Free-Radical-Mediated Formation of Trans-Cardiolipin Isomers, Analytical Approaches for Lipidomics and Consequences of the Structural Organization of Membranes †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Transesterification Procedure and Gas Chromatography (GC) Analysis
2.2. Photolytical Preparation of Mono-Trans CL (MT-CL)
2.3. Preparative Silver-Thin Layer Chromatography (Ag-TLC)
2.4. Preparation of Liposome
2.5. γ-Radiolysis Experiments
2.6. Lipid Extraction from Liposome Suspensions
2.7. Incubation Experiments under Oxidative Conditions
3. Results and Discussion
3.1. Analytical Protocol
3.2. MT-CL and T-CL Preparation by UV Photolysis
3.3. Isolation of MT-CL and T-CL as Ag-complexes
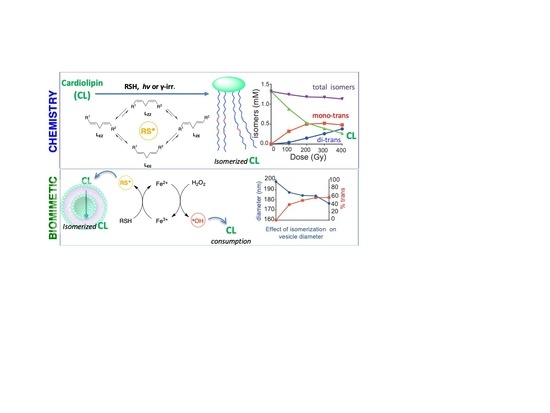
3.4. Kinetic and Product Studies by γ-Radiolysis of CL
3.5. Large Unilamellar Vesicles (LUV) Containing CL, MT-CL and T-CL
3.6. γ-Radiolysis of CL-Containing Liposomes
3.7. CL-Containing Liposomes Under Oxidative Conditions.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Oteng, A.-B.; Kersten, S. Mechanism of action of trans fatty acids. Adv. Nutr. 2019, 11, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Chatgilialoglu, C.; Ferreri, C.; Melchiorre, M.; Sansone, A.; Torreggiani, A. Lipid geometrical isomerism: From chemistry to biology and diagnostics. Chem. Rev. 2014, 114, 255–284. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.; Davies, S.S. Non enzymatic mechanisms of lipid oxidation. In Lipid Oxidation in Health and Disease; Spickett, C.M., Forman, H.J., Eds.; CRC Press: Boca Raton, FL, USA, 2015; Chapter 2. [Google Scholar] [CrossRef]
- Kuhnt, K.; Baehr, M.; Rohrer, C.; Jahreis, G. Trans fatty acid isomers and the trans-9/trans-11 index in fat containing foods. Eur. J. Lipid Sci. Technol. 2011, 113, 1281–1292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bannenberg, G.; Mallon, C.; Edwards, H.; Yeadon, D.; Yan, K.; Johnson, H.; Ismail, A. Omega-3 long-chain polyunsaturated fatty acid content and oxidation state of fish oil supplements in New Zealand. Sci. Rep. 2017, 7, 1488. [Google Scholar] [CrossRef] [Green Version]
- Menounou, G.; Giacometti, G.; Scanferlato, R.; Dambruoso, P.; Sansone, A.; Tueros, I.; Amézaga, J.; Chatgilialoglu, C.; Ferreri, C. Trans lipid library: Synthesis of docosahexaenoic acid (DHA) monotrans isomers and regioisomer identification in DHA-containing supplements. Chem. Res. Toxicol. 2018, 31, 191–200. [Google Scholar] [CrossRef]
- Houtkooper, R.H.; Vaz, F.M. Cardiolipin, the heart of mitochondrial metabolism. Cell Mol. Life Sci. 2008, 65, 2493–2506. [Google Scholar] [CrossRef]
- Mileykovskaya, E.; Dowhan, W. Cardiolipin membrane domains in prokaryotes and eukaryotes. Biochim. Biophys. Acta Biomembr. 2009, 1788, 2084–2091. [Google Scholar] [CrossRef] [Green Version]
- Paradies, G.; Paradies, V.; Ruggiero, F.M.; Petrosillo, G. Role of cardiolipin in mitochondrial function and dynamics in health and disease: Molecular and pharmacological aspects. Cells 2019, 8, 728. [Google Scholar] [CrossRef] [Green Version]
- Huang, K.C.; Ramamurthi, K.S. Macromolecules that prefer their membranes curvy. Mol. Microbiol. 2010, 76, 822–832. [Google Scholar] [CrossRef] [Green Version]
- Ye, C.; Shen, Z.; Greenberg, M.L. Cardiolipin remodeling: A regulatory hub for modulating cardiolipin metabolism and function. J. Bioenerg. Biomembr. 2016, 48, 113–123. [Google Scholar] [CrossRef] [Green Version]
- Sam, P.N.; Avery, E.; Claypool, S.M. Proteolytic control of lipid metabolism. ACS Chem. Biol. 2019, 14, 2406–2423. [Google Scholar] [CrossRef] [PubMed]
- Bradley, R.M.; Stark, K.D.; Duncan, R.E. Influence of tissue, diet, and enzymatic remodeling on cardiolipin fatty acyl profile. Mol. Nutr. Food. Res. 2016, 60, 1804–1818. [Google Scholar] [CrossRef] [PubMed]
- Kiebish, M.A.; Han, X.; Cheng, H.; Chuang, J.H.; Seyfried, T.N. Cardiolipin and electron transport chain abnormalities in mouse brain tumor mitochondria: Lipidomic evidence supporting the Warburg theory of cancer. J. Lipid Res. 2008, 49, 2545–2556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lokhmatikov, A.V.; Voskoboynikova, N.; Cherepanov, D.A.; Skulachev, M.V.; Steinhoff, H.-J.; Skulachev, V.P.; Mulkidjanian, A.Y. Impact of antioxidants on cardiolipin oxidation in liposomes: Why mitochondrial cardiolipin serves as an apoptotic signal? Oxid. Med. Cell. Long. 2016, 2016, 8679469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatgilialoglu, C.; Ferreri, C. Trans lipids: The free radical path. Acc. Chem. Res. 2005, 38, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Chatgilialoglu, C.; Bowry, V.W. Why not trans? Inhibited radical isomerization cycles and coupling chains of lipids and alkenes with alkane-thiols. J. Org. Chem. Soc. 2018, 83, 9178–9189. [Google Scholar] [CrossRef] [PubMed]
- Ferreri, C.; Costantino, C.; Perrotta, L.; Landi, L.; Mulazzani, Q.G.; Chatgilialoglu, C. Cis-trans isomerization of polyunsaturated fatty acid residues in phospholipids catalyzed by thiyl radicals. J. Am. Chem. Soc. 2001, 123, 4459–4468. [Google Scholar] [CrossRef]
- Chatgilialoglu, C.; Ferreri, C.; Guerra, M.; Samadi, A.; Bowry, V.W. The reaction of thiyl radical with methyl linoleate: Completing the picture. J. Am. Chem. Soc. 2017, 139, 4704–4714. [Google Scholar] [CrossRef]
- Tartaro Bujak, I.; Mihaljević, B.; Ferreri, C.; Chatgilialoglu, C. The influence of antioxidants in the thiyl radical induced lipid peroxidation and geometrical isomerization in micelles of linoleic acid. Free Radic. Res. 2016, 50, S18–S23. [Google Scholar] [CrossRef] [Green Version]
- Christie, W.W.; Han, X. Lipid Analysis, 4nd ed.; The Oily Press: Bridgewater, UK, 2010; Part 1; pp. 21–54. ISBN 9780955251245. [Google Scholar]
- Guan, Z.Z.; Söderberg, M.; Sindelar, P.; Edlund, C. Content and fatty acid composition of cardiolipin in the brain of patients with alzheimer’s disease. Neurochem. Int. 1994, 25, 295–300. [Google Scholar] [CrossRef]
- Lee, H.-J.; Mayette, J.; Rapoport, S.I.; Bazinet, R.P. Selective remodeling of cardiolipin fatty acids in the aged rat heart. Lipids Health Dis. 2006, 5, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fouret, G.; Tolika, E.; Lecomte, J.; Bonafos, B.; Aoun, M.; Murphy, M.P.; Ferreri, C.; Chatgilialoglu, C.; Dubreucq, E.; Coudray, C.; et al. The mitochondrial-targeted antioxidant, MitoQ, increases liver mitochondrial cardiolipin content in obesogenic diet-fed rats. Biochim. Biophys. Acta Bioenerg. 2015, 147, 1025–1035. [Google Scholar] [CrossRef] [PubMed]
- Hsu, F.-F.; Turk, J.; Rhoades, E.R.; Russell, D.G.; Shi, Y.; Groisman, E.A. Structural characterization of cardiolipin by tandem quadrupole and multiple-stage quadrupole ion-trap mass spectrometry with electrospray ionization. J. Am. Soc. Mass Spectr. 2005, 16, 491–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sparagna, G.C.; Johnson, C.A.; McCune, S.A.; Moore, R.L.; Murphy, R.C. Quantitation of cardiolipin molecular species in spontaneously hypertensive heart failure rats using electrospray ionization mass spectrometry. J. Lipid Res. 2005, 46, 1196–1204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malhotra, A.; Xu, Y.; Ren, M.; Schlame, M. Formation of molecular species of mitochondrial cardiolipin. 1. A novel transacylation mechanism to shuttle fatty acids between sn-1 and sn-2 positions of multiple phospholipid species. Biochim. Biophys. Acta 2009, 1791, 314–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ting, H.-C.; Chen, L.-T.; Chen, J.-Y.; Huang, Y.-L.; Xin, R.-C.; Chan, J.-F.; Hsu, Y.-H.H. Double bonds of unsaturated fatty acids differentially regulate mitochondrial cardiolipin remodeling. Lipids Health Dis. 2019, 18, 53. [Google Scholar] [CrossRef]
- Macias, L.A.; Feider, C.L.; Eberlin, L.S.; Brodbelt, J.S. Hybrid 193 nm ultraviolet photodissociation mass spectrometry localizes cardiolipin unsaturations. Anal. Chem. 2019, 91, 12509–12516. [Google Scholar] [CrossRef]
- Fox, B.G.; Lyle, K.S.; Rogge, C.E. Reactions of the diiron enzyme stearoyl-acyl carrier protein desaturase. Acc. Chem. Res. 2004, 37, 421–429. [Google Scholar] [CrossRef]
- Forman, B.M.; Chen, J.; Evans, R.M. Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors alpha and delta. Proc. Natl. Acad. Sci. USA 1997, 94, 4312–4317. [Google Scholar] [CrossRef] [Green Version]
- Jump, D.B.; Clarke, S.D. Regulation of gene expression by dietary fat. Annu. Rev. Nutr. 1999, 19, 63–90. [Google Scholar] [CrossRef]
- Ferreri, C.; Pierotti, S.; Barbieri, A.; Zambonin, L.; Landi, L.; Rasi, S.; Luisi, P.L.; Barigelletti, F.; Chatgilialoglu, C. Comparison of phosphatidylcholine vesicle properties related to geometrical isomerism. Photochem. Photobiol. 2006, 82, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Ferreri, C.; Pierotti, S.; Chatgilialoglu, C.; Barbieri, A.; Barigelletti, F. Probing the influence of cis-trans isomers on model lipid membrane fluidity using cis-parinaric acid and a stop-flow technique. Chem. Commun. 2006, 529–531. [Google Scholar] [CrossRef] [PubMed]
- Tyler, A.I.I.; Greenfield, J.L.; Seddon, J.M.; Brooks, N.J.; Purushothaman, S. Coupling phase behavior of fatty acid containing membranes to membrane bio-mechanics. Front. Cell Dev. Biol. 2019, 7, 187. [Google Scholar] [CrossRef] [PubMed]
- Chatgilialoglu, C.; Ferreri, C.; Torreggiani, A.; Renzone, G.; Salzano, A.M.; Scaloni, A. Radiation-induced reductive modifications of sulfur-containing amino acids within peptides and proteins. J. Proteomics 2011, 74, 2264–2273. [Google Scholar] [CrossRef]
- Ferreri, C.; Kratzsch, S.; Brede, O.; Marciniak, B.; Chatgilialoglu, C. Trans lipid formation induced by thiols in human monocytic leukemia cells. Free Radic. Biol. Med. 2005, 38, 1180–1187. [Google Scholar] [CrossRef]
- Cort, A.; Ozben, T.; Melchiorre, M.; Chatgilialoglu, C.; Ferreri, C.; Sansone, A. Effects of bleomycin and antioxidants on the fatty acid profile of testicular cancer cell membranes. Biochim. Biophys. Acta Biomembr. 2016, 1858, 434–441. [Google Scholar] [CrossRef]
- Zambonin, L.; Ferreri, C.; Cabrini, L.; Prata, C.; Chatgilialoglu, C.; Landi, L. Occurrence of trans fatty acids in rats fed a trans-free diet: A free radical-mediated formation? Free Radic. Biol. Med. 2006, 40, 1549–1556. [Google Scholar] [CrossRef]
- Marini, M.; Abruzzo, P.M.; Bolotta, A.; Veicsteinas, A.; Ferreri, C. Aerobic training affects fatty acid composition of erythrocyte membranes. Lipids Health Dis. 2011, 10, 188. [Google Scholar] [CrossRef] [Green Version]
- Ferreri, C.; Chatgilialoglu, C. Membrane Lipidomics for Personalized Health; Wiley: Chichester, UK, 2015. [Google Scholar]
- Sansone, A.; Melchiorre, M.; Chatgilialoglu, C.; Ferreri, C. Hexadecenoic fatty acid isomers: A chemical biology approach for human plasma biomarker development. Chem. Res. Toxicol. 2013, 26, 1703–1709. [Google Scholar] [CrossRef]
- Sansone, A.; Tolika, E.; Louka, M.; Sunda, V.; Deplano, S.; Melchiorre, M.; Anagnostopoulos, D.; Chatgilialoglu, C.; Formisano, C.; Di Micco, R.; et al. Hexadecenoic fatty acid isomers in human blood lipids and their relevance for the interpretation of lipidomic profiles. PLoS ONE 2016, 11, e0152378. [Google Scholar] [CrossRef] [Green Version]
- Ferreri, C.; Grabovskiy, S.A.; Aoun, M.; Melchiorre, M.; Kabal’nova, N.; Feillet-Coudray, C.; Fouret, G.; Coudray, C.; Chatgilialoglu, C. Trans fatty acids: Chemical synthesis of eicosapentaenoic acid isomers and detection in rats fed a deodorized fish oil diet. Chem. Res. Toxicol. 2012, 25, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Batzri, S.; Korn, E.D. Single bilayer liposomes prepared without sonication. Biochim. Biophys. Acta Biomembr. 1973, 298, 1015–1019. [Google Scholar] [CrossRef]
- Spinks, J.W.T.; Woods, R.J. An Introduction to Radiation Chemistry, 3rd ed.; John-Wiley and Sons, Inc.: New York, NY, USA, 1990; p. 100. [Google Scholar]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [PubMed]
- Ferreri, C.; Samadi, A.; Sassatelli, F.; Landi, L.; Chatgilialoglu, C. Regioselective cis−trans isomerization of arachidonic double bonds by thiyl radicals: the influence of phospholipid supramolecular organization. J. Am. Chem. Soc. 2004, 126, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Bus, J.; Sies, I.; Lie Ken Jie, M.S.F. 13C-NMR of methyl, methylene and carbonyl carbon atoms of methyl alkenoates and alkynoates. Chem. Phys. Lipids 1976, 17, 501–518. [Google Scholar] [CrossRef]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals ((⋅OH)/O−) in aqueous solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef] [Green Version]
- Ross, A.B.; Mallard, W.G.; Helman, W.P.; Buxton, G.V.; Huie, R.E.; Neta, P. NDRL-NIST Solution Kinetics Database: Ver. 3; Notre Dame Radiation Laboratory: Notre Dame, IN, USA; NIST Standard Reference Data: Gaithersburg, MD, USA, 1998. [Google Scholar]
- Miller, D.M.; Buettner, G.R.; Aust, S.D. Transition metals as catalysts of “autoxidation” reactions. Free Radic. Biol. Med. 1990, 8, 95–108. [Google Scholar] [CrossRef]
- Stohs, S.J.; Bagchi, D. Oxidative mechanisms in the toxicity of metal ions. Free Radic. Biol. Med. 1995, 18, 321–336. [Google Scholar] [CrossRef] [Green Version]
- Mihaljević, B.; Tartaro, I.; Ferreri, C.; Chatgilialoglu, C. Linoleic acid peroxidation vs. isomerization: A biomimetic model of free radical reactivity in the presence of thiols. Org. Biomol. Chem. 2011, 9, 3541–3548. [Google Scholar]
- Fu, M.; Zhang, W.; Wu, L.; Yang, G.; Li, H.; Wang, R. Hydrogen sulfide (H2S) metabolism in mitochondria and its regulatory role in energy production. Proc. Natl. Acad Sci. 2012, 109, 2943–2948. [Google Scholar] [CrossRef] [Green Version]
- Filipovic, M.R.; Zivanovic, J.; Alvarez, B.; Benerjee, R. Chemical biology of H2S signaling through persulfidation. Chem. Rev. 2018, 118, 1253–1337. [Google Scholar] [CrossRef] [PubMed]
- Lykakis, I.N.; Ferreri, C.; Chatgilialoglu, C. The sulfhydryl radical (HS• and S•–): A contender for the isomerization of double bonds in membrane lipids. Angew. Chem. Int. Ed. 2007, 46, 1914–1916. [Google Scholar] [CrossRef] [PubMed]





| Dose, Gy | Internal Control (% rel) 1,2 | CL Fatty Acid Residues (% rel) 2 | ||||||
|---|---|---|---|---|---|---|---|---|
| 9cis-18:1 | 9trans-18:1 | 11cis-18:1 | 11trans-18:1 | LZZ | LEZ | LZE | LEE | |
| 0 | 100 | 0 | 100 | 0 | 100 | 0 | 0 | 0 |
| 100 | 84.4 | 15.6 | 83.7 | 12.3 | 71.3 | 13.0 | 13.0 | 2.7 |
| 200 | 67.2 | 32.8 | 64.9 | 35.1 | 44.9 | 21.2 | 21.2 | 12.7 |
| 300 | 53.4 | 46.6 | 55.6 | 44.4 | 33.8 | 22.1 | 22.2 | 21.9 |
| 400 | 40.5 | 59.5 | 44.9 | 55.1 | 24.6 | 21.4 | 21.2 | 32.8 |
| Entry | Phospholipid 1 | Ratio | Diameter, nm | Polydispersity |
|---|---|---|---|---|
| 1 | POPC | - | 93.2 | 0.211 |
| 2 | POPC/CL | 3: 1 | 198 | 0.278 |
| 3 | POPC/MT-CL 2 | 3: 1 | 167 | 0.151 |
| 4 | POPC/T-CL 3 | 3: 1 | 146.1 | 0.394 |
| Dose, Gy | 18: 1 from POPC (% rel) 1 | CL fatty acid residues (% rel) 1 | ||||||
|---|---|---|---|---|---|---|---|---|
| 9cis-18:1 | 9trans-18:1 | 11cis-18:1 | 11trans-18:1 | LZZ | LEZ | LZE | LEE | |
| 0 | 100 | 0 | 100 | 0 | 100 | 0 | 0 | 0 |
| 100 | 65.4 | 34.6 | 70.9 | 29.1 | 65.3 | 14.6 | 8.8 | 11.3 |
| 200 | 50.7 | 49.3 | 56.2 | 43.8 | 51.4 | 17.8 | 10.7 | 20.1 |
| 300 | 38.8 | 61.2 | 49.2 | 50.8 | 44.5 | 18.5 | 11.5 | 25.5 |
| 400 | 34.6 | 65.4 | 43.0 | 57.0 | 44.2 | 17.9 | 11.1 | 26.8 |
| Dose, Gy | Diameter, nm | Polydispersity |
|---|---|---|
| 0 | 198 | 0.278 |
| 100 | 187.4 | 0.310 |
| 200 | 184.4 | 0.286 |
| 300 | 184 | 0.303 |
| 400 | 176.6 | 0.279 |
| Thiol, µM | 18:1 from POPC (% rel) 1 | 18:2 from CL1 | |||
|---|---|---|---|---|---|
| 9cis-18:1 2,3 | 9trans-18:1 2,3 | LZZ 3 | LEZ + LZE 3 | LZZ Consumption 3 | |
| - | 100 | - | 14.5 ± 0.9 | - | 85.0 ± 1.2 |
| HO(CH2)2SH, 10 | 99.6 | 0.4 | 70.0 ± 1.4 | 0.6 ± 0.1 | 29.4 ± 1.4 |
| HO(CH2)2SH, 100 | 99.0 | 1.0 | 48.2 ± 1.3 | 3.5 ± 0.4 | 48.3 ± 1.3 |
| H2S, 10 | 99.7 | 0.3 | 63.6 ± 1.2 | 0.2 ± 0.1 | 36.2 ± 1.2 |
| H2S, 100 | 99.1 | 0.9 | 44.5 ± 3.3 | 0.6 ± 0.1 | 54.9 ± 3.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vetica, F.; Sansone, A.; Meliota, C.; Batani, G.; Roberti, M.; Chatgilialoglu, C.; Ferreri, C. Free-Radical-Mediated Formation of Trans-Cardiolipin Isomers, Analytical Approaches for Lipidomics and Consequences of the Structural Organization of Membranes. Biomolecules 2020, 10, 1189. https://0-doi-org.brum.beds.ac.uk/10.3390/biom10081189
Vetica F, Sansone A, Meliota C, Batani G, Roberti M, Chatgilialoglu C, Ferreri C. Free-Radical-Mediated Formation of Trans-Cardiolipin Isomers, Analytical Approaches for Lipidomics and Consequences of the Structural Organization of Membranes. Biomolecules. 2020; 10(8):1189. https://0-doi-org.brum.beds.ac.uk/10.3390/biom10081189
Chicago/Turabian StyleVetica, Fabrizio, Anna Sansone, Cesare Meliota, Gessica Batani, Marinella Roberti, Chryssostomos Chatgilialoglu, and Carla Ferreri. 2020. "Free-Radical-Mediated Formation of Trans-Cardiolipin Isomers, Analytical Approaches for Lipidomics and Consequences of the Structural Organization of Membranes" Biomolecules 10, no. 8: 1189. https://0-doi-org.brum.beds.ac.uk/10.3390/biom10081189