Electro-Acupuncture Alleviates Cisplatin-Induced Anorexia in Rats by Modulating Ghrelin and Monoamine Neurotransmitters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Description of Study
2.3. Anorexia Model
2.4. Measurement of Body Weight and Daily Food Intake
2.5. Electro-acupuncture Treatment
2.6. Quantitative Analysis of Monoamine Neurotransmitters in Plasma
2.7. Measurement of Gastrointestinal Hormone Levels in the Plasma
2.8. RT-qPCR
2.9. Western Blotting Analysis
2.10. Statistical Analysis
3. Results
3.1. Evaluation of the Optimal EA Condition to Alleviate Cisplatin-Induced Anorexia
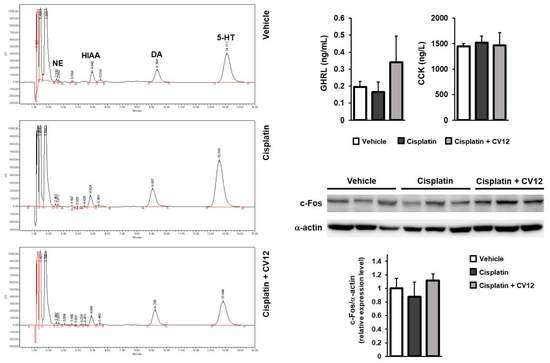
3.2. Plasma Monoamine Neurotransmitters’ Concentrations
3.3. Gastrointestinal Hormone Levels in Plasma
3.4. mRNA Expression Levels of GHRL, NPY, and POMC
3.5. c-Fos Expression in the NTS
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-HIAA | 5-hydroxyindoleacetic acid |
| 5-HT | 5-hydroxytryptamine |
| CCK | cholecystokinin |
| DA | dopamine |
| EA | electro-acupuncture |
| ELISA | enzyme-linked immunosorbent assay |
| GHRL | ghrelin |
| HPLC-ECD | high performance liquid chromatography-electrochemical detection |
| i.p. | intraperitoneal |
| NE | norepinephrine |
| NPY | neuropeptide Y |
| NTS | nucleus tractus solitarii |
| POMC | pro-opiomelanocortin |
| qRT-PCR | quantitative reverse transcription - polymerase chain reaction |
References
- Jung, K.W.; Won, Y.J.; Kong, H.J.; Lee, E.S. Cancer Statistics in Korea: Incidence, Mortality, Survival, and Prevalence in 2016. Cancer Res. Treat. 2019, 51, 417–430. [Google Scholar] [CrossRef] [Green Version]
- Jung, K.W.; Won, Y.J.; Kong, H.J.; Lee, E.S. Prediction of Cancer Incidence and Mortality in Korea, 2019. Cancer Res. Treat. 2019, 51, 431–437. [Google Scholar] [CrossRef]
- Tisdale, M.J. Cancer anorexia and cachexia. Nutrition 2001, 17, 438–442. [Google Scholar] [CrossRef]
- Laviano, A.; Koverech, A.; Seelaender, M. Assessing pathophysiology of cancer anorexia. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 340–345. [Google Scholar] [CrossRef]
- Muliawati, Y.; Haroen, H.; Rotty, L.A.W. Cancer anorexia-cachexia syndrome. Acta Med. Indones. Indones. J. Intern. Med. 2012, 44, 154–162. [Google Scholar]
- Laviano, A.; Meguid, M.M.; Rossi-Fanelli, F. Cancer anorexia: Clinical implications, pathogenesis, and therapeutic strategies. Lancet Oncol. 2003, 4, 686–694. [Google Scholar] [CrossRef]
- Laviano, A.; Meguid, M.M.; Inui, A.; Muscaritoli, M.; Rossi-Fanelli, F. Therapy insight: Cancer anorexia-cachexia syndrome—When all you can eat is yourself. Nat. Rev. Clin. Oncol. 2005, 2, 158–165. [Google Scholar] [CrossRef]
- Ezzo, J.; Richardson, R.M.; Vickers, A.; Allen, C.; Dibble, S.; Issell, B.F.; Lao, L.; Pearl, M.; Ramirez, G.; Roscoe, J.A.; et al. Acupuncture-point stimulation for chemotherapy-induced nausea or vomiting. Cochrane Database Syst. Rev. 2006. [Google Scholar] [CrossRef] [Green Version]
- Jeanette Ezzo, K.S. Antonius Schneider, Cochrane systematic reviews examine P6 acupuncture-point stimulation for nausea and vomiting. J. Altern. Complemen. Med. 2006, 12, 489–495. [Google Scholar] [CrossRef]
- Kim, M.; Kim, J.-E.; Kim, A.R.; Park, H.J.; Kwon, O.J.; Kim, B.K.; Cho, J.H.; Kim, J.H. Electroacupuncture for treating insomnia in patients with cancer: A study protocol for a randomised pilot clinical trial. BMJ Open 2017, 7, e016269. [Google Scholar] [CrossRef]
- Chen, H.; Liu, T.Y.; Kuai, L.; Zhu, J.; Wu, C.J.; Liu, L.M. Electroacupuncture treatment for pancreatic cancer pain: A randomized controlled trial. Pancreatology 2013, 13, 594–597. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Wang, L.; Shi, G.; Liu, L.; Pei, P.; Guo, J. Electroacupuncture alleviates cisplatin-induced nausea in rats. Acupunct. Med. 2016, 34, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, S.; Ye, F.; Yin, J.; Li, S.; Chen, J.D.Z. Ameliorating effects and mechanisms of chronic electroacupuncture at ST36 in a rodent model of dyspepsia induced by cisplatin. Neurogastroenterol. Motil. 2019, 31, e13474. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.S.; Huh, W.; Bang, Y.; Choi, H.J.; Baek, J.Y.; Song, J.H.; Kang, J.W.; Kim, T.H. Electroacupuncture for chemotherapy-induced anorexia through humoral appetite regulation: A preliminary experimental study. Exp. Ther. Med. 2019, 17, 2587–2597. [Google Scholar] [CrossRef]
- Li, Z.R. Experimental Acupuncture Science; China Press of Traditional Chinese Medicine: Beijing, China, 2003. [Google Scholar]
- Park, R.; Lee, K.I.; Kim, H.; Jang, M.; Ha, T.K.Q.; Oh, W.K.; Park, J. Reserpine treatment activates AMP activated protein kinase (AMPK). Nat. Prod. Sci. 2017, 23, 157–161. [Google Scholar] [CrossRef]
- Yoon, D.H.; Han, C.; Fang, Y.; Gundeti, S.; Han Lee, I.-S.; Song, W.O.; Hwang, K.-C.; Kim, T.W.; Sung, G.-H.; Park, H. Inhibitory activity of cordyceps bassiana extract on LPS-induced inflammation in RAW 264.7 cells by suppressing NF-κB activation. Nat. Prod. Sci. 2017, 23, 162–168. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, Z.; Qin, Y.; Niu, W.; Wang, Q.; Li, L.; Zhou, J. Analgesic effects of electroacupuncture at ST25 and CV12 in a rat model of postinflammatory irritable bowel syndrome visceral pain. Acupunct. Med. 2018, 36, 240–246. [Google Scholar] [CrossRef]
- Ge, A.X.; Ryan, M.E.; Giaccone, G.; Hughes, M.S.; Pavletic, S.Z. Acupuncture treatment for persistent hiccups in patients with cancer. J. Altern. Complement. Med. 2010, 16, 811–816. [Google Scholar] [CrossRef]
- Kim, K.; Lee, S. Intradermal Acupuncture Along with Analgesics for Pain Control in Advanced Cancer Cases: A Pilot, Randomized, Patient-Assessor-Blinded, Controlled Trial. Integr. Cancer Ther. 2018, 17, 1137–1143. [Google Scholar] [CrossRef]
- Ng, J.; Papandreou, A.; Heales, S.J.; Kurian, M.A. Monoamine neurotransmitter disorders--clinical advances and future perspectives. Nat. Rev. Neurol. 2015, 11, 567–584. [Google Scholar] [CrossRef]
- Beaudoin-Gobert, M.; Sgambato-Faure, V. Serotonergic pharmacology in animal models: From behavioral disorders to dyskinesia. Neuropharmacology 2014, 81, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Lee, J.; Jung, S.; Kim, J.W.; Shin, J.H.; Lee, H.J. The involvement of ginseng berry extract in blood flow via regulation of blood coagulation in rats fed a high-fat diet. J. Ginseng Res. 2017, 41, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Lam, D.D.; Przydzial, M.J.; Ridley, S.H.; Yeo, G.S.; Rochford, J.J.; O’Rahilly, S.; Heisler, L.K. Serotonin 5-HT2C receptor agonist promotes hypophagia via downstream activation of melanocortin 4 receptors. Endocrinology 2008, 149, 1323–1328. [Google Scholar] [CrossRef] [PubMed]
- Cambraia, R.P.B. Psychobiological aspects of feeding behavior. Rev. Nutr. 2004, 17, 217–225. [Google Scholar] [CrossRef]
- Ramos, E.J.B.; Suzuki, S.; Marks, D.; Inui, A.; Asakawa, A.; Meguid, M.M. Cancer anorexia-cachexia syndrome: Cytokines and neuropeptides. Curr. Opin. Clin. Nutr. Metab. Care 2004, 7, 427–434. [Google Scholar] [CrossRef]
- Yu, J.H.; Kim, M.S. Molecular mechanisms of appetite regulation. Diabetes Metab. J. 2012, 36, 391–398. [Google Scholar] [CrossRef]
- Yoichi Ueta, H.H. Etsuro Onuma, Yoh Takuwa, and Etsuro Ogata, Hypothalamic neuropeptides and appetite response in anorexia-cachexia animal. Endocr. J. 2007, 54, 831–838. [Google Scholar] [CrossRef]
- Yoshimura, M.; Matsuura, T.; Ohkubo, J.; Ohno, M.; Maruyama, T.; Ishikura, T.; Hashimoto, H.; Kakuma, T.; Yoshimatsu, H.; Terawaki, K.; et al. The gene expression of the hypothalamic feeding-regulating peptides in cisplatin-induced anorexic rats. Peptides 2013, 46, 13–19. [Google Scholar] [CrossRef]
- Holland, R.A.; Leonard, J.J.; Kensey, N.A.; Hannikainen, P.A.; De Jonghe, B.C. Cisplatin induces neuronal activation and increases central AMPA and NMDA receptor subunit gene expression in mice. Physiol. Behav. 2014, 136, 79–85. [Google Scholar] [CrossRef]
- Lee, J.H.; Min, D.S.; Lee, C.W.; Song, K.H.; Kim, Y.S.; Kim, H.P. Ginsenosides from Korean Red Ginseng ameliorate lung inflammatory responses: Inhibition of the MAPKs/NF-κB/c-Fos pathways. J. Ginseng Res. 2018, 42, 476–484. [Google Scholar] [CrossRef]
- Chen, B.; Hu, S.; Liu, B.; Zhao, T.; Li, B.; Liu, Y.; Li, M.; Pan, X.; Guo, Y.; Chen, Z.; et al. Efficacy and safety of electroacupuncture with different acupoints for chemotherapy-induced nausea and vomiting: Study protocol for a randomized controlled trial. Trials 2015, 16, 212. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Chen, B.; Zhang, Q.; Zhao, T.; Li, B.; Sha, T.; Zou, J.; Guo, Y.; Pan, X.; Guo, Y. Acupuncture with different acupoint combinations for chemotherapy-induced nausea and vomiting: Study protocol for a randomized controlled trial. BMC Complement. Altern. Med. 2016, 16, 441. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Guo, Y.; Zhao, X.; Gao, L.L.; Li, B.; Zhao, T.Y.; Zhang, Q.W.; Zou, J.X.; Li, M.Y.; Guo, Y.M.; et al. Efficacy differences of electroacupuncture with single acupoint or matching acupoints for chemotherapy-induced nausea and vomiting: Study protocol for a randomized controlled trial. Trials 2017, 18, 477. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yu, M.; Yang, G.; Wang, X.; Wang, H.; Zhang, C.; Xue, N.; Xu, W.; Fu, Q.; Yang, Z.; et al. Effect of acupuncture in prevention and treatment of chemotherapy-induced nausea and vomiting in patients with advanced cancer: Study protocol for a randomized controlled trial. Trials 2017, 18, 185. [Google Scholar] [CrossRef]





| Experimental Condition | Day −1 | Day 0 | Day 1 | Day 2 | Day 3 |
|---|---|---|---|---|---|
| 1st Experiment|Intensity of EA | |||||
| Cisplatin, i.p. | − | + | − | − | − |
| EA (low intensity stimulation) | − | − | + | + | + |
| EA (high intensity stimulation) | − | − | + | + | + |
| Body weight and food intake | + | + | + | + | + |
| 1st Experiment|Frequency of EA | |||||
| Cisplatin, i.p. | − | + | − | − | − |
| EA (10 Hz) | − | − | + | + | + |
| EA (100 Hz) | − | − | + | + | + |
| Body weight and food intake | + | + | + | + | + |
| 2nd Experiment|Pharmacological effects of the optimal EA conditions | |||||
| Cisplatin, i.p. | − | + | − | − | − |
| EA at non-acupoint | − | − | + | + | + |
| EA at CV12 (low intensity stimulation, 10 Hz) | − | − | + | + | + |
| Body weight and food intake | + | + | + | + | + |
| Blood, duodenum, and brain stem | − | − | − | − | + |
| Gene | Forward (5′ → 3′) | Reverse (5′ → 3′) |
|---|---|---|
| GHRL | AGCCCAGCAGAGAAAGGAAT | GTGGCTGCAGTTTAGCTGGT |
| NPY | TGTCTCAGGGCTGGATCTCT | TACTCCGCTCTGCGACACTA |
| POMC | GCTTCATGACCTCCGAGAAG | TCTTGATGATGGCGTTCTTG |
| β-actin | AAGTCCCTCACCCTCCCAAAAG | AAGCAATGCTGTCACCTTCCC |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baek, J.Y.; Trinh, T.A.; Huh, W.; Song, J.H.; Kim, H.Y.; Lim, J.; Kim, J.; Choi, H.J.; Kim, T.-H.; Kang, K.S. Electro-Acupuncture Alleviates Cisplatin-Induced Anorexia in Rats by Modulating Ghrelin and Monoamine Neurotransmitters. Biomolecules 2019, 9, 624. https://0-doi-org.brum.beds.ac.uk/10.3390/biom9100624
Baek JY, Trinh TA, Huh W, Song JH, Kim HY, Lim J, Kim J, Choi HJ, Kim T-H, Kang KS. Electro-Acupuncture Alleviates Cisplatin-Induced Anorexia in Rats by Modulating Ghrelin and Monoamine Neurotransmitters. Biomolecules. 2019; 9(10):624. https://0-doi-org.brum.beds.ac.uk/10.3390/biom9100624
Chicago/Turabian StyleBaek, Ji Yun, Tuy An Trinh, Wonsang Huh, Ji Hoon Song, Hyun Young Kim, Juhee Lim, Jinhee Kim, Hyun Jin Choi, Tae-Hun Kim, and Ki Sung Kang. 2019. "Electro-Acupuncture Alleviates Cisplatin-Induced Anorexia in Rats by Modulating Ghrelin and Monoamine Neurotransmitters" Biomolecules 9, no. 10: 624. https://0-doi-org.brum.beds.ac.uk/10.3390/biom9100624