Interaction of a Polyarginine Peptide with Membranes of Different Mechanical Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Methods
2.1.1. Monolayers at the Air-Water Interface
2.1.2. Large Unilamellar Vesicles (LUVs), Hydrodynamic Size and Z-Potential Measurements
2.1.3. Giant Unilamellar Vesicles (GUVs)
2.1.4. Optical Tweezer Setup
2.1.5. Bilayer Lipid Membrane (BLM)
3. Results
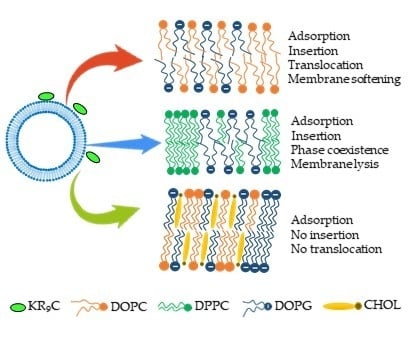
3.1. KR9C Recruits DOPG and Does Not Incorporate into Membranes with Cholesterol
3.2. KR9C Increases the Conductivity of DPPC/DOPG and DOPC/DOPG Membranes, without Affecting Those with Cholesterol
3.3. KR9C Adsorbs at the Surfaces of the Three Membrane Compositions
3.4. Adsorption of 5-FAM-KR9C on Membranes with Cholesterol Is Faster and Occurs to Higher Extents than on DOPC/DOPG Membranes
3.5. KR9C Softens DOPC/DOPG Membranes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Heimburg, T. Thermal Biophysics of Membranes; Wiley-VCH: Weinheim, Germany, 2007. [Google Scholar]
- De Almeida, R.F.M.; Joly, E. Crystallization around solid-like nanosized docks can explain the specificity, diversity, and stability of membrane microdomains. Front. Plant Sci. 2014, 5, 72. [Google Scholar] [CrossRef] [Green Version]
- Rosetti, C.M.; Mangiarotti, A.; Wilke, N. Sizes of lipid domains: What do we know from artificial lipid membranes? What are the possible shared features with membrane rafts in cells? Biochim. Biophys. Acta Biomembr. 2017, 1859, 789–802. [Google Scholar] [CrossRef]
- Alves, I.D.; Walrant, A.; Bechara, C.; Sagan, S. Is there anybody in there? On the mechanisms of wall crossing of cell penetrating peptides. Curr. Protein Pept. Sci. 2012, 13, 658–671. [Google Scholar] [CrossRef]
- Di Pisa, M.; Chassaing, G.; Swiecicki, J. Translocation Mechanism(s) of Cell-Penetrating Peptides: Biophysical Studies Using Artificial Membrane Bilayers. Biochemistry 2015, 54, 194–207. [Google Scholar] [CrossRef]
- Choi, Y.S.; David, A.E. Cell penetrating peptides and the mechanisms for intracellular entry. Curr. Pharm. Biotechnol 2014, 15, 192–199. [Google Scholar] [CrossRef]
- Borrelli, A.; Tornesello, A.L.; Tornesello, M.L.; Buonaguro, F.M. Cell Penetrating Peptides as Molecular Carriers for Anti-Cancer Agents. Molecules 2018, 23, 295. [Google Scholar] [CrossRef]
- McClorey, G.; Banerjee, S. Cell-Penetrating Peptides to Enhance Delivery of Oligonucleotide-Based Therapeutics. Biomedicines. 2018, 6, 51. [Google Scholar] [CrossRef]
- Derossi, D.; Joliot, A.H.; Chassaing, G.; Prochiantz, A. The Third Helix of the Antennapedia Homeodornain Translocates through Biological Membranes. Indian J. Chest Dis. Allied Sci. 1994, 269, 10444–10450. [Google Scholar]
- Vives, E.; Brodin, P.; LeBleu, B. A Truncated HIV-1 Tat Protein Basic Domain Rapidly Translocates through the Plasma Membrane and Accumulates in the Cell Nucleus. J. Boil. Chem. 1997, 272, 16010–16017. [Google Scholar] [CrossRef] [Green Version]
- Bechara, C.; Sagan, S. Cell-penetrating peptides: 20 years later, where do we stand? FEBS Lett. 2013, 587, 1693–1702. [Google Scholar] [CrossRef]
- Sakai, N.; Takeuchi, T.; Futaki, S.; Matile, S. Direct Observation of Anion-Mediated Translocation of Fluorescent Oligoarginine Carriers into and across Bulk Liquid and Anionic Bilayer Membranes. ChemBioChemochem 2005, 6, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Herce, H.D.; García, A.E.; Cardoso, M.C. Fundamental Molecular Mechanism for the Cellular Uptake of Guanidinium-Rich Molecules. J. Am. Chem. Soc. 2014, 136, 17459–17467. [Google Scholar] [CrossRef] [PubMed]
- Sakai, N.; Matile, S. Anion-Mediated Transfer of Polyarginine across Liquid and Bilayer Membranes. J. Am. Chem. Soc. 2003, 125, 14348–14356. [Google Scholar] [CrossRef] [PubMed]
- Vazdar, M.; Heyda, J.; Mason, P.E.; Tesei, G.; Allolio, C.; Lund, M.; Jungwirth, P. Arginine “Magic”: Guanidinium Like-Charge Ion Pairing from Aqueous Salts to Cell Penetrating Peptides. Accounts Chem. Res. 2018, 51, 1455–1464. [Google Scholar] [CrossRef]
- Via, M.A.; Del Pópolo, M.G.; Wilke, N. Negative Dipole Potentials and Carboxylic Polar Head Groups Foster the Insertion of Cell-Penetrating Peptides into Lipid Monolayers. Langmuir 2018, 34, 3102–3111. [Google Scholar] [CrossRef]
- Terrone, D.; Sang, S.L.W.; Roudaia, L.; Silvius, J.R. Penetratin and Related Cell-Penetrating Cationic Peptides Can Translocate Across Lipid Bilayers in the Presence of a Transbilayer Potential. Biochem. 2003, 42, 13787–13799. [Google Scholar] [CrossRef]
- Swiecicki, J.-M.; Bartsch, A.; Tailhades, J.; Di Pisa, M.; Heller, B.; Chassaing, G.; Mansuy, C.; Burlina, F.; Lavielle, S. The Efficacies of Cell-Penetrating Peptides in Accumulating in Large Unilamellar Vesicles Depend on their Ability To Form Inverted Micelles. ChemBioChem 2014, 15, 884–891. [Google Scholar] [CrossRef]
- Sharmin, S.; Islam, M.Z.; Karal, M.A.S.; Alam Shibly, S.U.; Dohra, H.; Yamazaki, M. Effects of Lipid Composition on the Entry of Cell-Penetrating Peptide Oligoarginine into Single Vesicles. Biochem. 2016, 55, 4154–4165. [Google Scholar] [CrossRef]
- Lorents, A.; Säälik, P.; Langel, Ü.; Pooga, M. Arginine-Rich Cell-Penetrating Peptides Require Nucleolin and Cholesterol-Poor Subdomains for Translocation across Membranes. Bioconjugate Chem. 2018, 29, 1168–1177. [Google Scholar] [CrossRef]
- Himeno, H.; Shimokawa, N.; Komura, S.; Andelman, D.; Hamada, T.; Takagi, M. Charge-induced phase separation in lipid membranes. Soft Matter 2014, 10, 7959–7967. [Google Scholar] [CrossRef] [Green Version]
- Smith, A.K.; Freed, J.H. Determination of Tie-Line Fields for Coexisting Lipid Phases: An ESR Study†. J. Phys. Chem. B 2009, 113, 3957–3971. [Google Scholar] [CrossRef]
- Kapoor, S.; Werkmüller, A.; Denter, C.; Zhai, Y.; Markgraf, J.; Weise, K.; Opitz, N.; Winter, R. Temperature–pressure phase diagram of a heterogeneous anionic model biomembrane system: Results from a combined calorimetry, spectroscopy and microscopy study. Biochim. Biophys. Acta Biomembr. 2011, 1808, 1187–1195. [Google Scholar] [CrossRef]
- Mangiarotti, A.; Genovese, D.M.; Naumann, C.A.; Monti, M.R.; Wilke, N. Hopanoids, like sterols, modulate dynamics, compaction, phase segregation and permeability of membranes. Biochim. Biophys. Acta Biomembr. 2019, 1861, 183060. [Google Scholar] [CrossRef]
- Mitchell, D.; Steinman, L.; Kim, D.; Fathman, C.; Rothbard, J. Polyarginine enters cells more efficiently than other polycationic homopolymers. J. Pept. Res. 2000, 56, 318–325. [Google Scholar] [CrossRef]
- Via, M.A.; Klug, J.; Wilke, N.; Mayorga, L.S.; Del Pópolo, M.G. The interfacial electrostatic potential modulates the insertion of cell-penetrating peptides into lipid bilayers. Phys. Chem. Chem. Phys. 2018, 20, 5180–5189. [Google Scholar] [CrossRef]
- Bartlett, G.R. Colorimetric Assay Phosphorylated for Free Glyceric Acids. J. Biol. Chem. 1958, 234, 469–471. [Google Scholar]
- Cámara, C.; Lurgo, F.E.; Fanani, M.L.; Wilke, N. Interaction of Dextran sulfate with anionic and cationic membranes. ACS Omega 2018, 3, 11673–11683. [Google Scholar] [CrossRef]
- Lee, W.M.; Reece, P.J.; Marchington, R.F.; Metzger, N.K.; Dholakia, K. Construction and calibration of an optical trap on a fluorescence optical microscope. Nat. Protoc. 2007, 2, 3226–3238. [Google Scholar] [CrossRef]
- Khan, M.S.; Dosoky, N.S.; Berdiev, B.K.; Williams, J.D. Electrochemical impedance spectroscopy for black lipid membranes fused with channel protein supported on solid-state nanopore. Eur. Biophys. J. 2016, 45, 843–852. [Google Scholar] [CrossRef]
- Corvalán, N.A.; Kembro, J.M.; Clop, P.D.; Perillo, M.A. Cholesterol favors the emergence of a long-range autocorrelated fluctuation pattern in voltage-induced ionic currents through lipid bilayers. Biochim. Biophys. Acta Biomembr. 2013, 1828, 1754–1764. [Google Scholar] [CrossRef] [Green Version]
- Sinner, E.-K.; Ritz, S.; Naumann, R.; Schiller, S.; Knoll, W. Chapter 7 Self-Assembled Tethered Bimolecular Lipid Membranes. Adv. Clin. Chem. 2009, 49, 159–179. [Google Scholar]
- Wodzinska, K.; Blicher, A.; Heimburg, T. The thermodynamics of lipid ion channel formation in the absence and presence of anesthetics. BLM experiments and simulations. Soft Matter 2009, 5, 3319. [Google Scholar] [CrossRef]
- Demel, R.; Van Kessel, W.G.; Zwaal, R.; Roelofsen, B.; Van Deenen, L. Relation between various phospholipase actions on human red cell membranes and the interfacial phospholipid pressure in monolayers. Biochim. Biophys. Acta Biomembr. 1975, 406, 97–107. [Google Scholar] [CrossRef] [Green Version]
- Marsh, D. Lateral pressure in membranes. Biochim. Biophys. Acta 1996, 1286, 183–223. [Google Scholar] [CrossRef]
- Mangiarotti, A.; Caruso, B.; Wilke, N. Phase coexistence in films composed of DLPC and DPPC: A comparison between different model membrane systems. Biochim. Biophys. Acta Biomembr. 2014, 1838, 1823–1831. [Google Scholar] [CrossRef] [Green Version]
- Ramírez, P.G.; Del Pópolo, M.G.; Vila, J.A.; Szleifer, I.; Longo, G.S. Adsorption and insertion of polyarginine peptides into membrane pores: The trade-off between electrostatics, acid-base chemistry and pore formation energy. J. Colloid Interface Sci. 2019, 552, 701–711. [Google Scholar] [CrossRef]
- Avci, F.G.; Akbulut, B.S.; Ozkirimli, E. Membrane Active Peptides and Their Biophysical Characterization. Biomol. 2018, 8, 77. [Google Scholar] [CrossRef]
- Guha, S.; Ghimire, J.; Wu, E.; Wimley, W.C. Mechanistic Landscape of Membrane-Permeabilizing Peptides. Chem. Rev. 2019, 119, 6040–6085. [Google Scholar] [CrossRef]
- Blicher, A.; Wodzinska, K.; Fidorra, M.; Winterhalter, M.; Heimburg, T. The Temperature Dependence of Lipid Membrane Permeability, its Quantized Nature, and the Influence of Anesthetics. Biophys. J. 2009, 96, 4581–4591. [Google Scholar] [CrossRef] [Green Version]
- Seeger, H.M.; Fidorra, M.; Heimburg, T. Domain Size and Fluctuations at Domain Interfaces in Lipid Mixtures. Macromol. Symp. 2005, 219, 85–96. [Google Scholar] [CrossRef]
- Himeno, H.; Ito, H.; Higuchi, Y.; Hamada, T.; Shimokawa, N.; Takagi, M. Coupling between pore formation and phase separation in charged lipid membranes. Phys. Rev. E 2015, 92, 1–12. [Google Scholar] [CrossRef]
- Lyubchenko, Y.L. (Ed.) An Introduction to Single Molecule Biophysics; CRC Press: Omaha, NE, USA, 2017. [Google Scholar]
- Lekkerkerker, H. The electric contribution to the curvature elastic moduli of charged fluid interfaces. Phys. A Stat. Mech. Its Appl. 1990, 167, 384–394. [Google Scholar] [CrossRef] [Green Version]
- Lekkerkerker, H. Contribution of the electric double layer to the curvature elasticity of charged amphiphilic monolayers. Phys. A Stat. Mech. Its Appl. 1989, 159, 319–328. [Google Scholar] [CrossRef] [Green Version]
- Bivas, I.; Ermakov, Y.A. Elasticity and Electrostatics of Amphiphilic Layers: Current State of the Theory and the Experiment. Adv. Planar Lipid Bilayers Liposomes 2007, 5, 313–343. [Google Scholar]
- May, S. Curvature elasticity and thermodynamic stability of electrically charged membranes. J. Chem. Phys. 1996, 105, 8314–8323. [Google Scholar] [CrossRef]
- Winterhalter, M.; Helfrich, W. Effect of surface charge on the curvature elasticity of membranes. J. Phys. Chem. 1988, 92, 6865–6867. [Google Scholar] [CrossRef]
- Winterhalter, M.; Helfrich, W. Bending elasticity of electrically charged bilayers: Coupled monolayers, neutral surfaces, and balancing stresses. J. Phys. Chem. 1992, 96, 327–330. [Google Scholar] [CrossRef]
- Vitkova, V.; Genova, J.; Finogeva, O.; Mitov, M.D.; Ermakov, Y.; Bivas, I. Surface charge effect on the bending elasticity of lipid bilayers. Comptes Rendus l’Academie Bulg. Des. Sci. 2004, 57, 25–30. [Google Scholar]
- Dimova, R. Recent developments in the field of bending rigidity measurements on membranes. Adv. Colloid Interface Sci. 2014, 208, 225–234. [Google Scholar] [CrossRef]
- Zhou, Y.; Raphael, R.M. Solution pH alters mechanical and electrical properties of phosphatidylcholine membranes: Relation between interfacial electrostatics, intramembrane potential, and bending elasticity. Biophys. J. 2007, 92, 2451–2462. [Google Scholar] [CrossRef]
- Mertins, O.; Dimova, R. Insights on the Interactions of Chitosan with Phospholipid Vesicles. Part II: Membrane Stiffening and Pore Formation. Langmuir 2013, 29, 14552–14559. [Google Scholar] [CrossRef]
- Derényi, I.; Koster, G.; van Duijn, M.M.; Czövek, A.; Dogterom, M.; Prost, J. Membrane Nanotubes. In Control. Nanoscale Motion; Springer: Berlin/Heidelberg, Germany, 2017; pp. 141–159. [Google Scholar]
- Häckl, W.; Seifert, U.; Sackmann, E. Elllects of Fully and Partially Solubilized Alnphiphiles on Bilayer Bending Stiffiuess and Telnperature Dependence of the Elllective Tension of Giant Vesicles. J. Phys. II France 1997, 7, 1141–1157. [Google Scholar] [CrossRef]
- Bouvrais, H.; Méléard, P.; Pott, T.; Jensen, K.J.; Brask, J.; Ipsen, J.H. Softening of POPC membranes by magainin. Biophys. Chem. 2008, 137, 7–12. [Google Scholar] [CrossRef] [Green Version]
- Fa, N.; Lins, L.; Courtoy, P.J.; Dufrêne, Y.; Van Der Smissen, P.; Brasseur, R.; Tyteca, D. Mingeot-Leclercq, M.P. Decrease of elastic moduli of DOPC bilayers induced by a macrolide antibiotic, azithromycin. Biochim. Biophys. Acta Biomembr. 2007, 1768, 1830–1838. [Google Scholar] [CrossRef]
- Vitkova, V.; Méléard, P.; Pott, T.; Bivas, I. Alamethicin influence on the membrane bending elasticity. Eur. Biophys. J. 2006, 35, 281–286. [Google Scholar]
- Pan, J.; Tieleman, D.P.; Nagle, J.F.; Kučerka, N.; Tristram-Nagle, S. Alamethicin in lipid bilayers: Combined use of X-ray scattering and MD simulations. Biochim. Biophys. Acta 2009, 1788, 1387–1397. [Google Scholar] [CrossRef] [Green Version]
- Pabst, G.; Danner, S.; Podgornik, R.; Katsaras, J.; Podgornik, R. Entropy-Driven Softening of Fluid Lipid Bilayers by Alamethicin. Langmuir 2007, 23, 11705–11711. [Google Scholar] [CrossRef]
- Shchelokovskyy, P.; Tristram-Nagle, S.; Dimova, R. Effect of the HIV-1 fusion peptide on the mechanical properties and leaflet coupling of lipid bilayers. New J. Phys. 2011, 13, 025004. [Google Scholar] [CrossRef] [Green Version]
- Tristram-Nagle, S.; Chan, R.; Kooijman, E.; Uppamoochikkal, P.; Qiang, W.; Weliky, D.P.; Nagle, J.F. HIV fusion peptide penetrates, disorders, and softens T-cell membrane mimics. J. Mol. Biol. 2010, 402, 139–153. [Google Scholar] [CrossRef]
- Grasso, G.; Muscat, S.; Rebella, M.; Morbiducci, U.; Audenino, A.; Danani, A.; Deriu, M.A. Cell penetrating peptide modulation of membrane biomechanics by Molecular dynamics. J. Biomech. 2018, 73, 137–144. [Google Scholar] [CrossRef]







| Membrane Composition | ∆ζ (mV) | P/L50 |
|---|---|---|
| DOPC/DOPG | 55 ± 1 | 0.030 ± 0.005 |
| DPPC/DOPG | 68 ± 2 | 0.017 ± 0.002 |
| DOPC/CHOL/DOPG | 108 ± 3 | 0.003 ± 0.001 |
| Membrane Composition | k1/103 s−1 | Γrim at Long Times | |
|---|---|---|---|
| 1 µM | 2.5 µM | ||
| DOPC/DOPG | 3.1 ± 0.7 | 1.18 ± 0.07 | 1.4 ± 0.1 |
| DOPC/CHOL/DOPG | 4.9 ± 0.9 | 1.7 ± 0.3 | 4 ± 1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crosio, M.A.; Via, M.A.; Cámara, C.I.; Mangiarotti, A.; Del Pópolo, M.G.; Wilke, N. Interaction of a Polyarginine Peptide with Membranes of Different Mechanical Properties. Biomolecules 2019, 9, 625. https://0-doi-org.brum.beds.ac.uk/10.3390/biom9100625
Crosio MA, Via MA, Cámara CI, Mangiarotti A, Del Pópolo MG, Wilke N. Interaction of a Polyarginine Peptide with Membranes of Different Mechanical Properties. Biomolecules. 2019; 9(10):625. https://0-doi-org.brum.beds.ac.uk/10.3390/biom9100625
Chicago/Turabian StyleCrosio, Matías A., Matías A. Via, Candelaria I. Cámara, Agustin Mangiarotti, Mario G. Del Pópolo, and Natalia Wilke. 2019. "Interaction of a Polyarginine Peptide with Membranes of Different Mechanical Properties" Biomolecules 9, no. 10: 625. https://0-doi-org.brum.beds.ac.uk/10.3390/biom9100625