Study of the Cluster Thinning Grape as a Source of Phenolic Compounds and Evaluation of Its Antioxidant Potential
Abstract
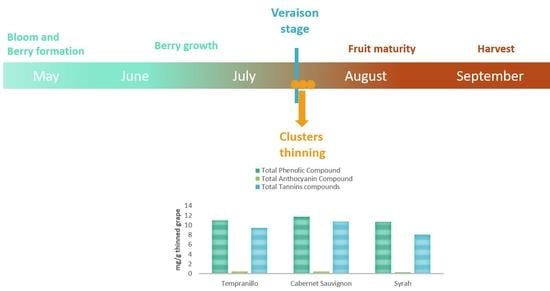
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Samples
2.3. Sample Preparation
2.4. Control Parameters
2.5. Total Phenolic Content
2.6. Individual Phenolic Compounds
2.7. Antioxidant Activity
2.8. Statistical Analysis
3. Results and Discussion
3.1. Control Parameters
3.2. Total Phenolic Content and Antioxidant Activity
3.2.1. Total Phenolic Content
3.2.2. Antioxidant Activity
3.3. Individual Phenolic Compounds
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Organisation of Vine and Wine (OIV). 2019 Statistical Report on World Vitiviniculture; OIV: Paris, France, 2019. [Google Scholar]
- Ruberto, G.; Renda, A.; Daquino, C.; Amico, V.; Spatafora, C.; Tringali, C.; Tommasi, N.D. Polyphenol constituents and antioxidant activity of grape pomace extracts from five Sicilian red grape cultivars. Food Chem. 2007, 100, 203–210. [Google Scholar] [CrossRef]
- Anastasiadi, M.; Pratsinis, H.; Kletsas, D.; Skaltsounis, A.L.; Haroutounian, S.A. Grape stem extracts: Polyphenolic content and assessment of their in vitro antioxidant properties. LWT Food Sci. Technol. 2012, 48, 316–322. [Google Scholar] [CrossRef]
- Mildner-Szkudlarz, S.; Bajerska, J.; Zawirska-Wojtasiak, R.; Górecka, D. White grape pomace as a source of dietary fibre and polyphenols and its effect on physical and nutraceutical characteristics of wheat biscuits. J. Sci. Food Agric. 2013, 93, 389–395. [Google Scholar] [CrossRef]
- Denny, C.; Lazarini, J.G.; Franchin, M.; Melo, P.S.; Pereira, G.E.; Massarioli, A.P.; Moreno, I.A.M.; Paschoal, J.A.R.; Alencar, S.M.; Rosalen, P.L. Bioprospection of Petit Verdot grape pomace as a source of anti-inflammatory compounds. J. Funct. Foods 2014, 8, 292–300. [Google Scholar] [CrossRef]
- Makris, D.P.; Boskou, G.; Andrikopoulos, N.K. Polyphenolic content and in vitro antioxidant characteristics of wine industry and other agri-food solid waste extracts. J. Food Compos. Anal. 2007, 20, 125–132. [Google Scholar] [CrossRef]
- Rockenbach, I.I.; Rodrigues, E.; Gonzaga, L.V.; Caliari, V.; Genovese, M.I.; Gonalves, A.E.D.S.S.; Fett, R. Phenolic compounds content and antioxidant activity in pomace from selected red grapes (Vitis vinifera L. and Vitis labrusca L.) widely produced in Brazil. Food Chem. 2011, 127, 174–179. [Google Scholar] [CrossRef]
- De la Cerda-Carrasco, A.; López-Solís, R.; Nuñez-Kalasic, H.; Peña-Neira, Á.; Obreque-Slier, E. Phenolic composition and antioxidant capacity of pomaces from four grape varieties (Vitis vinifera L.). J. Sci. Food Agric. 2015, 95, 1521–1527. [Google Scholar] [CrossRef]
- Rosales Soto, M.U.; Brown, K.; Ross, C.F. Antioxidant activity and consumer acceptance of grape seed flour-containing food products. Int. J. Food Sci. Technol. 2012, 47, 592–602. [Google Scholar] [CrossRef]
- Sharma, A.K.; Kumar, R.; Azad, Z.R.A.A.; Adsule, P.G. Use of fine wine lees for value addition in ice cream. J. Food Sci. Technol. 2015, 52, 592–596. [Google Scholar] [CrossRef]
- Tseng, A.; Zhao, Y. Wine grape pomace as antioxidant dietary fibre for enhancing nutritional value and improving storability of yogurt and salad dressing. Food Chem. 2013, 138, 356–365. [Google Scholar] [CrossRef]
- Vashisth, T.; Singh, R.K.; Pegg, R.B. Effects of drying on the phenolics content and antioxidant activity of muscadine pomace. LWT Food Sci. Technol. 2011, 44, 1649–1657. [Google Scholar] [CrossRef]
- Yu, J.; Ahmedna, M. Functional components of grape pomace: Their composition, biological properties and potential applications. Int. J. Food Sci. Technol. 2013, 48, 221–237. [Google Scholar] [CrossRef]
- Doshi, P.; Adsule, P.; Banerjee, K.; Oulkar, D. Phenolic compounds, antioxidant activity and insulinotropic effect of extracts prepared from grape (Vitis vinifera L.) byproducts. J. Food Sci. Technol. 2015, 52, 181–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drosou, C.; Kyriakopoulou, K.; Bimpilas, A.; Tsimogiannis, D.; Krokida, M. A comparative study on different extraction techniques to recover red grape pomace polyphenols from vinification byproducts. Ind. Crop. Prod. 2015, 75, 141–149. [Google Scholar] [CrossRef]
- Fontana, A.R.; Antoniolli, A.; Bottini, R. Grape pomace as a sustainable source of bioactive compounds: Extraction, characterization, and biotechnological applications of phenolics. J. Agric. Food Chem. 2013, 61, 8987–9003. [Google Scholar] [CrossRef] [PubMed]
- González-Centeno, M.R.; Jourdes, M.; Femenia, A.; Simal, S.; Rosselló, C.; Teissedre, P.L. Characterization of polyphenols and antioxidant potential of white grape pomace byproducts (Vitis vinifera L.). J. Agric. Food Chem. 2013, 61, 11579–11587. [Google Scholar] [CrossRef]
- Ky, I.; Lorrain, B.; Kolbas, N.; Crozier, A.; Teissedre, P.L. Wine by-Products: Phenolic characterization and antioxidant activity evaluation of grapes and grape pomaces from six different French grape varieties. Molecules 2014, 19, 482–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedroza, M.A.; Carmona, M.; Salinas, M.R.; Zalacain, A. Use of dehydrated waste grape skins as a natural additive for producing rosé Wines: Study of extraction conditions and evolution. J. Agric. Food Chem. 2011, 59, 10976–10986. [Google Scholar] [CrossRef] [PubMed]
- Rockenbach, I.I.; Gonzaga, L.V.; Rizelio, V.M.; Gonçalves, A.E.D.S.S.; Genovese, M.I.; Fett, R. Phenolic compounds and antioxidant activity of seed and skin extracts of red grape (Vitis vinifera and Vitis labrusca) pomace from Brazilian winemaking. Food Res. Int. 2011, 44, 897–901. [Google Scholar] [CrossRef]
- Sousa, E.C.; Uchôa-Thomaz, A.M.A.; Carioca, J.O.B.; de Morais, S.M.; de Lima, A.; Martins, C.G.; Alexandrino, C.D.; Ferreira, P.A.T.; Rodrigues, A.L.M.; Rodrigues, S.P.; et al. Chemical composition and bioactive compounds of grape pomace (Vitis vinifera L.), Benitaka variety, grown in the semiarid region of Northeast Brazil. Food Sci. Technol. 2014, 34, 135–142. [Google Scholar] [CrossRef] [Green Version]
- Alonso, Á.M.; Guillén, D.A.; Barroso, C.G.; Puertas, B.; García, A. Determination of antioxidant activity of wine byproducts and its correlation with polyphenolic content. J. Agric. Food Chem. 2002, 50, 5832–5836. [Google Scholar] [CrossRef]
- De Gaetano, G.; Di Castelnuovo, A.; Rotondo, S. Cardiovascular protective effect of moderate wine consumption: Evidence after the French Paradox. Sang. Thromb. Vaiss. 2005, 17, 47–60. [Google Scholar]
- De Nisco, M.; Manfra, M.; Bolognese, A.; Sofo, A.; Scopa, A.; Tenore, G.C.; Pagano, F.; Milite, C.; Russo, M.T. Nutraceutical properties and polyphenolic profile of berry skin and wine of Vitis vinifera L. (cv. Aglianico). Food Chem. 2013, 140, 623–629. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Kliewer, W.M.; Dokoozlian, N.K. Leaf area/crop weight ratios of grapevines: Influence on fruit composition and wine quality. Am. J. Enol. Vitic. 2005, 56, 170–181. [Google Scholar]
- Condurso, C.; Cincotta, F.; Tripodi, G.; Sparacio, A.; Giglio, D.M.L.; Sparla, S.; Verzera, A. Effects of cluster thinning on wine quality of Syrah cultivar (Vitis vinifera L.). Eur. Food Res. Technol. 2016, 242, 1719–1726. [Google Scholar] [CrossRef]
- Cortell, J.M.; Kennedy, J.A. Effect of shading on accumulation of flavonoid compounds in (Vitis vinifera L.) Pinot noir fruit and extraction in a model system. J. Agric. Food Chem. 2006, 54, 8510–8520. [Google Scholar] [CrossRef]
- Gil, M.; Esteruelas, M.; González, E.; Kontoudakis, N.; Jiménez, J.; Fort, F.; Canals, J.M.; Hermosín-Gutiérrez, I.; Zamora, F. Effect of two different treatments for reducing grape yield in Vitis vinifera cv Syrah on wine composition and quality: Berry thinning versus cluster thinning. J. Agric. Food Chem. 2013, 61, 4968–4978. [Google Scholar] [CrossRef] [PubMed]
- Garrido, I.; Uriarte, D.; Hernández, M.; Llerena, J.L.; Valdés, M.E.; Espinosa, F. The evolution of total phenolic compounds and antioxidant activities during ripening of grapes (Vitis vinifera L., cv. Tempranillo) grown in semiarid region: Effects of cluster thinning and water deficit. Int. J. Mol. Sci. 2016, 17, 1923. [Google Scholar] [CrossRef] [Green Version]
- Peña-Neira, Á.; Cáceres, A.; Pastenes, C. Low molecular weight phenolic and anthocyanin composition of grape skins from cv. Syrah (Vitis vinifera L.) in the Maipo Valley (Chile): Effect of clusters thinning and vineyard yield. Food Sci. Technol. Int. 2007, 13, 153–158. [Google Scholar] [CrossRef]
- Kok, D. Influences of pre- and post-veraison cluster thinning treatments on grape composition variables and monoterpene levels of Vitis vinifera L. cv. Sauvignon Blanc. J. Food Agric. Environ. 2011, 9, 22–26. [Google Scholar]
- Fanzone, M.; Zamora, F.; Jofré, V.; Assof, M.; Peña-Neira, Á. Phenolic composition of Malbec grape skins and seeds from valle de Uco (Mendoza, Argentina) during ripening. effect of cluster thinning. J. Agric. Food Chem. 2011, 59, 6120–6136. [Google Scholar] [CrossRef]
- García-Escudero, E.; López Martín, R.; Santamaría, P.; Zaballa, O.; Arbizu, J. El control del rendimiento por aclareo de racimos. Experiencias sobre el cv. “Mazuelo”. Zubía Monogr. 1995, 7, 53–64. [Google Scholar]
- Kontoudakis, N.; Esteruelas, M.; Fort, F.; Canals, J.M.; Zamora, F. Use of unripe grapes harvested during cluster thinning as a method for reducing alcohol content and pH of wine. Aust. J. Grape Wine Res. 2011, 17, 230–238. [Google Scholar] [CrossRef]
- Ryan, J.M.; Revilla, E. Anthocyanin composition of Cabernet Sauvignon and Tempranillo grapes at different stages of ripening. J. Agric. Food Chem. 2003, 51, 3372–3378. [Google Scholar] [CrossRef]
- Vian, M.A.; Tomao, V.; Coulomb, P.O.; Lacombe, J.M.; Dangles, O. Comparison of the anthocyanin composition during ripening of Syrah grapes grown using organic or conventional agricultural practices. J. Agric. Food Chem. 2006, 54, 5230–5235. [Google Scholar] [CrossRef]
- Coombe, B.G. The regulation of set and development of the grape berry. Acta Hortic. 1973, 34, 261–274. [Google Scholar] [CrossRef]
- Kyraleou, M.; Kallithraka, S.; Theodorou, N.; Teissedre, P.L.; Kotseridis, Y.; Koundouras, S. Changes in tannin composition of syrah grape skins and seeds during fruit ripening under contrasting water conditions. Molecules 2017, 22, 1453. [Google Scholar] [CrossRef] [Green Version]
- Navarro, S.; León, M.; Roca-Pérez, L.; Boluda, R.; García-Ferriz, L.; Pérez-Bermúdez, P.; Gavidia, I. Characterisation of Bobal and Crujidera grape cultivars, in comparison with Tempranillo and Cabernet Sauvignon: Evolution of leaf macronutrients and berry composition during grape ripening. Food Chem. 2008, 108, 182–190. [Google Scholar] [CrossRef]
- Fournand, D.; Vicens, A.; Sidhoum, L.; Souquet, J.M.; Moutounet, M.; Cheynier, V. Accumulation and extractability of grape skin tannins and anthocyanins at different advanced physiological stages. J. Agric. Food Chem. 2006, 54, 7331–7338. [Google Scholar] [CrossRef]
- Carrera, C.; Ruiz-Rodríguez, A.; Palma, M.; Barroso, C.G. Ultrasound assisted extraction of phenolic compounds from grapes. Anal. Chim. Acta 2012, 732, 100–104. [Google Scholar] [CrossRef]
- Mercurio, M.D.; Smith, P.A. Tannin Quantification in Red Grapes and Wine: Comparison of Polysaccharide- and Protein-based Tannin. J. Agric. Food Chem. 2008, 56, 5528–5537. [Google Scholar] [CrossRef]
- Sarneckis, C.J.; Dambergs, R.G.; Jones, P.; Mercurio, M.; Herderich, M.J.; Smith, P.A. Quantification of condensed tannins by precipitation with methyl cellulose: Development and validation of an optimised tool for grape and wine analysis. Aust. J. Grape Wine Res. 2006, 12, 39–49. [Google Scholar] [CrossRef]
- Carmona-Jiménez, Y.; García-Moreno, M.V.; Igartuburu, J.M.; Garcia Barroso, C. Simplification of the DPPH assay for estimating the antioxidant activity of wine and wine by-products. Food Chem. 2014, 165, 198–204. [Google Scholar] [CrossRef]
- Thomasset, S.C.; Berry, D.P.; Garcea, G.; Marczylo, T.; Steward, W.P.; Gescher, A.J. Dietary polyphenolic phytochemicals—Promising cancer chemopreventive agents in humans? A review of their clinical properties. Int. J. Cancer 2007, 120, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.A.; Troup, G.J.; Pilbrow, J.R.; Hutton, D.R.; Hewitt, D.; Hunter, C.R.; Ristic, R.; Iland, P.G.; Jones, G.P. Development of seed polyphenols in berries from Vitis vinifera L. cv. Shiraz. Aust. J. Grape Wine Res. 2000, 6, 244–254. [Google Scholar] [CrossRef]
- Dudoit, A.; Benbouguerra, N.; Richard, T.; Hornedo-Ortega, R.; Valls-Onayet, J.; Coussot, G.; Saucier, C. α-Glucosidase Inhibitory Activity of Tannat Grape Phenolic Extracts in Relation to Their Ripening Stages. Biomolecules 2020, 10, 1088. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Mu, L.; Yan, G.L.; Liang, N.N.; Pan, Q.H.; Wang, J.; Reeves, M.J.; Duan, C.Q. Biosynthesis of anthocyanins and their regulation in colored grapes. Molecules 2010, 15, 9057–9091. [Google Scholar] [CrossRef] [Green Version]


| Variety | pH | Brix Degree | Total Acidity |
|---|---|---|---|
| Tempranillo | |||
| LRS | 2.81 ± 0.02 a | 12.11 ± 0.00 a | 24.59 ± 0.20 c |
| IRS | 3.17 ± 0.06 b | 15.76 ± 0.01 b | 7.78 ± 0.45 b |
| HRS | 3.03 ± 0.01 b | 19.09 ± 0.01 c | 6.80 ± 1.23 a |
| Cabernet Sauvignon | |||
| LRS | 2.71 ± 0.02 a | 9.75 ± 0.00 a | 32.09 ± 0.54 c |
| IRS | 3.02 ± 0.03 b | 16.06 ± 0.00 b | 15.46 ± 0.41 b |
| HRS | 3.10 ± 0.03 b | 20.04 ± 0.00 c | 9.46 ± 0.37 a |
| Syrah | |||
| LRS | 2.73 ± 0.01 a | 10.11 ± 0.00 a | 31.39 ± 0.46 c |
| IRS | 3.05 ± 0.04 b | 15.38 ± 0.01 b | 15.22 ± 0.96 b |
| HRS | 3.06 ± 0.03 b | 17.97 ± 0.00 c | 9.60 ± 1.72 a |
| Variety | TAC | TTC | TPC | EC20 |
|---|---|---|---|---|
| Tempranillo | ||||
| LRS | 130 ± 18 a | 12.52 ± 0.24 c | 11.94 ± 0.16 b | 4.37 ± 0.04 a |
| IRS | 312 ± 8 b | 8.32 ± 0.44 b | 9.69 ± 0.37 a | 4.09 ± 0.04 a |
| HRS | 995 ± 60 c | 7.63 ± 0.26 a | 11.44 ± 0.26 b | 5.72 ± 0.21 b |
| Cabernet Sauvignon | ||||
| LRS | 41 ± 9 a | 14.00 ± 0.19 c | 12.88 ± 0.31 b | 3.61 ± 0.05 a |
| IRS | 293 ± 23 b | 12.03 ± 0.31 b | 11.25 ± 0.14 a | 2.47 ± 0.15 a |
| HRS | 1100 ± 61 c | 6.34 ± 0.38 a | 11.11 ± 0.38 b | 6.02 ± 0.13 b |
| Syrah | ||||
| LRS | 17 ± 4 a | 9.10 ± 0.15 c | 10.92 ± 0.12 b | 5.00 ± 0.02 a |
| IRS | 349 ± 21 b | 8.66 ± 0.36 b | 9.65 ± 0.51 a | 3.94 ± 0.05 a |
| HRS | 675 ± 11 c | 6.45 ± 0.12 a | 11.41 ± 0.34 b | 6.85 ± 0.39 b |
| TAC | TTC | TPC | EC20 | |
|---|---|---|---|---|
| TAC | −0.7373 (0.0005) | −0.0960 (0.7046) | 0.6452 (0.0038) | |
| TTC | −0.7373 (0.0005) | 0.5469 (0.0188) | −0.7616 (0.0002) | |
| TPC | −0.0960 (0.7046) | 0.5469 (0.0188) | 0.0335 (0.8949) | |
| EC20 | 0.6452 (0.0038) | −0.7616 (0.0002) | 0.0335 (0.8949) |
| Phenolic Compounds | TM LRS | TM IRS | TM HRS | CS LRS | CS IRS | CS HRS | SY LRS | SY IRS | SY HRS |
|---|---|---|---|---|---|---|---|---|---|
| Benzoic acids, cinnamic acids, and flavan-3-ols | |||||||||
| Protocatechuic acid | 296.89 ± 16.08 b | 39.55 ± 0.75 a | 58.65 ± 1.85 a | 325.67 ± 4.51 b | 70.84 ± 24.16 a | 107.78 ± 7.38 a | 151.42 ± 5.69 b | 34.97 ± 0.78 a | 105.56 ± 6.72 a |
| Tyrosol | 513.56 ± 29.62 b | 44.22 ± 2.71 a | N.Q. | 468.04 ± 40.79 b | 85.79 ± 6.96 a | N.Q. | 307.14 ± 8.22 b | 51.38 ± 0.55 a | N.Q. |
| Vanillic acid | 16.98 ± 0.06 b | 5.33 ± 0.40 a | N.Q. | 22.06 ± 0.14 b | 10.96 ± 0.08 a | N.Q. | 15.54 ± 0.17 b | 6.49 ± 0.13 a | N.Q. |
| Syringic acid | N.Q. | N.Q. | 1.84 ± 0.11 | N.Q. | N.Q. | N.Q. | N.Q. | N.Q. | N.Q. |
| Chlorogenic acid | 14.11 ± 0.53 a | 14.03 ± 4.74 a | N.Q. | 11.22 ± 1.56 a | 11.12 ± 1.78 a | N.D. | N.Q. | 10.63 ± 1.68 a | N.Q. |
| Caffeic acid | 81.45 ± 4.74 b | 6.44 ± 0.64 a | 21.09 ± 0.90 a | 76.94 ± 0.44 b | 12.23 ± 0.17 a | 21.04 ± 2.29 a | 42.30 ± 0.70 b | N.Q. | 12.36 ± 1.33 a |
| Ferulic acid | 6.90 ± 0.10 b | 6.25 ± 0.24 a | 21.16 ± 0.75 c | 7.56 ± 0.06 b | 6.36 ± 0.50 a | N.D. | 6.55 ± 0.10 b | 5.85 ± 0.70 a | 21.19 ± 0.90 c |
| Catechin | 1689.54 ± 116.41 c | 111.12 ± 1.20 b | 65.86 ± 3.24 a | 2881.42 ± 163.58 c | 1026.81 ± 76.80 b | 168.98 ± 2.05 a | 1493.32 ± 85.92 c | 357.35 ± 10.53 b | 94.63 ± 6.51 a |
| Epicatechin | 1091.25 ± 10.91 b | 64.25 ± 4.58 a | 39.21 ± 2.22 a | 2279.02 ± 5.58 b | 380.51 ± 30.14 a | 88.31 ± 1.26 a | 2297.10 ± 42.49 b | 281.97 ± 16.28 a | 90.44 ± 1.40 a |
| Flavonols | |||||||||
| Myricetin-3-glucuronide | 9.65 ± 0.12 a | 12.53 ± 0.50 b | 20.13 ± 1.35 c | 8.85 ± 0.25 a | 11.27 ± 0.23 b | 28.34 ± 0.77 c | 8.70 ± 0.30 a | 11.98 ± 1.43 b | 22.53 ± 0.89 c |
| Myricetin-3-glucoside | 13.62 ± 0.76 a | 15.12 ± 1.16 a | 16.03 ± 1.22 b | 17.05 ± 0.35 a | 15.09 ± 0.13 a | 21.36 ± 2.21 b | 15.67 ± 1.53 a | 10.63 ± 1.24 a | 17.57 ± 0.47 b |
| Quercetin-3-glucuronide | 8.65 ± 0.12 b | 7.42 ± 0.21 a | 8.47 ± 0.05 b | 7.99 ± 0.16 b | 6.97 ± 0.53 a | 8.50 ± 0.50 b | 8.18 ± 0.26 b | 6.90 ± 0.98 a | 8.17 ± 0.52 b |
| Quercetin-3-rutinoside | 8.64 ± 0.18 b | 8.18 ± 0.00 a | 9.67 ± 0.31 c | 8.75 ± 0.36 b | 7.51 ± 0.19 a | 9.38 ± 0.61 c | 8.63 ± 0.03 b | 7.68 ± 1.41 a | 9.08 ± 0.58 c |
| Kaempherol-3-glucoside | 7.95 ± 0.18 a | 13.00 ± 0.63 b | 21.76 ± 1.05 c | 8.25 ± 0.57 a | 10.08 ± 0.64 b | 17.43 ± 1.17 c | 8.07 ± 0.21 a | 12.41 ± 0.80 b | 19.51 ± 0.15 c |
| Anthocyanins | |||||||||
| Delphinidin-3-glucoside | 15.81 ± 0.07 a | 22.09 ± 0.78 a | 58.32 ± 4.97 b | 7.64 ± 0.68 a | 18.29 ± 0.28 a | 44.96 ± 2.79 b | 4.88 ± 0.16 a | 7.94 ± 0.12 a | 21.20 ± 0.62 b |
| Cyanidin-3-glucoside | 11.06 ± 0.26 a | 5.94 ± 0.18 a | 21.41 ± 0.47 b | 6.26 ± 0.00 a | 4.58 ± 0.04 a | 18.21 ± 0.19 b | 4.07 ± 0.05 a | 4.12 ± 0.19 a | 16.26 ± 0.39 b |
| Petunidin-3-glucoside | 15.65 ± 0.90 a | 23.78 ± 0.80 a | 64.67 ± 6.25 b | 6.89 ± 0.26 a | 15.90 ± 0.42 a | 38.51 ± 1.12 b | 5.93 ± 0.11 a | 13.45 ± 0.25 a | 24.79 ± 0.55 b |
| Peonidin-3-glucoside | 19.63 ± 0.34 a | 15.01 ± 0.67 a | 89.60 ± 2.87 b | 6.66 ± 0.25 a | 18.24 ± 0.45 a | 109.35 ± 1.22 b | 5.73 ± 0.10 a | 24.22 ± 1.73 a | 78.53 ± 0.21 b |
| Malvidin-3-glucoside | 41.34 ± 1.89 a | 97.19 ± 2.33 b | 328.23 ± 19.87 c | 19.13 ± 0.03 a | 93.21 ± 3.15 b | 335.20 ± 11.38 c | 17.74 ± 0.43 a | 100.40 ± 2.91 b | 171.40 ± 0.99 c |
| Malvidin-3-acetylglucoside | 9.69 ± 0.05 a | 34.48 ± 0.78 a | 69.50 ± 1.26 b | 16.60 ± 0.21 a | 109.61 ± 1.65 a | 265.41 ± 13.01 b | 8.39 ± 0.58 a | 60.98 ± 1.79 a | 99.30 ± 2.05 b |
| Malvidin-3-cis-p-coumaroylglucoside | 8.76 ± 0.12 a | 21.42 ± 1.14 b | 62.01 ± 4.02 c | 5.57 ± 0.13 a | 11.99 ± 1.10 b | 35.24 ± 0.74 c | 5.94 ± 0.21 a | 29.41 ± 1.70 b | 48.80 ± 1.60 c |
| Malvidin-3-caffeoylglucoside | 5.06 ± 0.24 a | 28.85 ± 1.25 b | 43.88 ± 5.49 c | 4.50 ± 0.05 a | 12.97 ± 1.24 b | 28.37 ± 1.44 c | 4.78 ± 0.13 a | 33.75 ± 1.01 b | 32.32 ± 3.34 c |
| Petunidin-3-p-coumaroylglucoside | 7.92 ± 0.54 a | 26.03 ± 1.45 b | 59.79 ± 0.85 c | 5.02 ± 0.20 a | 10.88 ± 2.31 b | 27.09 ± 2.27 c | 5.96 ± 0.42 a | 19.23 ± 4.16 b | 37.03 ± 0.66 c |
| Malvidin-3-trans-p-coumaroylglucoside | 10.62 ± 0.19 a | 70.78 ± 5.52 b | 224.78 ± 8.06 c | 6.52 ± 0.10 a | 30.90 ± 2.14 b | 162.55 ± 15.36 c | 7.77 ± 0.54 a | 58.58 ± 2.28 b | 187.70 ± 2.76 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carmona-Jiménez, Y.; Palma, M.; Guillén-Sánchez, D.A.; García-Moreno, M.V. Study of the Cluster Thinning Grape as a Source of Phenolic Compounds and Evaluation of Its Antioxidant Potential. Biomolecules 2021, 11, 227. https://0-doi-org.brum.beds.ac.uk/10.3390/biom11020227
Carmona-Jiménez Y, Palma M, Guillén-Sánchez DA, García-Moreno MV. Study of the Cluster Thinning Grape as a Source of Phenolic Compounds and Evaluation of Its Antioxidant Potential. Biomolecules. 2021; 11(2):227. https://0-doi-org.brum.beds.ac.uk/10.3390/biom11020227
Chicago/Turabian StyleCarmona-Jiménez, Yolanda, Miguel Palma, Dominico A. Guillén-Sánchez, and M. Valme García-Moreno. 2021. "Study of the Cluster Thinning Grape as a Source of Phenolic Compounds and Evaluation of Its Antioxidant Potential" Biomolecules 11, no. 2: 227. https://0-doi-org.brum.beds.ac.uk/10.3390/biom11020227