Kingham, P.J.; Gardiner, N.J.; Wiberg, M.; Terenghi, G. Phenotypic and functional characteristics of mesenchymal stem cells differentiated along a Schwann cell lineage. Glia 2006, 54, 840–849. [Google Scholar] [CrossRef] [PubMed]
- De Ugarte, D.A.; Morizono, K.; Elbarbary, A.; Alfonso, Z.; Zuk, P.A.; Zhu, M.; Dragoo, J.L.; Ashjian, P.; Thomas, B.; Benhaim, P.; et al. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs 2003, 174, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Strem, B.M.; Hicok, K.C.; Zhu, M.; Wulur, I.; Alfonso, Z.; Schreiber, R.E.; Fraser, J.K.; Hedrick, M.H. Multipotential differentiation of adipose tissue-derived stem cells. Keio J. Med. 2005, 54, 132–141. [Google Scholar] [CrossRef] [Green Version]
- Cruciani, S.; Santaniello, S.; Montella, A.; Ventura, C.; Maioli, M. Orchestrating stem cell fate: Novel tools for regenerative medicine. World J. Stem Cells 2019, 11, 464–475. [Google Scholar] [CrossRef] [PubMed]
- Mazzini, L.; Ferrari, D.; Andjus, P.R.; Buzanska, L.; Cantello, R.; De Marchi, F.; Gelati, M.; Giniatullin, R.; Glover, J.C.; Grilli, M.; et al. Advances in stem cell therapy for amyotrophic lateral sclerosis. Expert Opin. Biol. Ther. 2018, 18, 865–881. [Google Scholar] [CrossRef]
- Kern, S.; Eichler, H.; Stoeve, J.; Kluter, H.; Bieback, K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells (Dayt. Ohio) 2006, 24, 1294–1301. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; De Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell 2002, 13, 4279–4295. [Google Scholar] [CrossRef]
- El Atat, O.; Antonios, D.; Hilal, G.; Hokayem, N.; Abou-Ghoch, J.; Hashim, H.; Serhal, R.; Hebbo, C.; Moussa, M.; Alaaeddine, N. An Evaluation of the Stemness, Paracrine, and Tumorigenic Characteristics of Highly Expanded, Minimally Passaged Adipose-Derived Stem Cells. PLoS ONE 2016, 11, e0162332. [Google Scholar] [CrossRef]
- Aust, L.; Devlin, B.; Foster, S.J.; Halvorsen, Y.D.; Hicok, K.; du Laney, T.; Sen, A.; Willingmyre, G.D.; Gimble, J.M. Yield of human adipose-derived adult stem cells from liposuction aspirates. Cytotherapy 2004, 6, 7–14. [Google Scholar] [CrossRef]
- McIntosh, K.R.; Lopez, M.J.; Borneman, J.N.; Spencer, N.D.; Anderson, P.A.; Gimble, J.M. Immunogenicity of allogeneic adipose-derived stem cells in a rat spinal fusion model. Tissue Eng. Part A 2009, 15, 2677–2686. [Google Scholar] [CrossRef]
- Kingham, P.J.; Kalbermatten, D.F.; Mahay, D.; Armstrong, S.J.; Wiberg, M.; Terenghi, G. Adipose-derived stem cells differentiate into a Schwann cell phenotype and promote neurite outgrowth in vitro. Exp. Neurol. 2007, 207, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Kingham, P.J.; Kolar, M.K.; Novikova, L.N.; Novikov, L.N.; Wiberg, M. Stimulating the neurotrophic and angiogenic properties of human adipose-derived stem cells enhances nerve repair. Stem Cells Dev. 2014, 23, 741–754. [Google Scholar] [CrossRef] [PubMed]
- Bucan, V.; Vaslaitis, D.; Peck, C.T.; Strauss, S.; Vogt, P.M.; Radtke, C. Effect of Exosomes from Rat Adipose-Derived Mesenchymal Stem Cells on Neurite Outgrowth and Sciatic Nerve Regeneration After Crush Injury. Mol. Neurobiol. 2019, 56, 1812–1824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Georgiou, M.; Golding, J.P.; Loughlin, A.J.; Kingham, P.J.; Phillips, J.B. Engineered neural tissue with aligned, differentiated adipose-derived stem cells promotes peripheral nerve regeneration across a critical sized defect in rat sciatic nerve. Biomaterials 2015, 37, 242–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sowa, Y.; Kishida, T.; Imura, T.; Numajiri, T.; Nishino, K.; Tabata, Y.; Mazda, O. Adipose-Derived Stem Cells Promote Peripheral Nerve Regeneration In Vivo without Differentiation into Schwann-Like Lineage. Plast. Reconstr. Surg. 2016, 137, 318e–330e. [Google Scholar] [CrossRef]
- Saller, M.M.; Huettl, R.E.; Mayer, J.M.; Feuchtinger, A.; Krug, C.; Holzbach, T.; Volkmer, E. Validation of a novel animal model for sciatic nerve repair with an adipose-derived stem cell loaded fibrin conduit. Neural Regen. Res. 2018, 13, 854–861. [Google Scholar] [CrossRef]
- Kappos, E.A.; Engels, P.E.; Tremp, M.; Meyer zu Schwabedissen, M.; di Summa, P.; Fischmann, A.; von Felten, S.; Scherberich, A.; Schaefer, D.J.; Kalbermatten, D.F. Peripheral Nerve Repair: Multimodal Comparison of the Long-Term Regenerative Potential of Adipose Tissue-Derived Cells in a Biodegradable Conduit. Stem Cells Dev. 2015, 24, 2127–2141. [Google Scholar] [CrossRef]
- Klein, S.M.; Vykoukal, J.; Li, D.P.; Pan, H.L.; Zeitler, K.; Alt, E.; Geis, S.; Felthaus, O.; Prantl, L. Peripheral Motor and Sensory Nerve Conduction following Transplantation of Undifferentiated Autologous Adipose Tissue-Derived Stem Cells in a Biodegradable U.S. Food and Drug Administration-Approved Nerve Conduit. Plast. Reconstr. Surg. 2016, 138, 132–139. [Google Scholar] [CrossRef]
- Erba, P.; Mantovani, C.; Kalbermatten, D.F.; Pierer, G.; Terenghi, G.; Kingham, P.J. Regeneration potential and survival of transplanted undifferentiated adipose tissue-derived stem cells in peripheral nerve conduits. J. Plast. Reconstr. Aesthetic Surg. Jpras 2010, 63, e811–e817. [Google Scholar] [CrossRef]
- di Summa, P.G.; Kingham, P.J.; Raffoul, W.; Wiberg, M.; Terenghi, G.; Kalbermatten, D.F. Adipose-derived stem cells enhance peripheral nerve regeneration. J. Plast. Reconstr. Aesthetic Surg. Jpras 2010, 63, 1544–1552. [Google Scholar] [CrossRef]
- Hoyng, S.A.; De Winter, F.; Gnavi, S.; de Boer, R.; Boon, L.I.; Korvers, L.M.; Tannemaat, M.R.; Malessy, M.J.; Verhaagen, J. A comparative morphological, electrophysiological and functional analysis of axon regeneration through peripheral nerve autografts genetically modified to overexpress BDNF, CNTF, GDNF, NGF, NT3 or VEGF. Exp. Neurol. 2014, 261, 578–593. [Google Scholar] [CrossRef] [PubMed]
- Faroni, A.; Mobasseri, S.A.; Kingham, P.J.; Reid, A.J. Peripheral nerve regeneration: Experimental strategies and future perspectives. Adv. Drug Deliv. Rev. 2015, 82–83, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Belanger, K.; Dinis, T.M.; Taourirt, S.; Vidal, G.; Kaplan, D.L.; Egles, C. Recent Strategies in Tissue Engineering for Guided Peripheral Nerve Regeneration. Macromol. Biosci. 2016, 16, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Madduri, S.; Papaloizos, M.; Gander, B. Synergistic effect of GDNF and NGF on axonal branching and elongation in vitro. Neurosci. Res. 2009, 65, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Hobson, M.I. Increased vascularisation enhances axonal regeneration within an acellular nerve conduit. Ann. R. Coll. Surg. Engl. 2002, 84, 47–53. [Google Scholar] [PubMed]
- Sondell, M.; Sundler, F.; Kanje, M. Vascular endothelial growth factor is a neurotrophic factor which stimulates axonal outgrowth through the flk-1 receptor. Eur. J. Neurosci. 2000, 12, 4243–4254. [Google Scholar] [CrossRef]
- Sanchez, A.; Wadhwani, S.; Grammas, P. Multiple neurotrophic effects of VEGF on cultured neurons. Neuropeptides 2010, 44, 323–331. [Google Scholar] [CrossRef] [Green Version]
- Calvo, P.M.; Pastor, A.M.; de la Cruz, R.R. Vascular endothelial growth factor: An essential neurotrophic factor for motoneurons? Neural Regen. Res. 2018, 13, 1181–1182. [Google Scholar] [CrossRef]
- Muratori, L.; Gnavi, S.; Fregnan, F.; Mancardi, A.; Raimondo, S.; Perroteau, I.; Geuna, S. Evaluation of Vascular Endothelial Growth Factor (VEGF) and Its Family Member Expression After Peripheral Nerve Regeneration and Denervation. Anat. Rec. 2018, 301, 1646–1656. [Google Scholar] [CrossRef] [Green Version]
- Madduri, S.; di Summa, P.; Papaloizos, M.; Kalbermatten, D.; Gander, B. Effect of controlled co-delivery of synergistic neurotrophic factors on early nerve regeneration in rats. Biomaterials 2010, 31, 8402–8409. [Google Scholar] [CrossRef]
- Wood, M.D.; MacEwan, M.R.; French, A.R.; Moore, A.M.; Hunter, D.A.; Mackinnon, S.E.; Moran, D.W.; Borschel, G.H.; Sakiyama-Elbert, S.E. Fibrin matrices with affinity-based delivery systems and neurotrophic factors promote functional nerve regeneration. Biotechnol. Bioeng. 2010, 106, 970–979. [Google Scholar] [CrossRef] [PubMed]
- Kalbermatten, D.F.; Pettersson, J.; Kingham, P.J.; Pierer, G.; Wiberg, M.; Terenghi, G. New fibrin conduit for peripheral nerve repair. J. Reconstr. Microsurg. 2009, 25, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, J.; Kalbermatten, D.; McGrath, A.; Novikova, L.N. Biodegradable fibrin conduit promotes long-term regeneration after peripheral nerve injury in adult rats. J. Plast. Reconstr. Aesthetic Surg. Jpras 2010, 63, 1893–1899. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Rosen, J.M. The role of undifferentiated adipose-derived stem cells in peripheral nerve repair. Neural Regen. Res. 2018, 13, 757–763. [Google Scholar] [CrossRef]
- Orbay, H.; Uysal, A.C.; Hyakusoku, H.; Mizuno, H. Differentiated and undifferentiated adipose-derived stem cells improve function in rats with peripheral nerve gaps. J. Plast. Reconstr. Aesthetic Surg. Jpras 2012, 65, 657–664. [Google Scholar] [CrossRef]
- Watanabe, Y.; Sasaki, R.; Matsumine, H.; Yamato, M.; Okano, T. Undifferentiated and differentiated adipose-derived stem cells improve nerve regeneration in a rat model of facial nerve defect. J. Tissue Eng. Regen. Med. 2017, 11, 362–374. [Google Scholar] [CrossRef]
- Radtke, C.; Schmitz, B.; Spies, M.; Kocsis, J.D.; Vogt, P.M. Peripheral glial cell differentiation from neurospheres derived from adipose mesenchymal stem cells. Int. J. Dev. Neurosci. Off. J. Int. Soc. Dev. Neurosci. 2009, 27, 817–823. [Google Scholar] [CrossRef]
- Haghighat, A.; Akhavan, A.; Hashemi-Beni, B.; Deihimi, P.; Yadegari, A.; Heidari, F. Adipose derived stem cells for treatment of mandibular bone defects: An autologous study in dogs. Dent. Res. J. 2011, 8, S51–S57. [Google Scholar]
- Fang, Y.; Zhang, Y.; Zhou, J.; Cao, K. Adipose-derived mesenchymal stem cell exosomes: A novel pathway for tissues repair. Cell Tissue Bank. 2019, 20, 153–161. [Google Scholar] [CrossRef]
- Santiago, L.Y.; Clavijo-Alvarez, J.; Brayfield, C.; Rubin, J.P.; Marra, K.G. Delivery of adipose-derived precursor cells for peripheral nerve repair. Cell Transplant. 2009, 18, 145–158. [Google Scholar] [CrossRef]
- Salgado, A.J.; Reis, R.L.; Sousa, N.J.; Gimble, J.M. Adipose tissue derived stem cells secretome: Soluble factors and their roles in regenerative medicine. Curr. Stem Cell Res. Ther. 2010, 5, 103–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marconi, S.; Castiglione, G.; Turano, E.; Bissolotti, G.; Angiari, S.; Farinazzo, A.; Constantin, G.; Bedogni, G.; Bedogni, A.; Bonetti, B. Human adipose-derived mesenchymal stem cells systemically injected promote peripheral nerve regeneration in the mouse model of sciatic crush. Tissue Eng. Part A 2012, 18, 1264–1272. [Google Scholar] [CrossRef] [PubMed]
- Bellei, B.; Migliano, E.; Tedesco, M.; Caputo, S.; Papaccio, F.; Lopez, G.; Picardo, M. Adipose tissue-derived extracellular fraction characterization: Biological and clinical considerations in regenerative medicine. Stem Cell Res. Ther. 2018, 9, 207. [Google Scholar] [CrossRef] [PubMed]
- Vasilev, G.; Ivanova, M.; Ivanova-Todorova, E.; Tumangelova-Yuzeir, K.; Krasimirova, E.; Stoilov, R.; Kyurkchiev, D. Secretory factors produced by adipose mesenchymal stem cells downregulate Th17 and increase Treg cells in peripheral blood mononuclear cells from rheumatoid arthritis patients. Rheumatol. Int. 2019, 39, 819–826. [Google Scholar] [CrossRef]
- Madduri, S.; Papaloizos, M.; Gander, B. Trophically and topographically functionalized silk fibroin nerve conduits for guided peripheral nerve regeneration. Biomaterials 2010, 31, 2323–2334. [Google Scholar] [CrossRef]
- Lee, J.Y.; Giusti, G.; Friedrich, P.F.; Bishop, A.T.; Shin, A.Y. Effect of Vascular Endothelial Growth Factor Administration on Nerve Regeneration after Autologous Nerve Grafting. J. Reconstr. Microsurg. 2016, 32, 183–188. [Google Scholar] [CrossRef]
- Pereira Lopes, F.R.; Lisboa, B.C.; Frattini, F.; Almeida, F.M.; Tomaz, M.A.; Matsumoto, P.K.; Langone, F.; Lora, S.; Melo, P.A.; Borojevic, R.; et al. Enhancement of sciatic nerve regeneration after vascular endothelial growth factor (VEGF) gene therapy. Neuropathol. Appl. Neurobiol. 2011, 37, 600–612. [Google Scholar] [CrossRef]
- Xia, B.; Lv, Y. Dual-delivery of VEGF and NGF by emulsion electrospun nanofibrous scaffold for peripheral nerve regeneration. Mater. Sci. Eng. CMater. Biol. Appl. 2018, 82, 253–264. [Google Scholar] [CrossRef]
- Lisi, A.; Briganti, E.; Ledda, M.; Losi, P.; Grimaldi, S.; Marchese, R.; Soldani, G. A combined synthetic-fibrin scaffold supports growth and cardiomyogenic commitment of human placental derived stem cells. PLoS ONE 2012, 7, e34284. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi, M.R.; Riazifar, M.; Pone, E.J.; Yeri, A.; Van Keuren-Jensen, K.; Lasser, C.; Lotvall, J.; Zhao, W. Isolation and characterization of microvesicles from mesenchymal stem cells. Methods (San DiegoCalif.) 2019. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prautsch, K.M.; Degrugillier, L.; Schaefer, D.J.; Guzman, R.; Kalbermatten, D.F.; Madduri, S. Ex-Vivo Stimulation of Adipose Stem Cells by Growth Factors and Fibrin-Hydrogel Assisted Delivery Strategies for Treating Nerve Gap-Injuries. Bioengineering 2020, 7, 42. https://0-doi-org.brum.beds.ac.uk/10.3390/bioengineering7020042
Prautsch KM, Degrugillier L, Schaefer DJ, Guzman R, Kalbermatten DF, Madduri S. Ex-Vivo Stimulation of Adipose Stem Cells by Growth Factors and Fibrin-Hydrogel Assisted Delivery Strategies for Treating Nerve Gap-Injuries. Bioengineering. 2020; 7(2):42. https://0-doi-org.brum.beds.ac.uk/10.3390/bioengineering7020042
Chicago/Turabian StylePrautsch, Katharina M., Lucas Degrugillier, Dirk J. Schaefer, Raphael Guzman, Daniel F. Kalbermatten, and Srinivas Madduri. 2020. "Ex-Vivo Stimulation of Adipose Stem Cells by Growth Factors and Fibrin-Hydrogel Assisted Delivery Strategies for Treating Nerve Gap-Injuries" Bioengineering 7, no. 2: 42. https://0-doi-org.brum.beds.ac.uk/10.3390/bioengineering7020042