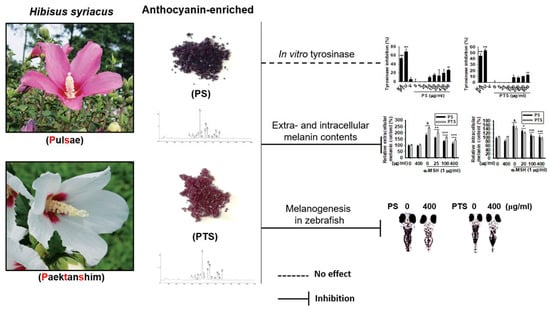
Anthocyanins from Hibiscus syriacus L. Inhibit Melanogenesis by Activating the ERK Signaling Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Extraction of PS and PTS
2.2. Regents and Antibodies
2.3. Cell Culture
2.4. Cell Viability
2.5. Flow Cytometry Analysis
2.6. Mushroom Tyrosinase Assay
2.7. Measurement of Extracellular and Intracellular Melanin Content
2.8. Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
2.9. Western Blotting Analysis
2.10. Maintenance and Phenotype Evaluation of Zebrafish
2.11. Determination of Cardiotoxicity in Zebrafish
2.12. UPLC-QT of MS for Flavonoid Analysis
2.13. Statistical Analysis
3. Results
3.1. PS and PTS Do Not Induce Cytotoxicity in B16F10 Cells
3.2. PS and PTS Decrease Extracellular and Intracellular Melanin Production in α-MSH-Stimulated B16F10 Cells
3.3. PS and PTS Do Not Downregulate Mushroom Tyrosinase Activity in Vitro
3.4. PS and PTS Inhibit the Expression of MITF and Tyrosinase in α-MSH-Stimulated B16F10 Cells
3.5. PS and PTS Inhibit Melanin Synthesis in Zebrafish Larvae
3.6. PS- and PTS-induced ERK Phosphorylation Downregulates MITF Expression
3.7. The ERK Signaling Pathway Downregulates Melanogenesis in PS- and PTS-treated B16F10 Cells and Zebrafish Larvae
3.8. Tentative Identification of Metabolites in PS and PTS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Zabierowski, S.E.; Baubet, V.; Himes, B.; Li, L.; Fukunaga-Kalabis, M.; Patel, S.; McDaid, R.; Guerra, M.; Gimotty, P.; Dahmane, N.; et al. Direct reprogramming of melanocytes to neural crest stem-like cells by one defined factor. Stem Cells 2011, 29, 1752–1762. [Google Scholar] [CrossRef]
- Videira, I.F.; Moura, D.F.; Magina, S. Mechanisms regulating melanogenesis. An. Bras. Dermatol. 2013, 88, 76–83. [Google Scholar] [CrossRef]
- Ebanks, J.P.; Wickett, R.R.; Boissy, R.E. Mechanisms regulating skin pigmentation: The rise and fall of complexion coloration. Int. J. Mol. Sci. 2009, 10, 4066–4087. [Google Scholar] [CrossRef]
- Togsverd-Bo, K.; Philipsen, P.A.; Haedersdal, M.; Wulf, H.C.O. Skin autofluorescence reflects individual seasonal UV exposure, skin photodamage and skin cancer development in organ transplant recipients. J. Photochem. Photobiol. B 2018, 178, 577–583. [Google Scholar] [CrossRef]
- Kumari, S.; Thng, S.T.G.; Verma, N.K.; Gautam, H.K. Melanogenesis inhibitors. Acta Derm. Venereol. 2018, 98, 924–931. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Manickam, M.; Jung, S.H. Downregulation of melanogenesis: Drug discovery and therapeutic options. Drug Discov. Today 2017, 22, 282–298. [Google Scholar] [CrossRef]
- del Marmol, V.; Beermann, F. Tyrosinase and related proteins in mammalian pigmentation. FEBS Lett. 1996, 381, 165–168. [Google Scholar] [CrossRef]
- Liu, J.J.; Fisher, D.E. Lighting a path to pigmentation: Mechanisms of MITF induction by UV. Pigment Cell Melanoma Res. 2010, 23, 741–745. [Google Scholar] [CrossRef]
- Vachtenheim, J.; Borovansky, J. “Transcription physiology” of pigment formation in melanocytes: Central role of MITF. Exp. Dermatol. 2010, 19, 617–627. [Google Scholar] [CrossRef]
- Roh, E.; Yun, C.Y.; Young Yun, J.; Park, D.; Doo Kim, N.; Yeon Hwang, B.; Jung, S.H.; Park, S.K.; Kim, Y.B.; Han, S.B.; et al. cAMP-binding site of PKA as a molecular target of bisabolangelone against melanocyte-specific hyperpigmented disorder. J. Investig. Dermatol. 2013, 133, 1072–1079. [Google Scholar] [CrossRef]
- Rodriguez, C.I.; Setaluri, V. Cyclic AMP (cAMP) signaling in melanocytes and melanoma. Arch. Biochem. Biophys. 2014, 563, 22–27. [Google Scholar] [CrossRef]
- Kim, Y.H.; Im, A.R.; Park, B.K.; Paek, S.H.; Choi, G.; Kim, Y.R.; Whang, W.K.; Lee, K.H.; Oh, S.E.; Lee, M.Y. Antidepressant-like and neuroprotective effects of ethanol extract from the root bark of Hibiscus syriacus L. BioMed Res. Int. 2018, 2018, 7383869. [Google Scholar] [CrossRef]
- Wang, R.; Chen, T.; Zhao, B.; Fan, R.; Ji, K.; Yu, X.; Wang, X.; Dong, C. FGF21 regulates melanogenesis in alpaca melanocytes via ERK1/2-mediated MITF downregulation. Biochem. Biophys. Res. Commun. 2017, 490, 466–471. [Google Scholar] [CrossRef]
- Bellei, B.; Pitisci, A.; Catricala, C.; Larue, L.; Picardo, M. Wnt/beta-catenin signaling is stimulated by alpha-melanocyte-stimulating hormone in melanoma and melanocyte cells: Implication in cell differentiation. Pigment Cell Melanoma Res. 2011, 24, 309–325. [Google Scholar] [CrossRef]
- Guo, H.; Xing, Y.; Liu, Y.; Luo, Y.; Deng, F.; Yang, T.; Yang, K.; Li, Y. Wnt/beta-catenin signaling pathway activates melanocyte stem cells in vitro and in vivo. J. Dermatol. Sci. 2016, 83, 45–51. [Google Scholar] [CrossRef]
- di Martino, O.; Tito, A.; De Lucia, A.; Cimmino, A.; Cicotti, F.; Apone, F.; Colucci, G.; Calabro, V. Hibiscus syriacus extract from an established cell culture stimulates skin wound healing. BioMed Res. Int. 2017, 2017, 7932019. [Google Scholar] [CrossRef]
- Yang, J.E.; Ngo, H.T.T.; Hwang, E.; Seo, S.A.; Park, S.W.; Yi, T.H. Dietary enzyme-treated Hibiscus syriacus L. protects skin against chronic UVB-induced photoaging via enhancement of skin hydration and collagen synthesis. Arch. Biochem. Biophys. 2019, 662, 190–200. [Google Scholar] [CrossRef]
- Hsu, R.J.; Hsu, Y.C.; Chen, S.P.; Fu, C.L.; Yu, J.C.; Chang, F.W.; Chen, Y.H.; Liu, J.M.; Ho, J.Y.; Yu, C.P. The triterpenoids of Hibiscus syriacus induce apoptosis and inhibit cell migration in breast cancer cells. BMC Complement. Altern. Med. 2015, 15, 65. [Google Scholar] [CrossRef]
- Shi, L.S.; Wu, C.H.; Yang, T.C.; Yao, C.W.; Lin, H.C.; Chang, W.L. Cytotoxic effect of triterpenoids from the root bark of Hibiscus syriacus. Fitoterapia 2014, 97, 184–191. [Google Scholar] [CrossRef]
- Kwon, S.W.; Hong, S.S.; Kim, J.I.; Ahn, I.H. Antioxidant properties of heat-treated Hibiscus syriacus. Izv. Akad. Nauk Seriya Biol. 2003, 1, 20–21. [Google Scholar]
- Siegrist, W.; Eberle, A.N. In situ melanin assay for MSH using mouse B16 melanoma cells in culture. Anal. Biochem. 1986, 159, 191–197. [Google Scholar] [CrossRef]
- Lajis, A.F.B. A zebrafish embryo as an animal model for the treatment of hyperpigmentation in cosmetic dermatology medicine. Medicina (Kaunas) 2018, 54, 35. [Google Scholar] [CrossRef]
- Karunarathne, W.; Molagoda, I.M.N.; Kim, M.S.; Choi, Y.H.; Oren, M.; Park, E.K.; Kim, G.Y. Flumequine-mediated upregulation of p38 MAPK and JNK results in melanogenesis in B16F10 cells and zebrafish larvae. Biomolecules 2019, 9, 596. [Google Scholar] [CrossRef]
- Duckworth, H.W.; Coleman, J.E. Physicochemical and kinetic properties of mushroom tyrosinase. J. Biol. Chem. 1970, 245, 1613–1625. [Google Scholar]
- Hu, D.N. Methodology for evaluation of melanin content and production of pigment cells in vitro. Photochem. Photobiol. 2008, 84, 645–649. [Google Scholar] [CrossRef]
- Cornet, C.; Calzolari, S.; Minana-Prieto, R.; Dyballa, S.; van Doornmalen, E.; Rutjes, H.; Savy, T.; D’Amico, D.; Terriente, J. ZeGlobalTox: An innovative approach to address organ drug toxicity using zebrafish. Int. J. Mol. Sci. 2017, 18, 864. [Google Scholar] [CrossRef]
- Zhang, C.; Willett, C.; Fremgen, T. Zebrafish: An animal model for toxicological studies. Curr. Protoc. Toxicol. 2003, 17, 1–7. [Google Scholar]
- Kim, E.S.; Park, S.J.; Goh, M.J.; Na, Y.J.; Jo, D.S.; Jo, Y.K.; Shin, J.H.; Choi, E.S.; Lee, H.K.; Kim, J.Y.; et al. Mitochondrial dynamics regulate melanogenesis through proteasomal degradation of MITF via ROS-ERK activation. Pigment Cell Melanoma Res. 2014, 27, 1051–1062. [Google Scholar] [CrossRef]
- Lee, Y.M.; Yoon, Y.; Yoon, H.; Park, H.M.; Song, S.; Yeum, K.J. Dietary anthocyanins against obesity and inflammation. Nutrients 2017, 9, 1089. [Google Scholar] [CrossRef]
- Ito, C.; Oki, T.; Yoshida, T.; Nanba, F.; Yamada, K.; Toda, T. Characterisation of proanthocyanidins from black soybeans: Isolation and characterisation of proanthocyanidin oligomers from black soybean seed coats. Food Chem. 2013, 141, 2507–2512. [Google Scholar] [CrossRef]
- Koh, K.; Youn, J.E.; Kim, H.S. Identification of anthocyanins in black soybean (Glycine max (L.) Merr.) varieties. J. Food Sci. Technol. 2014, 51, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Kim, H.W.; Kwon, Y.S.; Kim, H.K.; Sung, S.H. Fast and simple discriminative analysis of anthocyanins-containing berries using LC/MS spectral data. Phytochem. Anal. 2017, 28, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Putta, S.; Yarla, N.S.; Peluso, I.; Tiwari, D.K.; Reddy, G.V.; Giri, P.V.; Kumar, N.; Malla, R.; Rachel, V.; Bramhachari, P.V.; et al. Anthocyanins: Multi-target agents for prevention and therapy of chronic diseases. Curr. Pharm. Des. 2017, 23, 6321–6346. [Google Scholar] [CrossRef]
- Bonesi, M.; Leporini, M.; Tenuta, M.C.; Tundis, R. The role of anthocyanins in drug discovery: Recent developments. Curr. Drug Discov. Technol. 2019. [Google Scholar] [CrossRef]
- Naseri, R.; Farzaei, F.; Haratipour, P.; Nabavi, S.F.; Habtemariam, S.; Farzaei, M.H.; Khodarahmi, R.; Tewari, D.; Momtaz, S. Anthocyanins in the management of metabolic syndrome: A pharmacological and biopharmaceutical review. Front. Pharmacol. 2018, 9, 1310. [Google Scholar] [CrossRef]
- Grajeda-Iglesias, C.; Figueroa-Espinoza, M.C.; Barouh, N.; Barea, B.; Fernandes, A.; de Freitas, V.; Salas, E. Isolation and characterization of anthocyanins from Hibiscus sabdariffa flowers. J. Nat. Prod. 2016, 79, 1709–1718. [Google Scholar] [CrossRef]
- Piovesana, A.; Rodrigues, E.; Norena, C.P.Z. Composition analysis of carotenoids and phenolic compounds and antioxidant activity from Hibiscus calyces (Hibiscus sabdariffa L.) by HPLC-DAD-MS/MS. Phytochem. Anal. 2019, 30, 208–217. [Google Scholar] [CrossRef]
- Chang, Y.C.; Huang, K.X.; Huang, A.C.; Ho, Y.C.; Wang, C.J. Hibiscus anthocyanins-rich extract inhibited LDL oxidation and oxLDL-mediated macrophages apoptosis. Food Chem. Toxicol. 2006, 44, 1015–1023. [Google Scholar] [CrossRef]
- Maciel, L.G.; do Carmo, M.A.V.; Azevedo, L.; Daguer, H.; Molognoni, L.; de Almeida, M.M.; Granato, D.; Rosso, N.D. Hibiscus sabdariffa anthocyanins-rich extract: Chemical stability, in vitro antioxidant and antiproliferative activities. Food Chem. Toxicol. 2018, 113, 187–197. [Google Scholar] [CrossRef]
- Wang, C.J.; Wang, J.M.; Lin, W.L.; Chu, C.Y.; Chou, F.P.; Tseng, T.H. Protective effect of Hibiscus anthocyanins against tert-butyl hydroperoxide-induced hepatic toxicity in rats. Food Chem. Toxicol. 2000, 38, 411–416. [Google Scholar] [CrossRef]
- Munoz-Munoz, J.L.; Garcia-Molina, F.; Varon, R.; Garcia-Ruiz, P.A.; Tudela, J.; Garcia-Canovas, F.; Rodriguez-Lopez, J.N. Suicide inactivation of the diphenolase and monophenolase activities of tyrosinase. IUBMB Life 2010, 62, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Zolghadri, S.; Bahrami, A.; Hassan Khan, M.T.; Munoz-Munoz, J.; Garcia-Molina, F.; Garcia-Canovas, F.; Saboury, A.A. A comprehensive review on tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jhan, J.K.; Chung, Y.C.; Chen, G.H.; Chang, C.H.; Lu, Y.C.; Hsu, C.K. Anthocyanin contents in the seed coat of black soya bean and their anti-human tyrosinase activity and antioxidative activity. Int. J. Cosmet. Sci. 2016, 38, 319–324. [Google Scholar] [CrossRef]
- Yu, L.G.; Packman, L.C.; Weldon, M.; Hamlett, J.; Rhodes, J.M. Protein phosphatase 2A, a negative regulator of the ERK signaling pathway, is activated by tyrosine phosphorylation of putative HLA class II-associated protein I (PHAPI)/pp32 in response to the antiproliferative lectin, jacalin. J. Biol. Chem. 2004, 279, 41377–41383. [Google Scholar] [CrossRef]
- Buffet, C.; Hecale-Perlemoine, K.; Bricaire, L.; Dumont, F.; Baudry, C.; Tissier, F.; Bertherat, J.; Cochand-Priollet, B.; Raffin-Sanson, M.L.; Cormier, F.; et al. DUSP5 and DUSP6, two ERK specific phosphatases, are markers of a higher MAPK signaling activation in BRAF mutated thyroid cancers. PLoS ONE 2017, 12, e0184861. [Google Scholar] [CrossRef]
- Kidger, A.M.; Keyse, S.M. The regulation of oncogenic Ras/ERK signalling by dual-specificity mitogen activated protein kinase phosphatases (MKPs). Semin. Cell Dev. Biol. 2016, 50, 125–132. [Google Scholar] [CrossRef] [Green Version]
- Urness, L.D.; Li, C.; Wang, X.; Mansour, S.L. Expression of ERK signaling inhibitors Dusp6, Dusp7, and Dusp9 during mouse ear development. Dev. Dyn. 2008, 237, 163–169. [Google Scholar] [CrossRef]
- Davis, M.I.; Ronesi, J.; Lovinger, D.M. A predominant role for inhibition of the adenylate cyclase/protein kinase A pathway in ERK activation by cannabinoid receptor 1 in N1E-115 neuroblastoma cells. J. Biol. Chem. 2003, 278, 48973–48980. [Google Scholar] [CrossRef]
- Chen, X.Y.; Zhou, J.; Luo, L.P.; Han, B.; Li, F.; Chen, J.Y.; Zhu, Y.F.; Chen, W.; Yu, X.P. Black rice anthocyanins suppress metastasis of breast cancer cells by targeting RAS/RAF/MAPK pathway. BioMed Res. Int. 2015, 2015, 414250. [Google Scholar] [CrossRef]
- Wang, L.S.; Stoner, G.D. Anthocyanins and their role in cancer prevention. Cancer Lett. 2008, 269, 281–290. [Google Scholar] [CrossRef] [Green Version]








| NO. | I.D. | Molecular Formula | Retention Time (min) | Calculated Ion (m/z) | Fragments | PubChem CID |
|---|---|---|---|---|---|---|
| 1 | Cyanidin-3-O-galactoside | C12H21O11+ | 4.34 | 449.1084 | 259, 287, 421 | 441699 |
| 2 | Cyanidin-3-O-glucoside | C21H21O11+ | 4.43 | 449.1084 | 259,287. 421 | 44256715 |
| 3 | Orientin-7-O-glucoside | C27H30O16 | 4.61 | 609.1456 | 327, 357, 447 | 44257973 |
| 4 | Cyanidin-3,5-O-diglucoside | C27H31O16+ | 4.87 | 611.1612 | 259, 287, 449 | 44256718 |
| 5 | Isoorientinm-4′-O-glucoside | C27H30O16 | 5.21 | 609.1456 | 193, 285, 299, 327, 357, 447 | 44257975 |
| 6 | Isovitexin-4′-O-glucoside | C27H30O15 | 5.32 | 593.1506 | 116, 447 | 154105 |
| 7 | Vitexin-4′-O-glucoside-2″-O-rhamnoside | C33H40O19 | 5.43 | 739.2086 | 431, 447, 593 | 44257755 |
| 8 | Isovitexin-7-O-glucoside (saponarin) | C27H30O15 | 5.54 | 593.1506 | 283, 311, 431 | 441381 |
| 9 | Apigenin-8-C-β-D-glucopyranoside (Vitexin) | C21H20O10 | 6.02 | 431.0987 | 283, 311 | 5280441 |
| 10 | Isovitexin-2″-O-rhamnoside | C27H30O14 | 6.20 | 577.1557 | 293, 311, 431 | 44257672 |
| 11 | Apigenin-6-C-β-D-glucopyranoside (Isovitexin) | C21H20O10 | 6.28 | 431.0978 | 283, 311, 341 | 162350 |
| 12 | Apigenin-6-C-glucoside-7-(6″-O-acetyl)-glucoside | C29H32O16 | 6.50 | 635.1671 | 431 | 44257840 |
| 13 | Kaempferol-O-glucoside derivative | C31H34O18 | 6.94 | 693.1612 | 227, 255, 284, 300, 311 | *N.F. |
| 14 | Kaempferol-7-O-glucoside | C21H20O11 | 7.23 | 447.0927 | 227, 255, 285 | 10095180 |
| 15 | Kaempferol-3-O-glucoside | C21H20O11 | 7.45 | 447.0927 | 151, 257, 285 | 44258798 |
| 16 | Apigenin-7-O-glucoside | C21H20O11 | 7.53 | 431.0978 | 268, 271 | 44257792 |
| 17 | Kaempferol-3-(6″-acetylglucoside) | C23H22O12 | 7.85 | 489.1033 | 227,255, 284, 429 | 44258855 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karunarathne, W.A.H.M.; Molagoda, I.M.N.; Park, S.R.; Kim, J.W.; Lee, O.-K.; Kwon, H.Y.; Oren, M.; Choi, Y.H.; Ryu, H.W.; Oh, S.-R.; et al. Anthocyanins from Hibiscus syriacus L. Inhibit Melanogenesis by Activating the ERK Signaling Pathway. Biomolecules 2019, 9, 645. https://0-doi-org.brum.beds.ac.uk/10.3390/biom9110645
Karunarathne WAHM, Molagoda IMN, Park SR, Kim JW, Lee O-K, Kwon HY, Oren M, Choi YH, Ryu HW, Oh S-R, et al. Anthocyanins from Hibiscus syriacus L. Inhibit Melanogenesis by Activating the ERK Signaling Pathway. Biomolecules. 2019; 9(11):645. https://0-doi-org.brum.beds.ac.uk/10.3390/biom9110645
Chicago/Turabian StyleKarunarathne, Wisurumuni Arachchilage Hasitha Maduranga, Ilandarage Menu Neelaka Molagoda, Sang Rul Park, Jeong Woon Kim, Oh-Kyu Lee, Hae Yun Kwon, Matan Oren, Yung Hyun Choi, Hyung Won Ryu, Sei-Ryang Oh, and et al. 2019. "Anthocyanins from Hibiscus syriacus L. Inhibit Melanogenesis by Activating the ERK Signaling Pathway" Biomolecules 9, no. 11: 645. https://0-doi-org.brum.beds.ac.uk/10.3390/biom9110645