177Lu-PSMA-617 Therapy in Mice, with or without the Antioxidant α1-Microglobulin (A1M), Including Kidney Damage Assessment Using 99mTc-MAG3 Imaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Radiolabeling
2.2. Cell Culturing
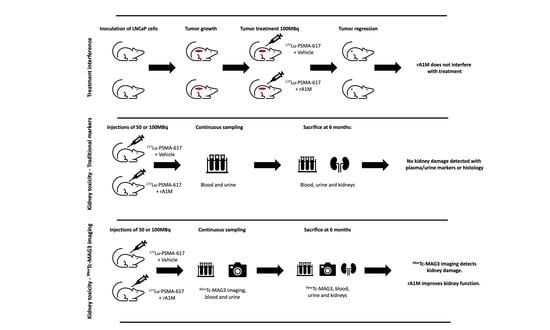
2.3. In Vivo Studies
2.4. [99mTc]Tc-MAG3 and [177Lu]Lu-PSMA-617 Imaging
2.5. WBC, RBC and Platelet Counts
2.6. Functional Urine and Serum/Plasma Markers
2.7. RT-qPCR in Kidney Tissue
2.8. Statistical Analysis of Functional Urine and Serum/Plasma Markers and RT-qPCR
2.9. Histopathology
2.10. Dosimetry
3. Results
3.1. [177Lu]Lu-PSMA-617 and [99mTc]Tc-MAG3 Imaging
3.2. Biochemical Kidney Damage Markers, Gene Expression and Histopathology
3.3. Dosimetry
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- James, N.D.; Spears, M.R.; Clarke, N.W.; Dearnaley, D.P.; De Bono, J.S.; Gale, J.; Hetherington, J.; Hoskin, P.J.; Jones, R.J.; Laing, R.; et al. Survival with Newly Diagnosed Metastatic Prostate Cancer in the “Docetaxel Era”: Data from 917 Patients in the Control Arm of the STAMPEDE Trial (MRC PR08, CRUK/06/019). Eur. Urol. 2015, 67, 1028–1038. [Google Scholar] [CrossRef] [Green Version]
- Tolkach, Y.; Gevensleben, H.; Bundschuh, R.; Koyun, A.; Huber, D.; Kehrer, C.; Hecking, T.; Keyver-Paik, M.D.; Kaiser, C.; Ahmadzadehfar, H.; et al. Prostate-specific membrane antigen in breast cancer: A comprehensive evaluation of expression and a case report of radionuclide therapy. Breast Cancer Res. Treat. 2018, 169, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Heitkötter, B.; Steinestel, K.; Trautmann, M.; Grünewald, I.; Barth, P.; Gevensleben, H.; Bögemann, M.; Wardelmann, E.; Hartmann, W.; Rahbar, K.; et al. Neovascular PSMA expression is a common feature in malignant neoplasms of the thyroid. Oncotarget 2018, 9, 9867–9874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, S.S.; Reuter, V.E.; Heston, W.D.; Gaudin, P.B. Metastatic renal cell carcinoma neovasculature expresses prostate-specific membrane antigen. Urology 2001, 57, 801–805. [Google Scholar] [CrossRef]
- Rahbar, K.; Ahmadzadehfar, H.; Kratochwil, C.; Haberkorn, U.; Schäfers, M.; Essler, M.; Baum, R.P.; Kulkarni, H.R.; Schmidt, M.; Drzezga, A.; et al. German Multicenter Study Investigating 177Lu-PSMA-617 Radioligand Therapy in Advanced Prostate Cancer Patients. J. Nucl. Med. 2017, 58, 85–90. [Google Scholar] [CrossRef] [Green Version]
- Hofman, M.S.; Violet, J.; Hicks, R.J.; Ferdinandus, J.; Thang, S.P.; Akhurst, T.; Iravani, A.; Kong, G.; Ravi Kumar, A.; Murphy, D.G.; et al. [(177)Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): A single-centre, single-arm, phase 2 study. Lancet Oncol. 2018, 19, 825–833. [Google Scholar] [CrossRef]
- Emmett, L.; Crumbaker, M.; Ho, B.; Willowson, K.; Eu, P.; Ratnayake, L.; Epstein, R.; Blanksby, A.; Horvath, L.; Guminski, A.; et al. Results of a Prospective Phase 2 Pilot Trial of (177)Lu-PSMA-617 Therapy for Metastatic Castration-Resistant Prostate Cancer Including Imaging Predictors of Treatment Response and Patterns of Progression. Clin. Genitourin. Cancer 2019, 17, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Bodei, L.; Ćwikla, J.B.; Kidd, M.; Modlin, I.M. The role of peptide receptor radionuclide therapy in advanced/metastatic thoracic neuroendocrine tumors. J. Thorac. Dis. 2017, 9, S1511–S1523. [Google Scholar] [CrossRef] [Green Version]
- Valkema, R.; Pauwels, S.A.; Kvols, L.K.; Kwekkeboom, D.J.; Jamar, F.; de Jong, M.; Barone, R.; Walrand, S.; Kooij, P.P.; Bakker, W.H.; et al. Long-term follow-up of renal function after peptide receptor radiation therapy with (90)Y-DOTA(0),Tyr(3)-octreotide and (177)Lu-DOTA(0), Tyr(3)-octreotate. J. Nucl. Med. 2005, 46 (Suppl. 1), 83s–91s. [Google Scholar]
- Bodei, L.; Cremonesi, M.; Ferrari, M.; Pacifici, M.; Grana, C.M.; Bartolomei, M.; Baio, S.M.; Sansovini, M.; Paganelli, G. Long-term evaluation of renal toxicity after peptide receptor radionuclide therapy with 90Y-DOTATOC and 177Lu-DOTATATE: The role of associated risk factors. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 1847–1856. [Google Scholar] [CrossRef]
- Bodei, L.; Kidd, M.; Paganelli, G.; Grana, C.M.; Drozdov, I.; Cremonesi, M.; Lepensky, C.; Kwekkeboom, D.J.; Baum, R.P.; Krenning, E.P.; et al. Long-term tolerability of PRRT in 807 patients with neuroendocrine tumours: The value and limitations of clinical factors. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 5–19. [Google Scholar] [CrossRef]
- Rolleman, E.J.; Melis, M.; Valkema, R.; Boerman, O.C.; Krenning, E.P.; de Jong, M. Kidney protection during peptide receptor radionuclide therapy with somatostatin analogues. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 1018–1031. [Google Scholar] [CrossRef]
- Emami, B.; Purdy, J.; Manolis, J.; Barest, G.; Cheng, E.; Coia, L.; Doppke, K.; Galvin, J.; LoSasso, T.; Matthews, J. Three-dimensional treatment planning for lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 1991, 21, 217–227. [Google Scholar] [CrossRef]
- Bergsma, H.; Konijnenberg, M.W.; van der Zwan, W.A.; Kam, B.L.R.; Teunissen, J.J.M.; Kooij, P.P.; Mauff, K.A.L.; Krenning, E.P.; Kwekkeboom, D.J. Nephrotoxicity after PRRT with (177)Lu-DOTA-octreotate. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1802–1811. [Google Scholar] [CrossRef] [Green Version]
- Åkerström, B.; Maghzal, G.J.; Winterbourn, C.C.; Kettle, A.J. The lipocalin α1-microglobulin has radical scavenging activity. J. Biol. Chem. 2007, 282, 31493–31503. [Google Scholar] [CrossRef] [Green Version]
- Allhorn, M.; Klapyta, A.; Åkerström, B. Redox properties of the lipocalin α1-microglobulin: Reduction of cytochrome c, hemoglobin, and free iron. Free Radic. Biol. Med. 2005, 38, 557–567. [Google Scholar] [CrossRef] [Green Version]
- Larsson, J.; Allhorn, M.; Kerstrom, B. The lipocalin alpha(1)-microglobulin binds heme in different species. Arch. Biochem. Biophys. 2004, 432, 196–204. [Google Scholar] [CrossRef]
- Tejler, L.; Eriksson, S.; Grubb, A.; Åstedt, B. Production of protein HC by human fetal liver explants. Biochim. Biophys. Acta (BBA) Gen. Subj. 1978, 542, 506–514. [Google Scholar] [CrossRef]
- DeMars, D.D.; Katzmann, J.A.; Kimlinger, T.K.; Calore, J.D.; Tracy, R.P. Simultaneous measurement of total and IgA-conjugated alpha 1-microglobulin by a combined immunoenzyme/immunoradiometric assay technique. Clin. Chem. 1989, 35, 766–772. [Google Scholar] [CrossRef]
- Wester, L.; Fast, J.; Labuda, T.; Cedervall, T.; Wingårdh, K.; Olofsson, T.; Åkerström, B. Carbohydrate groups of α1-microglobulin are important for secretion and tissue localization but not for immunological properties. Glycobiology 2000, 10, 891–900. [Google Scholar] [CrossRef] [Green Version]
- Larsson, J.; Wingardh, K.; Berggard, T.; Davies, J.R.; Logdberg, L.; Strand, S.E.; Akerstrom, B. Distribution of iodine 125-labeled alpha1-microglobulin in rats after intravenous injection. J. Lab. Clin. Med. 2001, 137, 165–175. [Google Scholar] [CrossRef]
- Olsson, M.G.; Olofsson, T.; Tapper, H.; Akerstrom, B. The lipocalin alpha1-microglobulin protects erythroid K562 cells against oxidative damage induced by heme and reactive oxygen species. Free Radic. Res. 2008, 42, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Kristiansson, A.; Bergwik, J.; Alattar, A.G.; Flygare, J.; Gram, M.; Hansson, S.R.; Olsson, M.L.; Storry, J.R.; Allhorn, M.; Åkerström, B. Human radical scavenger α(1)-microglobulin protects against hemolysis in vitro and α(1)-microglobulin knockout mice exhibit a macrocytic anemia phenotype. Free Radic. Biol. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Akerstrom, B.; Rosenlof, L.; Hagerwall, A.; Rutardottir, S.; Ahlstedt, J.; Johansson, M.E.; Erlandsson, L.; Allhorn, M.; Gram, M. rA1M-035, a Physicochemically Improved Human Recombinant alpha1-Microglobulin, Has Therapeutic Effects in Rhabdomyolysis-Induced Acute Kidney Injury. Antioxid. Redox Signal. 2019, 30, 489–504. [Google Scholar] [CrossRef] [Green Version]
- Wester-Rosenlof, L.; Casslen, V.; Axelsson, J.; Edstrom-Hagerwall, A.; Gram, M.; Holmqvist, M.; Johansson, M.E.; Larsson, I.; Ley, D.; Marsal, K.; et al. A1M/alpha1-microglobulin protects from heme-induced placental and renal damage in a pregnant sheep model of preeclampsia. PLoS ONE 2014, 9, e86353. [Google Scholar] [CrossRef]
- Naav, A.; Erlandsson, L.; Axelsson, J.; Larsson, I.; Johansson, M.; Wester-Rosenlof, L.; Morgelin, M.; Casslen, V.; Gram, M.; Akerstrom, B.; et al. A1M Ameliorates Preeclampsia-Like Symptoms in Placenta and Kidney Induced by Cell-Free Fetal Hemoglobin in Rabbit. PLoS ONE 2015, 10, e0125499. [Google Scholar] [CrossRef] [Green Version]
- Sverrisson, K.; Axelsson, J.; Rippe, A.; Gram, M.; Akerstrom, B.; Hansson, S.R.; Rippe, B. Extracellular fetal hemoglobin induces increases in glomerular permeability: Inhibition with alpha1-microglobulin and tempol. Am. J. Physiol. Ren. Physiol. 2014, 306, F442–F448. [Google Scholar] [CrossRef] [Green Version]
- Kristiansson, A.; Davidsson, S.; Johansson, M.E.; Piel, S.; Elmér, E.; Hansson, M.J.; Åkerström, B.; Gram, M. α1-Microglobulin (A1M) Protects Human Proximal Tubule Epithelial Cells from Heme-Induced Damage In Vitro. Int. J. Mol. Sci. 2020, 21, 5825. [Google Scholar] [CrossRef]
- Olsson, M.G.; Nilsson, E.J.C.; Rutardóttir, S.; Paczesny, J.; Pallon, J.; Åkerström, B. Bystander Cell Death and Stress Response is Inhibited by the Radical Scavenger α1-Microglobulin in Irradiated Cell Cultures. Radiat. Res. 2010, 174, 590–600. [Google Scholar] [CrossRef]
- Ahlstedt, J.; Tran, T.A.; Strand, F.; Holmqvist, B.; Strand, S.-E.; Gram, M.; Åkerström, B. Biodistribution and pharmacokinetics of recombinant α1-microglobulin and its potential use in radioprotection of kidneys. Am. J. Nucl. Med. Mol. Imaging 2015, 5, 333–347. [Google Scholar]
- Ahlstedt, J.; Tran, T.A.; Strand, S.-E.; Gram, M.; Åkerström, B. Human Anti-Oxidation Protein A1M—A Potential Kidney Protection Agent in Peptide Receptor Radionuclide Therapy. Int. J. Mol. Sci. 2015, 16, 30309–30320. [Google Scholar] [CrossRef]
- Kristiansson, A.; Ahlstedt, J.; Holmqvist, B.; Brinte, A.; Tran, T.A.; Forssell-Aronsson, E.; Strand, S.E.; Gram, M.; Akerstrom, B. Protection of Kidney Function with Human Antioxidation Protein alpha1-Microglobulin in a Mouse (177)Lu-DOTATATE Radiation Therapy Model. Antioxid. Redox Signal. 2019, 30, 1746–1759. [Google Scholar] [CrossRef] [Green Version]
- Andersson, C.K.; Shubbar, E.; Schüler, E.; Åkerström, B.; Gram, M.; Forssell-Aronsson, E.B. Recombinant α1-Microglobulin Is a Potential Kidney Protector in 177Lu-Octreotate Treatment of Neuroendocrine Tumors. J. Nucl. Med. 2019, 60, 1600–1604. [Google Scholar] [CrossRef]
- Kratochwil, C.; Giesel, F.L.; Stefanova, M.; Benešová, M.; Bronzel, M.; Afshar-Oromieh, A.; Mier, W.; Eder, M.; Kopka, K.; Haberkorn, U. PSMA-Targeted Radionuclide Therapy of Metastatic Castration-Resistant Prostate Cancer with 177Lu-Labeled PSMA-617. J. Nucl. Med. 2016, 57, 1170–1176. [Google Scholar] [CrossRef] [Green Version]
- Gram, M.; Anderson, U.D.; Johansson, M.E.; Edstrom-Hagerwall, A.; Larsson, I.; Jalmby, M.; Hansson, S.R.; Akerstrom, B. The Human Endogenous Protection System against Cell-Free Hemoglobin and Heme Is Overwhelmed in Preeclampsia and Provides Potential Biomarkers and Clinical Indicators. PLoS ONE 2015, 10, e0138111. [Google Scholar] [CrossRef] [Green Version]
- Sánchez, D.; Martínez, S.; Lindqvist, A.; Åkerström, B.; Falkenberg, C. Expression of the AMBP gene transcript and its two protein products, α1-microglobulin and bikunin, in mouse embryogenesis. Mech. Dev. 2002, 117, 293–298. [Google Scholar] [CrossRef]
- Greenwood, F.C.; Hunter, W.M.; Glover, J.S. The preparation of I-131-labelled human growth hormone of high specific radioactivity. Biochem. J. 1963, 89, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Umbricht, C.A.; Köster, U.; Bernhardt, P.; Gracheva, N.; Johnston, K.; Schibli, R.; Van der Meulen, N.P.; Müller, C. Alpha-PET for Prostate Cancer: Preclinical investigation using 149 Tb-PSMA-617. Sci. Rep. 2019, 9, 17800. [Google Scholar] [CrossRef] [Green Version]
- Tantawy, M.N.; Jiang, R.; Wang, F.; Takahashi, K.; Peterson, T.E.; Zemel, D.; Hao, C.-M.; Fujita, H.; Harris, R.C.; Quarles, C.C. Assessment of renal function in mice with unilateral ureteral obstruction using 99 m Tc-MAG3 dynamic scintigraphy. BMC Nephrol. 2012, 13, 168. [Google Scholar] [CrossRef] [Green Version]
- Larsson, E.; Ljungberg, M.; Strand, S.-E.; Jönsson, B.-A. Monte Carlo calculations of absorbed doses in tumours using a modified MOBY mouse phantom for pre-clinical dosimetry studies. Acta Oncol. 2011, 50, 973–980. [Google Scholar] [CrossRef]
- Benešová, M.; Schäfer, M.; Bauder-Wüst, U.; Afshar-Oromieh, A.; Kratochwil, C.; Mier, W.; Haberkorn, U.; Kopka, K.; Eder, M. Preclinical evaluation of a tailor-made DOTA-conjugated PSMA inhibitor with optimized linker moiety for imaging and endoradiotherapy of prostate cancer. J. Nucl. Med. 2015, 56, 914–920. [Google Scholar] [CrossRef] [Green Version]
- Kratochwil, C.; Schmidt, K.; Afshar-Oromieh, A.; Bruchertseifer, F.; Rathke, H.; Morgenstern, A.; Haberkorn, U.; Giesel, F.L. Targeted alpha therapy of mCRPC: Dosimetry estimate of 213 Bismuth-PSMA-617. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 31–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kabasakal, L.; Toklu, T.; Yeyin, N.; Demirci, E.; Abuqbeitah, M.; Ocak, M.; Aygün, A.; Karayel, E.; Pehlivanoğlu, H.; Selçuk, N.A. Lu-177-PSMA-617 prostate-specific membrane antigen inhibitor therapy in patients with castration-resistant prostate cancer: Stability, bio-distribution and dosimetry. Mol. Imaging Radionucl. Ther. 2017, 26, 62. [Google Scholar] [CrossRef]
- Scarpa, L.; Buxbaum, S.; Kendler, D.; Fink, K.; Bektic, J.; Gruber, L.; Decristoforo, C.; Uprimny, C.; Lukas, P.; Horninger, W. The 68 Ga/177 Lu theragnostic concept in PSMA targeting of castration-resistant prostate cancer: Correlation of SUV max values and absorbed dose estimates. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 788–800. [Google Scholar] [CrossRef] [PubMed]
- Violet, J.; Jackson, P.; Ferdinandus, J.; Sandhu, S.; Akhurst, T.; Iravani, A.; Kong, G.; Kumar, A.R.; Thang, S.P.; Eu, P. Dosimetry of 177Lu-PSMA-617 in metastatic castration-resistant prostate cancer: Correlations between pretherapeutic imaging and whole-body tumor dosimetry with treatment outcomes. J. Nucl. Med. 2019, 60, 517–523. [Google Scholar] [CrossRef] [Green Version]
- Roberts, J.; Chen, B.; Curtis, L.M.; Agarwal, A.; Sanders, P.W.; Zinn, K.R. Detection of early changes in renal function using 99mTc-MAG3 imaging in a murine model of ischemia-reperfusion injury. Am. J. Physiol. Ren. Physiol. 2007, 293, F1408–F1412. [Google Scholar] [CrossRef] [Green Version]
- Matteucci, F.; Mezzenga, E.; Caroli, P.; Di Iorio, V.; Sarnelli, A.; Celli, M.; Fantini, L.; Moretti, A.; Galassi, R.; De Giorgi, U. Reduction of 68 Ga-PSMA renal uptake with mannitol infusion: Preliminary results. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 2189–2194. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.T. Radionuclides in nephrourology, part 1: Radiopharmaceuticals, quality control, and quantitative indices. J. Nucl. Med. 2014, 55, 608–615. [Google Scholar] [CrossRef] [Green Version]





| Strain | Xenografted | 100 MBq + rA1M | 100MBq + Vehicle | 50 MBq + rA1M | 50 Mbq + Vehicle |
|---|---|---|---|---|---|
| BALB/cAnNRj | No | n = 15 | n = 15 | n = 15 | n = 15 |
| BALB/cAnNRj-Foxn1nu/nu | LNCaP cells | n = 14 | n = 14 | - | - |
| [99mTc]Tc-MAG3 SPECT | [177Lu]Lu-PSMA-617 SPECT | Blood Cell Counts | RT-qPCR | Functional Urine and Serum/Plasma Markers | |
| BALB/cAnNRj | Yes | Yes (kidney dosimetry) | No | Yes | Yes |
| BALB/cAnNRj-Foxn1nu/nu | No | Yes (tumor uptake) | Yes | Yes | Yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kristiansson, A.; Örbom, A.; Ahlstedt, J.; Karlsson, H.; Zedan, W.; Gram, M.; Åkerström, B.; Strand, S.-E.; Altai, M.; Strand, J.; et al. 177Lu-PSMA-617 Therapy in Mice, with or without the Antioxidant α1-Microglobulin (A1M), Including Kidney Damage Assessment Using 99mTc-MAG3 Imaging. Biomolecules 2021, 11, 263. https://0-doi-org.brum.beds.ac.uk/10.3390/biom11020263
Kristiansson A, Örbom A, Ahlstedt J, Karlsson H, Zedan W, Gram M, Åkerström B, Strand S-E, Altai M, Strand J, et al. 177Lu-PSMA-617 Therapy in Mice, with or without the Antioxidant α1-Microglobulin (A1M), Including Kidney Damage Assessment Using 99mTc-MAG3 Imaging. Biomolecules. 2021; 11(2):263. https://0-doi-org.brum.beds.ac.uk/10.3390/biom11020263
Chicago/Turabian StyleKristiansson, Amanda, Anders Örbom, Jonas Ahlstedt, Helena Karlsson, Wahed Zedan, Magnus Gram, Bo Åkerström, Sven-Erik Strand, Mohamed Altai, Joanna Strand, and et al. 2021. "177Lu-PSMA-617 Therapy in Mice, with or without the Antioxidant α1-Microglobulin (A1M), Including Kidney Damage Assessment Using 99mTc-MAG3 Imaging" Biomolecules 11, no. 2: 263. https://0-doi-org.brum.beds.ac.uk/10.3390/biom11020263