The Quest for Phenolic Compounds from Macroalgae: A Review of Extraction and Identification Methodologies
Abstract
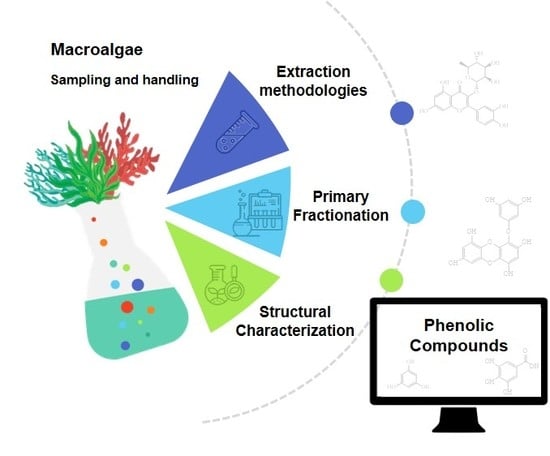
:1. Introduction
2. Wide Chemical Diversity of Phenolic Compounds Present in Macroalgae
2.1. Simple Phenolic Compounds
2.2. Phlorotannins
2.3. Biological Activities of Macroalgae Phenolic Compounds
3. Extraction of Phenolic Compounds from Macroalgae
3.1. Sampling Procedures and Sample Handling before Phenolic Compounds Extraction
3.2. Solvent Selection
3.3. Extraction Methodologies
3.3.1. Conventional Solid–Liquid Extraction
3.3.2. Novel Extraction Methodologies
Enzyme Assisted Extraction
Accelerated Solvent Extraction
Microwave-Assisted Extraction
Ultrasound-Assisted Extraction
Supercritical Fluid Extraction
4. Analysis of Phenolic Rich Crude Extracts
4.1. Global Analysis by Spectrophotometric Analysis
4.2. Direct Spectroscopic Analysis of Crude Extracts
5. Primary Fractionation of Macroalgae Polar Crude Extracts
5.1. Solubility-Base Separation
5.2. Adsorption-Base Separation
5.3. Other Primary Separation Methods
6. Preparative Chromatographic Techniques for Phenolic Compounds Isolation
6.1. Column Chromatography
6.2. High-Performance Liquid Chromatography
6.3. Other Preparative Chromatographic Techniques
7. Phenolic Compounds Identification
7.1. High-Performance Liquid Chromatography-Tandem-Mass Spectrometry
Phlorotannins Mass Spectrometry
7.2. NMR Analysis of Isolated Phenolic Compounds from Macroalgae Extracts
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (Poly)phenolics in Human Health: Structures, Bioavailability, and Evidence of Protective Effects Against Chronic Diseases. Antioxid. Redox Signal. 2012, 18, 1818–1892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uysal, S.; Aktumsek, A.; Picot-Allain, C.M.N.; Unuvar, H.; Mollica, A.; Georgiev, M.I.; Zengin, G.; Mahomoodally, M.F. Biological, chemical and in silico fingerprints of Dianthus calocephalus Boiss.: A novel source for rutin. Food Chem. Toxicol. 2018, 113, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, E.M.C.; Silva, S.; Santos, S.A.O.; Silvestre, A.J.D.; Duarte, M.F.; Saraiva, J.A.; Pintado, M. Antimicrobial activity of pomegranate peel extracts performed by high pressure and enzymatic assisted extraction. Food Res. Int. 2019, 115, 167–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, S.A.O.; Vilela, C.; Freire, C.S.R.; Neto, C.P.; Silvestre, A.J.D. Ultra-high performance liquid chromatography coupled to mass spectrometry applied to the identification of valuable phenolic compounds from eucalyptus wood. J. Chromatogr. B 2013, 938, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Abu-Ghannam, N. Bioactive potential and possible health effects of edible brown seaweeds. Trends Food Sci. Technol. 2011, 22, 315–326. [Google Scholar] [CrossRef] [Green Version]
- Abdala-Díaz, R.T.; Cabello-Pasini, A.; Pérez-Rodríguez, E.; Conde Álvarez, R.M.; Figueroa, F.L. Daily and seasonal variations of optimum quantum yield and phenolic compounds in Cystoseira tamariscifolia (Phaeophyta). Mar. Biol. 2006, 148, 459–465. [Google Scholar] [CrossRef]
- Martins, C.D.L.; Ramlov, F.; Nocchi Carneiro, N.P.; Gestinari, L.M.; dos Santos, B.F.; Bento, L.M.; Lhullier, C.; Gouvea, L.; Bastos, E.; Horta, P.A.; et al. Antioxidant properties and total phenolic contents of some tropical seaweeds of the Brazilian coast. J. Appl. Phycol. 2013, 25, 1179–1187. [Google Scholar] [CrossRef]
- Anaëlle, T.; Serrano Leon, E.; Laurent, V.; Elena, I.; Mendiola, J.A.; Stéphane, C.; Nelly, K.; Stéphane, L.B.; Luc, M.; Valérie, S.-P. Green improved processes to extract bioactive phenolic compounds from brown macroalgae using Sargassum muticum as model. Talanta 2013, 104, 44–52. [Google Scholar] [CrossRef]
- Catarino, M.D.; Silva, A.M.S.; Mateus, N.; Cardoso, S.M. Optimization of phlorotannins extraction from Fucus vesiculosus and evaluation of their potential to prevent metabolic disorders. Mar. Drugs 2019, 17, 162. [Google Scholar] [CrossRef] [Green Version]
- Steevensz, A.J.; Mackinnon, S.L.; Hankinson, R.; Craft, C.; Connan, S.; Stengel, D.B.; Melanson, J.E. Profiling phlorotannins in brown macroalgae by liquid chromatography-high resolution mass spectrometry. Phytochem. Anal. 2011, 23, 547–553. [Google Scholar] [CrossRef]
- Machu, L.; Misurcova, L.; Ambrozova, J.V.; Orsavova, J.; Mlcek, J.; Sochor, J.; Jurikova, T. Phenolic content and antioxidant capacity in algal food products. Molecules 2015, 20, 1118–1133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabeena Farvin, K.H.; Jacobsen, C. Phenolic compounds and antioxidant activities of selected species of seaweeds from Danish coast. Food Chem. 2013, 138, 1670–1681. [Google Scholar] [CrossRef] [PubMed]
- Hayat, S.; Atta-ur-Rahman; Choudhary, M.I.; Khan, K.M.; Abbaskhan, A. Two New Cinnamic Acid Esters from Marine Brown Alga Spatoglossum variabile. Chem. Pharm. Bull. (Tokyo) 2002, 50, 1297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yarnpakdee, S.; Benjakul, S.; Senphan, T. Antioxidant Activity of the Extracts from Freshwater Macroalgae (Cladophora glomerata) Grown in Northern Thailand and Its Preventive Effect against Lipid Oxidation of Refrigerated Eastern Little Tuna Slice. Turk. J. Fish. Aquat. Sci. 2018, 19, 209–219. [Google Scholar]
- Santoso, J.; Yoshie, Y.; Suzuki, T. The distribution and profile of nutrients and catechins of some Indonesian seaweeds. Fish. Sci. 2002, 68, 1647–1648. [Google Scholar] [CrossRef] [Green Version]
- Yoshie Stark, Y.; Hsieh, Y.P.; Suzuki, T. Distribution of flavonoids and related compounds from seaweeds in Japan. J. Tokyo Univ. Fish. 2003, 89, 1–6. [Google Scholar]
- Rajauria, G.; Foley, B.; Abu-Ghannam, N. Identification and characterization of phenolic antioxidant compounds from brown Irish seaweed Himanthalia elongata using LC-DAD–ESI-MS/MS. Innov. Food Sci. Emerg. Technol. 2016, 37, 261–268. [Google Scholar] [CrossRef]
- Klejdus, B.; Lojková, L.; Plaza, M.; Šnóblová, M.; Štěrbová, D. Hyphenated technique for the extraction and determination of isoflavones in algae: Ultrasound-assisted supercritical fluid extraction followed by fast chromatography with tandem mass spectrometry. J. Chromatogr. A 2010, 1217, 7956–7965. [Google Scholar] [CrossRef]
- Olate-Gallegos, C.; Barriga, A.; Vergara, C.; Fredes, C.; García, P.; Giménez, B.; Robert, P. Identification of Polyphenols from Chilean Brown Seaweeds Extracts by LC-DAD-ESI-MS/MS. J. Aquat. Food Prod. Technol. 2019, 28, 375–391. [Google Scholar] [CrossRef]
- Maheswari, M.U.; Reena, A.; Sivaraj, C. GC-MS analysis, antioxidant and antibacterial activity of the brown algae, Padina tetrastromatica. Int. J. Pharm. Sci. Res. 2018, 9, 298–304. [Google Scholar]
- Hartmann, A.; Ganzera, M.; Karsten, U.; Skhirtladze, A.; Stuppner, H. Phytochemical and analytical characterization of novel sulfated coumarins in the marine green macroalga Dasycladus vermicularis (Scopoli) Krasser. Molecules 2018, 23, 2735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flodin, C. 4-Hydroxybenzoic acid: A likely precursor of 2,4,6-tribromophenol in Ulva lactuca. Phytochemistry 1999, 51, 249–255. [Google Scholar] [CrossRef]
- Choudhary, M.I.; Hayat, S.; Khan, A.M.; Ahmed, A. Two New Aurones from Marine Brown Alga Spatoglossum variabile. Chem. Pharm. Bull. (Tokyo) 2001, 49, 105–107. [Google Scholar]
- De Carvalho, L.R.; Roque, N.F. Fenóis halogenados e/ou sulfatados de macroalgas marinhas. Quim. Nova 2000, 23, 757–764. [Google Scholar] [CrossRef]
- Jensen, A.; Ragan, M.A. 1,2,3,5-tetrahydroxybenzene 2,5-disulfate ester: The “phenolic precursor” in gelbstoff-forming exudates from the marine brown alga Ascophyllum nodosum (L.) Lejol. Tetrahedron Lett. 1978, 19, 847–850. [Google Scholar] [CrossRef]
- Birtić, S.; Dussort, P.; Pierre, F.X.; Bily, A.C.; Roller, M. Carnosic acid. Phytochemistry 2015, 115, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Barwell, C.J.; Blunden, G.; Manandhar, P.D. Isolation and characterization of brown algal polyphenols as inhibitors of α-amylase, lipase and trypsin. J. Appl. Phycol. 1989, 1, 319–323. [Google Scholar] [CrossRef]
- Heffernan, N.; Brunton, N.P.; FitzGerald, R.J.; Smyth, T.J. Profiling of the molecular weight and structural isomer abundance of macroalgae-derived phlorotannins. Mar. Drugs 2015, 13, 509–528. [Google Scholar] [CrossRef]
- Li, Y.-X.; Wijesekara, I.; Li, Y.; Kim, S.-K. Phlorotannins as bioactive agents from brown algae. Process Biochem. 2011, 46, 2219–2224. [Google Scholar] [CrossRef]
- Singh, I.P.; Bharate, S.B. Phloroglucinol compounds of natural origin. Nat. Prod. Rep. 2006, 23, 558–591. [Google Scholar] [CrossRef]
- Singh, I.P.; Sidana, J. Phlorotannins. In Functional Ingredients from Algae for Foods and Nutraceuticals; Elsevier: New Delhi, India, 2013; pp. 181–204. ISBN 9780857095121. [Google Scholar]
- Lee, M.S.; Shin, T.; Utsuki, T.; Choi, J.S.; Byun, D.S.; Kim, H.R. Isolation and identification of phlorotannins from Ecklonia stolonifera with antioxidant and hepatoprotective properties in tacrine-treated HepG2 cells. J. Agric. Food Chem. 2012, 60, 5340–5349. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Ko, J.-Y.; Oh, J.-Y.; Kim, C.-Y.; Lee, H.-J.; Kim, J.; Jeon, Y.-J. Preparative isolation and purification of phlorotannins from Ecklonia cava using centrifugal partition chromatography by one-step. Food Chem. 2014, 158, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Koivikko, R.; Loponen, J.; Pihlaja, K.; Jormalainen, V. High-performance liquid chromatographic analysis of phlorotannins from the brown alga Fucus vesiculosus. Phytochem. Anal. Int. J. Plant Chem. Biochem. Tech. 2007, 18, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Ferreres, F.; Lopes, G.; Gil-Izquierdo, A.; Andrade, P.B.; Sousa, C.; Mouga, T.; Valentão, P. Phlorotannin extracts from fucales characterized by HPLC-DAD-ESI-MSN: Approaches to hyaluronidase inhibitory capacity and antioxidant properties. Mar. Drugs 2012, 10, 2766–2781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montero, L.; Herrero, M.; Ibáñez, E.; Cifuentes, A. Separation and characterization of phlorotannins from brown algae Cystoseira abies-marina by comprehensive two-dimensional liquid chromatography. Electrophoresis 2014, 35, 1644–1651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koch, M.; Glombitza, K.W.; Eckhard, G. Phlorotannins of phaeophycea Laminaria ochroleuca. Phytochemistry 1980, 19, 1821–1823. [Google Scholar] [CrossRef]
- Kim, S.M.; Kang, S.W.; Jeon, J.-S.; Jung, Y.-J.; Kim, W.-R.; Kim, C.Y.; Um, B.-H. Determination of major phlorotannins in Eisenia bicyclis using hydrophilic interaction chromatography: Seasonal variation and extraction characteristics. Food Chem. 2013, 138, 2399–2406. [Google Scholar] [CrossRef]
- Glombitza, K.-W.; Kno, W. Sulphated phlorotannins from the brown alga Pleurophycus gardneri. Phytochemistry 1992, 31, 279–281. [Google Scholar] [CrossRef]
- Glombitza, K.-W.; Schmidt, A. Nonhalogenated and Halogenated Phlorotannins from the Brown Alga Carpophyllum angustifolium. J. Nat. Prod. 1999, 62, 1238–1240. [Google Scholar] [CrossRef]
- Sailler, B.; Glombitza, K.W. Halogenated phlorethols and fucophlorethols from the brown alga Cystophora retroflexa. Nat. Toxins 1999, 7, 57–62. [Google Scholar] [CrossRef]
- De Corato, U.; Salimbeni, R.; De Pretis, A.; Avella, N.; Patruno, G. Antifungal activity of crude extracts from brown and red seaweeds by a supercritical carbon dioxide technique against fruit postharvest fungal diseases. Postharvest Biol. Technol. 2017, 131, 16–30. [Google Scholar] [CrossRef]
- Glombitza, K.-W.; Keusgen, M.; Hauperich, S. Fucophlorethols from the brown algae Sargassum spinuligerum and Cystophora torulosa. Phytochemistry 1997, 46, 1417–1422. [Google Scholar] [CrossRef]
- Vissers, A.M.; Caligiani, A.; Sforza, S.; Vincken, J.-P.; Gruppen, H. Phlorotannin Composition of Laminaria digitata. Phytochem. Anal. 2017, 28, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Glombitza, K.-W.; Gerstberger, G. Phlorotannins with dibenzodioxin structural elements from the brown alga Eisenia arborea. Phytochemistry 1985, 24, 543–551. [Google Scholar] [CrossRef]
- Koch, M.; Gregson, R.P. Brominated phlorethols and nonhalogenated phlorotannins from the brown alga Cystophora congesta. Phytochemistry 1984, 23, 2633–2637. [Google Scholar] [CrossRef]
- Ragan, M.A.; Glombitza, K.-W. Phlorotannins, brown algal polyphenols. In Progress in Phycological Research; Round, F.E., Chapman, D.J., Eds.; Biopress: Bristol, UK, 1982; Volume 4, pp. 129–241. ISBN 094873700X. [Google Scholar]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef] [Green Version]
- Sanjeewa, K.K.A.; Kim, E.A.; Son, K.T.; Jeon, Y.J. Bioactive properties and potentials cosmeceutical applications of phlorotannins isolated from brown seaweeds: A review. J. Photochem. Photobiol. B Biol. 2016, 162, 100–105. [Google Scholar] [CrossRef]
- Montero, L.; Sánchez-Camargo, A.D.P.; Ibáñez, E.; Gilbert-López, B. Phenolic Compounds from Edible Algae: Bioactivity and Health Benefits. Curr. Med. Chem. 2018, 25, 4808–4826. [Google Scholar] [CrossRef]
- Naczk, M.; Shahidi, F. Extraction and analysis of phenolics in food. J. Chromatogr. A 2004, 1054, 95–111. [Google Scholar] [CrossRef]
- Liazid, A.; Palma, M.; Brigui, J.; Barroso, C.G. Investigation on phenolic compounds stability during microwave-assisted extraction. J. Chromatogr. A 2007, 1140, 29–34. [Google Scholar] [CrossRef]
- Palma, M.; Piñeiro, Z.; Barroso, C.G. Stability of phenolic compounds during extraction with superheated solvents. J. Chromatogr. A 2001, 921, 169–174. [Google Scholar] [CrossRef]
- Safari, P.; Rezaei, M.; Shaviklo, A.R. The optimum conditions for the extraction of antioxidant compounds from the Persian gulf green algae (Chaetomorpha sp.) using response surface methodology. J. Food Sci. Technol. 2015, 52, 2974–2981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadam, S.U.; Tiwari, B.K.; Smyth, T.J.; O’Donnell, C.P. Optimization of ultrasound assisted extraction of bioactive components from brown seaweed Ascophyllum nodosum using response surface methodology. Ultrason. Sonochem. 2015, 23, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, R.; Chitra, L.; Penislusshiyan, S.; Palvannan, T. Exploring bioactive fraction of Sargassum wightii: In vitro elucidation of angiotensin-I-converting enzyme inhibition and antioxidant potential. Int. J. Food Prop. 2018, 21, 674–684. [Google Scholar] [CrossRef] [Green Version]
- Houchi, S.; Mahdadi, R.; Khenchouche, A.; Song, J.; Zhang, W.; Pang, X.; Zhang, L.; Sandalli, C.; Du, G. Investigation of common chemical components and inhibitory effect on GES-type β-lactamase (GES22) in methanolic extracts of Algerian seaweeds. Microb. Pathog. 2019, 126, 56–62. [Google Scholar] [CrossRef]
- Lopes, G.; Barbosa, M.; Vallejo, F.; Gil-Izquierdo, Á.; Andrade, P.B.; Valentão, P.; Pereira, D.M.; Ferreres, F. Profiling phlorotannins from Fucus spp. of the Northern Portuguese coastline: Chemical approach by HPLC-DAD-ESI/MSn and UPLC-ESI-QTOF/MS. Algal Res. 2018, 29, 113–120. [Google Scholar] [CrossRef]
- Hermund, D.B.; Plaza, M.; Turner, C.; Jónsdóttir, R.; Kristinsson, H.G.; Jacobsen, C.; Nielsen, K.F. Structure dependent antioxidant capacity of phlorotannins from Icelandic Fucus vesiculosus by UHPLC-DAD-ECD-QTOFMS. Food Chem. 2018, 240, 904–909. [Google Scholar] [CrossRef] [Green Version]
- Phasanasophon, K.; Kim, S.M. Antioxidant and Cosmeceutical Activities of Agarum cribrosum Phlorotannin Extracted by Ultrasound Treatment. Nat. Prod. Commun. 2019, 13, 1934578X1801300. [Google Scholar] [CrossRef]
- Imbs, T.I.; Silchenko, A.S.; Fedoreev, S.A.; Isakov, V.V.; Ermakova, S.P.; Zvyagintseva, T.N. Fucoidanase inhibitory activity of phlorotannins from brown algae. Algal Res. 2018, 32, 54–59. [Google Scholar] [CrossRef]
- Parys, S.; Kehraus, S.; Krick, A.; Glombitza, K.-W.; Carmeli, S.; Klimo, K.; Gerhäuser, C.; König, G.M. In vitro chemopreventive potential of fucophlorethols from the brown alga Fucus vesiculosus L. by anti-oxidant activity and inhibition of selected cytochrome P450 enzymes. Phytochemistry 2010, 71, 221–229. [Google Scholar] [CrossRef]
- Rajauria, G. Optimization and validation of reverse phase HPLC method for qualitative and quantitative assessment of polyphenols in seaweed. J. Pharm. Biomed. Anal. 2018, 148, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Parys, S.; Rosenbaum, A.; Kehraus, S.; Reher, G.; Glombitza, K.W.; König, G.M. Evaluation of quantitative methods for the determination of polyphenols in algal extracts. J. Nat. Prod. 2007, 70, 1865–1870. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, K.; Joseph, D.; Praveen, N.K. Antioxidant activities and phenolic contents of three red seaweeds (Division: Rhodophyta) harvested from the Gulf of Mannar of Peninsular India. J. Food Sci. Technol. 2015, 52, 1924–1935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ling, A.L.M.; Yasir, S.; Matanjun, P.; Abu Bakar, M.F. Effect of different drying techniques on the phytochemical content and antioxidant activity of Kappaphycus alvarezii. J. Appl. Phycol. 2015, 27, 1717–1723. [Google Scholar] [CrossRef]
- Gupta, S.; Cox, S.; Abu-Ghannam, N. Effect of different drying temperatures on the moisture and phytochemical constituents of edible Irish brown seaweed. LWT-Food Sci. Technol. 2011, 44, 1266–1272. [Google Scholar] [CrossRef] [Green Version]
- Cruces, E.; Rojas-Lillo, Y.; Ramirez-Kushel, E.; Atala, E.; López-Alarcón, C.; Lissi, E.; Gómez, I. Comparison of different techniques for the preservation and extraction of phlorotannins in the kelp Lessonia spicata (Phaeophyceae): Assays of DPPH, ORAC-PGR, and ORAC-FL as testing methods. J. Appl. Phycol. 2016, 28, 573–580. [Google Scholar] [CrossRef]
- Leyton, A.; Pezoa-Conte, R.; Barriga, A.; Buschmann, A.H.; Mäki-Arvela, P.; Mikkola, J.-P.; Lienqueo, M.E. Identification and efficient extraction method of phlorotannins from the brown seaweed Macrocystis pyrifera using an orthogonal experimental design. Algal Res. 2016, 16, 201–208. [Google Scholar] [CrossRef]
- Lopes, G.; Sousa, C.; Silva, L.R.; Pinto, E.; Andrade, P.B.; Bernardo, J.; Mouga, T.; Valentão, P. Can phlorotannins purified extracts constitute a novel pharmacological alternative for microbial infections with associated inflammatory conditions? PLoS ONE 2012, 7, e31145. [Google Scholar] [CrossRef]
- Leyton, A.; Pezoa-Conte, R.; Mäki-Arvela, P.; Mikkola, J.-P.; Lienqueo, M.E. Improvement in carbohydrate and phlorotannin extraction from Macrocystis pyrifera using carbohydrate active enzyme from marine Alternaria sp. as pretreatment. J. Appl. Phycol. 2017, 29, 2039–2048. [Google Scholar] [CrossRef]
- Onofrejová, L.; Vasícková, J.; Klejdus, B.; Stratil, P.; Misurcová, L.; Krácmar, S.; Kopecký, J.; Vacek, J. Bioactive phenols in algae: The application of pressurized-liquid and solid-phase extraction techniques. J. Pharm. Biomed. Anal. 2010, 51, 464–470. [Google Scholar] [CrossRef]
- Sugiura, Y.; Matsuda, K.; Yamada, Y.; Nishikawa, M.; Shioya, K.; Katsuzaki, H.; Imai, K.; Amano, H. Isolation of a new anti-allergic phlorotannin, phlorofucofuroeckol-B, from an Edible Brown Alga, Eisenia arborea. Biosci. Biotechnol. Biochem. 2006, 70, 2807–2811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, S.A.O.; Vilela, C.; Freire, C.S.R.; Abreu, M.H.; Rocha, S.M.; Silvestre, A.J.D. Chlorophyta and Rhodophyta macroalgae: A source of health promoting phytochemicals. Food Chem. 2015, 183, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.A.O.; Oliveira, C.S.D.; Trindade, S.S.; Abreu, M.H.; Rocha, S.S.M.; Silvestre, A.J.D. Bioprospecting for lipophilic-like components of five Phaeophyta macroalgae from the Portuguese coast. J. Appl. Phycol. 2016, 28, 3151–3158. [Google Scholar] [CrossRef]
- Kim, Y.C.; Ren, B.A.; Na, Y.Y.; Taek, J.N.; Jae, S.C. Hepatoprotective constituents of the edible brown alga Ecklonia stolonifera on tacrine-induced cytotoxicity in Hep G2 cells. Arch. Pharm. Res. 2005, 28, 1376–1380. [Google Scholar] [CrossRef]
- Nwosu, F.; Morris, J.; Lund, V.A.; Stewart, D.; Ross, H.A.; McDougall, G.J. Anti-proliferative and potential anti-diabetic effects of phenolic-rich extracts from edible marine algae. Food Chem. 2011, 126, 1006–1012. [Google Scholar] [CrossRef]
- Tierney, M.S.; Smyth, T.J.; Rai, D.K.; Soler-Vila, A.; Croft, A.K.; Brunton, N. Enrichment of polyphenol contents and antioxidant activities of Irish brown macroalgae using food-friendly techniques based on polarity and molecular size. Food Chem. 2013, 139, 753–761. [Google Scholar] [CrossRef]
- Koivikko, R.; Loponen, J.; Honkanen, T.; Jormalainen, V. Contents of soluble, cell-wall-bound and exuded phlorotannins in the brown alga Fucus vesiculosus, with implications on their ecological functions. J. Chem. Ecol. 2005, 31, 195–212. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Jónsdóttir, R.; Liu, H.; Gu, L.; Kristinsson, H.G.; Raghavan, S.; Ólafsdóttir, G. Antioxidant capacities of phlorotannins extracted from the brown algae Fucus vesiculosus. J. Agric. Food Chem. 2012, 60, 5874–5883. [Google Scholar] [CrossRef]
- Passos, H.; Freire, M.G.; Coutinho, J.A.P. Ionic liquid solutions as extractive solvents for value-added compounds from biomass. Green Chem. 2014, 16, 4786–4815. [Google Scholar] [CrossRef] [Green Version]
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural deep eutectic solvents-Solvents for the 21st century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071. [Google Scholar] [CrossRef]
- Dai, Y.; Van Spronsen, J.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Ionic liquids and deep eutectic solvents in natural products research: Mixtures of solids as extraction solvents. J. Nat. Prod. 2013, 76, 2162–2173. [Google Scholar] [CrossRef] [PubMed]
- Saltmarsh, M.; Santos-Buelga, C.; Williamson, G. (Eds.) Methods in Polyphenol Analysis; RSC: Great Britain, UK, 2003; ISBN 978-0-85404-580-8. [Google Scholar]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Chowdhury, M.T.H.; Bangoura, I.; Kang, J.-Y.; Cho, J.Y.; Joo, J.; Choi, Y.S.; Hwang, D.S.; Hong, Y.-K. Comparison of Ecklonia cava, Ecklonia stolonifera and Eisenia bicyclis for phlorotannin extraction. J. Environ. Biol. 2014, 35, 713–719. [Google Scholar] [PubMed]
- Tierney, M.S.; Smyth, T.J.; Hayes, M.; Soler-Vila, A.; Croft, A.K.; Brunton, N. Influence of pressurised liquid extraction and solid-liquid extraction methods on the phenolic content and antioxidant activities of Irish macroalgae. Int. J. Food Sci. Technol. 2013, 48, 860–869. [Google Scholar] [CrossRef]
- Pantidos, N.; Boath, A.; Lund, V.; Conner, S.; McDougall, G.J. Phenolic-rich extracts from the edible seaweed, Ascophyllum nodosum, inhibit α-amylase and α-glucosidase: Potential anti-hyperglycemic effects. J. Funct. Foods 2014, 10, 201–209. [Google Scholar] [CrossRef]
- Moshfegh, A.; Salehzadeh, A.; Sadat Shandiz, S.A.; Shafaghi, M.; Naeemi, A.S.; Salehi, S. Phytochemical Analysis, Antioxidant, Anticancer and Antibacterial Properties of the Caspian Sea Red Macroalgae, Laurencia caspica. Iran. J. Sci. Technol. Trans. A Sci. 2019, 43, 49–56. [Google Scholar] [CrossRef]
- Heffernan, N.; Smyth, T.J.; Fitzgerald, R.J.; Soler-Vila, A.; Brunton, N. Antioxidant activity and phenolic content of pressurised liquid and solid-liquid extracts from four Irish origin macroalgae. Int. J. Food Sci. Technol. 2014, 49, 1765–1772. [Google Scholar] [CrossRef]
- Zenthoefer, M.; Geisen, U.; Hofmann-Peiker, K.; Fuhrmann, M.; Kerber, J.; Kirchhöfer, R.; Hennig, S.; Peipp, M.; Geyer, R.; Piker, L.; et al. Isolation of polyphenols with anticancer activity from the Baltic Sea brown seaweed Fucus vesiculosus using bioassay-guided fractionation. J. Appl. Phycol. 2017, 29, 2021–2037. [Google Scholar] [CrossRef]
- Bernardini, G.; Minetti, M.; Polizzotto, G.; Biazzo, M.; Santucci, A. Pro-Apoptotic Activity of French Polynesian Padina pavonica Extract on Human Osteosarcoma Cells. Mar. Drugs 2018, 16, 504. [Google Scholar] [CrossRef] [Green Version]
- Domozych, D.S. Algal Cell Walls. In eLS.; John Wiley & Sons, Ltd.: Chichester, UK, 2011. [Google Scholar]
- Stern, J.L.; Hagerman, A.E.; Steinberg, P.D.; Mason, P.K. Phlorotannins-protein interactions. J. Chem. Ecol. 1996, 22, 1877–1899. [Google Scholar] [CrossRef]
- Rodrigues, D.; Sousa, S.; Silva, A.; Amorim, M.; Pereira, L.; Rocha-Santos, T.A.P.; Gomes, A.M.P.; Duarte, A.C.; Freitas, A.C. Impact of enzyme-and ultrasound-assisted extraction methods on biological properties of red, brown, and green seaweeds from the Central West Coast of Portugal. J. Agric. Food Chem. 2015, 63, 3177–3188. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Camargo, A.D.P.; Montero, L.; Stiger-Pouvreau, V.; Tanniou, A.; Cifuentes, A.; Herrero, M.; Ibáñez, E. Considerations on the use of enzyme-assisted extraction in combination with pressurized liquids to recover bioactive compounds from algae. Food Chem. 2016, 192, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Jónsdóttir, R.; Kristinsson, H.G.; Hreggvidsson, G.O.; Jónsson, J.Ó.; Thorkelsson, G.; Ólafsdóttir, G. Enzyme-enhanced extraction of antioxidant ingredients from red algae Palmaria palmata. LWT-Food Sci. Technol. 2010, 43, 1387–1393. [Google Scholar] [CrossRef]
- Olivares-Molina, A.; Fernández, K. Comparison of different extraction techniques for obtaining extracts from brown seaweeds and their potential effects as angiotensin I-converting enzyme (ACE) inhibitors. J. Appl. Phycol. 2016, 28, 1295–1302. [Google Scholar] [CrossRef]
- Siriwardhana, N.; Kim, K.-N.; Lee, K.-W.; Kim, S.-H.; Ha, J.-H.; Song, C.B.; Lee, J.-B.; Jeon, Y.-J. Optimisation of hydrophilic antioxidant extraction from Hizikia fusiformis by integrating treatments of enzymes, heat and pH control. Int. J. Food Sci. Technol. 2008, 43, 587–596. [Google Scholar] [CrossRef]
- Charoensiddhi, S.; Franco, C.; Su, P.; Zhang, W. Improved antioxidant activities of brown seaweed Ecklonia radiata extracts prepared by microwave-assisted enzymatic extraction. J. Appl. Phycol. 2015, 27, 2049–2058. [Google Scholar] [CrossRef]
- Park, S.R.; Kim, J.H.; Jang, H.D.; Yang, S.Y.; Kim, Y.H. Inhibitory activity of minor phlorotannins from Ecklonia cava on α-glucosidase. Food Chem. 2018, 257, 128–134. [Google Scholar] [CrossRef]
- Tanna, B.; Choudhary, B.; Mishra, A. Metabolite profiling, antioxidant, scavenging and anti-proliferative activities of selected tropical green seaweeds reveal the nutraceutical potential of Caulerpa spp. Algal Res. 2018, 36, 96–105. [Google Scholar] [CrossRef]
- Richter, B.E.; Jones, B.A.; Ezzell, J.L.; Porter, N.L.; Avdalovic, N.; Pohl, C. Accelerated Solvent Extraction: A Technique for Sample Preparation. Anal. Chem. 1996, 68, 1033–1039. [Google Scholar] [CrossRef]
- Montero, L.; Sánchez-Camargo, A.P.; García-Cañas, V.; Tanniou, A.; Stiger-Pouvreau, V.; Russo, M.; Rastrelli, L.; Cifuentes, A.; Herrero, M.; Ibáñez, E. Anti-proliferative activity and chemical characterization by comprehensive two-dimensional liquid chromatography coupled to mass spectrometry of phlorotannins from the brown macroalga Sargassum muticum collected on North-Atlantic coasts. J. Chromatogr. A 2016, 1428, 115–125. [Google Scholar] [CrossRef]
- Otero, P.; López-Martínez, M.I.; García-Risco, M.R. Application of pressurized liquid extraction (PLE) to obtain bioactive fatty acids and phenols from Laminaria ochroleuca collected in Galicia (NW Spain). J. Pharm. Biomed. Anal. 2019, 164, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Heavisides, E.; Rouger, C.; Reichel, A.F.; Ulrich, C.; Wenzel-Storjohann, A.; Sebens, S.; Tasdemir, D. Seasonal Variations in the Metabolome and Bioactivity Profile of Fucus vesiculosus Extracted by an Optimised, Pressurised Liquid Extraction Protocol. Mar. Drugs 2018, 16, 503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Routray, W.; Orsat, V. Microwave-Assisted Extraction of Flavonoids: A Review. Food Bioprocess Technol. 2012, 5, 409–424. [Google Scholar] [CrossRef]
- He, Z.; Chen, Y.; Chen, Y.; Liu, H.; Yuan, G.; Fan, Y.; Chen, K. Optimization of the microwave-assisted extraction of phlorotannins from Saccharina japonica Aresch and evaluation of the inhibitory effects of phlorotannin-containing extracts on HepG2 cancer cells. Chin. J. Oceanol. Limnol. 2013, 31, 1045–1054. [Google Scholar] [CrossRef]
- Li, Z.; Wang, B.; Zhang, Q.; Qu, Y.; Xu, H.; Li, G. Preparation and antioxidant property of extract and semipurified fractions of Caulerpa racemosa. J. Appl. Phycol. 2012, 24, 1527–1536. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, J.; Fan, J.; Clark, J.; Shen, P.; Li, Y.; Zhang, C. Microwave assisted extraction of phenolic compounds from four economic brown macroalgae species and evaluation of their antioxidant activities and inhibitory effects on α-amylase, α-glucosidase, pancreatic lipase and tyrosinase. Food Res. Int. 2018, 113, 288–297. [Google Scholar] [CrossRef]
- Michalak, I.; Tuhy, Ł.; Chojnacka, K. Seaweed extract by microwave assisted extraction as plant growth biostimulant. Open Chem. 2015, 13, 1183–1195. [Google Scholar] [CrossRef]
- Pérez, L.; Conde, E.; Domínguez, H. Microwave hydrodiffusion and gravity processing of Sargassum muticum. Process Biochem. 2014, 49, 981–988. [Google Scholar] [CrossRef]
- Luque-García, J.; Luque de Castro, M. Ultrasound: A powerful tool for leaching. TrAC Trends Anal. Chem. 2003, 22, 41–47. [Google Scholar] [CrossRef]
- Kadam, S.U.; Tiwari, B.K.; O’Connell, S.; O’Donnell, C.P. Effect of Ultrasound Pretreatment on the Extraction Kinetics of Bioactives from Brown Seaweed (Ascophyllum nodosum). Sep. Sci. Technol. 2015, 50, 670–675. [Google Scholar] [CrossRef]
- Zhou, X.; Yi, M.; Ding, L.; He, S.; Yan, X. Isolation and Purification of a Neuroprotective Phlorotannin from the Marine Algae Ecklonia maxima by Size Exclusion and High-Speed Counter-Current Chromatography. Mar. Drugs 2019, 17, 212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, H.; Doan, T.; Ha, T.; Kim, H.; Lee, B.; Pham, H.; Cho, T.; Oh, W.; Cho, H.M.; Doan, T.P.; et al. Dereplication by High-Performance Liquid Chromatography (HPLC) with Quadrupole-Time-of-Flight Mass Spectroscopy (qTOF-MS) and Antiviral Activities of Phlorotannins from Ecklonia cava. Mar. Drugs 2019, 17, 149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Topuz, O.K.; Gokoglu, N.; Yerlikaya, P.; Ucak, I.; Gumus, B. Optimization of Antioxidant Activity and Phenolic Compound Extraction Conditions from Red Seaweed (Laurencia obtuse). J. Aquat. Food Prod. Technol. 2016, 25, 414–422. [Google Scholar] [CrossRef]
- Agregán, R.; Munekata, P.E.S.; Franco, D.; Dominguez, R.; Carballo, J.; Lorenzo, J.M. Phenolic compounds from three brown seaweed species using LC-DAD–ESI-MS/MS. Food Res. Int. 2017, 99, 979–985. [Google Scholar] [CrossRef]
- Morán-Santibañez, K.; Peña-Hernández, M.; Cruz-Suárez, L.; Ricque-Marie, D.; Skouta, R.; Vasquez, A.; Rodríguez-Padilla, C.; Trejo-Avila, L. Virucidal and Synergistic Activity of Polyphenol-Rich Extracts of Seaweeds against Measles Virus. Viruses 2018, 10, 465. [Google Scholar] [CrossRef] [Green Version]
- Lin, G.; Liu, X.; Yan, X.; Liu, D.; Yang, C.; Liu, B.; Huang, Y.; Zhao, C. Role of green macroalgae Enteromorpha prolifera Polyphenols in the modulation of gene expression and intestinal microflora profiles in Type 2 diabetic mice. Int. J. Mol. Sci. 2018, 20, 25. [Google Scholar] [CrossRef] [Green Version]
- Reverchon, E.; De Marco, I. Supercritical fluid extraction and fractionation of natural matter. J. Supercrit. Fluids 2006, 38, 146–166. [Google Scholar] [CrossRef]
- Herrero, M.; Mendiola, J.A.; Cifuentes, A.; Ibáñez, E. Supercritical fluid extraction: Recent advances and applications. J. Chromatogr. A 2010, 1217, 2495–2511. [Google Scholar] [CrossRef] [Green Version]
- Díaz-Reinoso, B.; Moure, A.; Domínguez, H.; Parajó, J.C. Supercritical CO2 Extraction and Purification of Compounds with Antioxidant Activity. J. Agric. Food Chem. 2006, 54, 2441–2469. [Google Scholar] [CrossRef]
- Lang, Q. Supercritical fluid extraction in herbal and natural product studies—A practical review. Talanta 2001, 53, 771–782. [Google Scholar] [CrossRef]
- Conde, E.; Moure, A.; Domínguez, H. Supercritical CO2 extraction of fatty acids, phenolics and fucoxanthin from freeze-dried Sargassum muticum. J. Appl. Phycol. 2014, 27, 957–964. [Google Scholar] [CrossRef]
- Roh, M.K.; Uddin, M.S.; Chun, B.S. Extraction of fucoxanthin and polyphenol from Undaria pinnatifida using supercritical carbon dioxide with co-solvent. Biotechnol. Bioprocess Eng. 2008, 13, 724–729. [Google Scholar] [CrossRef]
- Michalak, I.; Górka, B.; Wieczorek, P.P.; Rój, E.; Lipok, J.; Łęska, B.; Messyasz, B.; Wilk, R.; Schroeder, G.; Dobrzyńska-Inger, A.; et al. Supercritical fluid extraction of algae enhances levels of biologically active compounds promoting plant growth. Eur. J. Phycol. 2016, 51, 243–252. [Google Scholar] [CrossRef]
- Kosanić, M.; Ranković, B.; Stanojković, T. Biological activities of two macroalgae from Adriatic coast of Montenegro. Saudi J. Biol. Sci. 2015, 22, 390–397. [Google Scholar] [CrossRef] [Green Version]
- Folin, O.; Denis, W. On phosphotungstic-phosphomolybdic compounds as color reagents. J. Biol. Chem. 1912, 2, 239–243. [Google Scholar]
- Folin, O.; Ciocalteau, V. Tyrosine and Tryptophane in Proteins. J. Biol. Chem. 1927, 73, 627–648. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Stern, J.L.; Hagerman, A.E.; Steinberg, P.D.; Winter, F.C.; Estes, J.A. A new assay for quantifying brown algal phlorotannins and comparisons to previous methods. J. Chem. Ecol. 1996, 22, 1273–1293. [Google Scholar] [CrossRef]
- Price, M.L.; Butler, L.G. Rapid Visual Estimation and Spectrophotometric Determination of Tannin Content of Sorghum Grain. J. Agric. Food Chem. 1977, 25, 1268–1273. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Magnusson, M.; Mata, L.; Wang, N.; Zhao, J.; de Nys, R.; Paul, N.A. Manipulating antioxidant content in macroalgae in intensive land-based cultivation systems for functional food applications. Algal Res. 2015, 8, 153–160. [Google Scholar] [CrossRef]
- Celis-Plá, P.S.M.; Martínez, B.; Quintano, E.; García-Sánchez, M.; Pedersen, A.; Navarro, N.P.; Copertino, M.S.; Mangaiyarkarasi, N.; Mariath, R.; Figueroa, F.L.; et al. Short-term ecophysiological and biochemical responses of Cystoseira tamariscifolia and Ellisolandia elongata to environmental changes. Aquat. Biol. 2014, 22, 227–243. [Google Scholar] [CrossRef] [Green Version]
- Celis-Plá, P.S.M.; Korbee, N.; Gómez-Garreta, A.; Figueroa, F.L. Seasonal photoacclimation patterns in the intertidal macroalga Cystoseira tamariscifolia (Ochrophyta). Sci. Mar. 2014, 78, 377–388. [Google Scholar] [CrossRef] [Green Version]
- Shahidi, F.; Naczk, M. Phenolics in Food and Nutraceuticals; CRC Press: Boca Raton, FL, USA, 2006; ISBN 0203508734. [Google Scholar]
- Waterman, P.G.; Mole, S. Analysis of Phenolic Plant Metabolites; Wiley: Oxford, UK, 1994. [Google Scholar]
- Paiva, L.; Lima, E.; Neto, A.I.; Baptista, J. Angiotensin I-converting enzyme (ACE) inhibitory activity of Fucus spiralis macroalgae and influence of the extracts storage temperature-A short report. J. Pharm. Biomed. Anal. 2016, 131, 503–507. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.N.; Cheung, P.C.K.; Ooi, V.E.C.; Ang, P.O. Evaluation of antioxidative activity of extracts from a brown seaweed, Sargassum siliquastrum. J. Agric. Food Chem. 2002, 50, 3862–3866. [Google Scholar] [CrossRef]
- Rajauria, G.; Jaiswal, A.K.; Abu-Ghannam, N.; Gupta, S. Antimicrobial, antioxidant and free radical-scavenging capacity of brown seaweed Himanthalia elongata from western coast of Ireland. J. Food Biochem. 2013, 37, 322–335. [Google Scholar] [CrossRef]
- Wang, B.-G.; Zhang, W.-W.; Duan, X.-J.; Li, X.-M. In vitro antioxidative activities of extract and semi-purified fractions of the marine red alga, Rhodomela confervoides (Rhodomelaceae). Food Chem. 2009, 113, 1101–1105. [Google Scholar] [CrossRef]
- Tanniou, A.; Vandanjon, L.; Incera, M.; Leon, E.S.; Husa, V.; Le Grand, J.; Nicolas, J.-L.; Poupart, N.; Kervarec, N.; Engelen, A.; et al. Assessment of the spatial variability of phenolic contents and associated bioactivities in the invasive alga Sargassum muticum sampled along its European range from Norway to Portugal. J. Appl. Phycol. 2014, 26, 1215–1230. [Google Scholar] [CrossRef] [Green Version]
- Breton, F.; Cerantola, S.; Gall, E.A. Distribution and radical scavenging activity of phenols in Ascophyllum nodosum (Phaeophyceae). J. Exp. Mar. Bio. Ecol. 2011, 399, 167–172. [Google Scholar] [CrossRef]
- Liu, B.; Kongstad, K.T.; Wiese, S.; Jäger, A.K.; Staerk, D. Edible seaweed as future functional food: Identification of α-glucosidase inhibitors by combined use of high-resolution α-glucosidase inhibition profiling and HPLC-HRMS-SPE-NMR. Food Chem. 2016, 203, 16–22. [Google Scholar] [CrossRef]
- Jégou, C.; Kervarec, N.; Cérantola, S.; Bihannic, I.; Stiger-Pouvreau, V. NMR use to quantify phlorotannins: The case of Cystoseira tamariscifolia, a phloroglucinol-producing brown macroalga in Brittany (France). Talanta 2015, 135, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Audibert, L.; Fauchon, M.; Blanc, N.; Hauchard, D.; Ar Gall, E. Phenolic compounds in the brown seaweed Ascophyllum nodosum: Distribution and radical-scavenging activities. Phytochem. Anal. 2010, 21, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Parys, S.; Kehraus, S.; Pete, R.; Küpper, F.C.; Glombitza, K.W.; König, G.M. Seasonal variation of polyphenolics in Ascophyllum nodosum (Phaeophyceae). Eur. J. Phycol. 2009, 44, 331–338. [Google Scholar] [CrossRef]
- Steinberg, P.D.; Van Altena, I. Tolerance of marine invertebrate herbivores to brown algal phlorotannins in temperate Australasia. Ecol. Monogr. 1992, 62, 189–222. [Google Scholar] [CrossRef]
- Jung, H.A.; Jin, S.E.; Ahn, B.R.; Lee, C.M.; Choi, J.S. Anti-inflammatory activity of edible brown alga Eisenia bicyclis and its constituents fucosterol and phlorotannins in LPS-stimulated RAW264.7 macrophages. Food Chem. Toxicol. 2013, 59, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.-C.; Cha, S.-H.; Heo, S.-J.; Lee, S.-H.; Kang, S.-M.; Jeon, Y.-J. Protective effect of Ecklonia cava on UVB-induced oxidative stress: In vitro and in vivo zebrafish model. J. Appl. Phycol. 2011, 23, 697–708. [Google Scholar] [CrossRef]
- Zou, Y.; Qian, Z.-J.; Li, Y.; Kim, M.-M.; Lee, S.-H.; Kim, S.-K. Antioxidant effects of phlorotannins isolated from Ishige okamurae in free radical mediated oxidative systems. J. Agric. Food Chem. 2008, 56, 7001–7009. [Google Scholar] [CrossRef]
- Glombitza, K.W.; Keusgen, M. Fuhalols and deshydroxyfuhalols from the brown alga Sargassum spinuligerum. Phytochemistry 1995, 38, 987–995. [Google Scholar] [CrossRef]
- Heffernan, N.; Smyth, T.J.; Soler-Villa, A.; Fitzgerald, R.J.; Brunton, N.P. Phenolic content and antioxidant activity of fractions obtained from selected Irish macroalgae species (Laminaria digitata, Fucus serratus, Gracilaria gracilis and Codium fragile). J. Appl. Phycol. 2014, 27, 519–530. [Google Scholar] [CrossRef]
- De Oliveira, A.L.L.; Da Silva, D.B.; Lopes, N.P.; Debonsi, H.M.; Yokoya, N.S. Chemical constituents from red algae Bostrychia radicans (Rhodomelaceae): New amides and phenolic compounds. Quim. Nova 2012, 35, 2186–2188. [Google Scholar] [CrossRef] [Green Version]
- Kang, M.-C.; Kim, E.; Kang, S.-M.; Wijesinghe, W.A.J.P.; Yang, X.; Kang, N.; Jeon, Y. Thermostability of a marine polyphenolic antioxidant dieckol, derived from the brown seaweed Ecklonia cava. Algae 2012, 27, 205–213. [Google Scholar] [CrossRef]
- Soto, M.L.; Moure, A.; Domínguez, H.; Parajó, J.C. Recovery, concentration and purification of phenolic compounds by adsorption: A review. J. Food Eng. 2011, 105, 1–27. [Google Scholar] [CrossRef]
- Yotsu-Yamashita, M.; Kondo, S.; Segawa, S.; Lin, Y.C.; Toyohara, H.; Ito, H.; Konoki, K.; Cho, Y.; Uchida, T. Isolation and structural determination of two novel phlorotannins from the brown alga Ecklonia kurome okamura, and their radical scavenging activities. Mar. Drugs 2013, 11, 165–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fairhead, V.A.; Amsler, C.D.; McClintock, J.B.; Baker, B.J. Variation in phlorotannin content within two species of brown macroalgae (Desmarestia anceps and D. menziesii) from the Western Antarctic Peninsula. Polar Biol. 2005, 28, 680–686. [Google Scholar] [CrossRef]
- Ragan, M.A. The high molecular weight polyphloroglucinols of the marine brown alga Fucus vesiculosus L.: Degradative analysis. Can. J. Chem. 1985, 63, 294–303. [Google Scholar] [CrossRef] [Green Version]
- McInnes, A.G.; Ragan, M.A.; Smith, D.G.; Walter, J.A. The high molecular weight polyphloroglucinols of the marine brown alga Fucus vesiculosus L. 1 H and 13 C nuclear magnetic resonance spectroscopy. Can. J. Chem. 1985, 63, 304–313. [Google Scholar] [CrossRef] [Green Version]
- Belanche, A.; Jones, E.; Parveen, I.; Newbold, C.J. A Metagenomics Approach to Evaluate the Impact of Dietary Supplementation with Ascophyllum nodosum or Laminaria digitata on Rumen Function in Rusitec Fermenters. Font. Microbiol. 2016, 7, 299. [Google Scholar] [CrossRef]
- Salgado, L.T.; Cinelli, L.P.; Viana, N.B.; de Carvalho, R.T.; de Souza Mourao, P.A.; Teixeira, V.L.; Farina, M.; Amado Filho, G.M. A vanadium bromoperoxidase catalyzes the formation of high-molecular-weight complexes between brown algal phenolic substances and alginates. J. Phycol. 2009, 45, 193–202. [Google Scholar] [CrossRef]
- Tierney, M.S.; Soler-Vila, A.; Rai, D.K.; Croft, A.K.; Brunton, N.P.; Smyth, T.J. UPLC-MS profiling of low molecular weight phlorotannin polymers in Ascophyllum nodosum, Pelvetia canaliculata and Fucus spiralis. Metabolomics 2014, 10, 524–535. [Google Scholar] [CrossRef]
- Kirke, D.A.; Smyth, T.J.; Rai, D.K.; Kenny, O.; Stengel, D.B. The chemical and antioxidant stability of isolated low molecular weight phlorotannins. Food Chem. 2017, 221, 1104–1112. [Google Scholar] [CrossRef]
- Truus, K.; Vaher, M.; Koel, M.; Mähar, A.; Taure, I. Analysis of bioactive ingredients in the brown alga Fucus vesiculosus by capillary electrophoresis and neutron activation analysis. Anal. Bioanal. Chem. 2004, 379, 849–852. [Google Scholar] [CrossRef] [PubMed]
- Vaher, M.; Koel, M. Separation of polyphenolic compounds extracted from plant matrices using capillary electrophoresis. J. Chromatogr. A 2003, 990, 225–230. [Google Scholar] [CrossRef]
- Yoon, J.-S.; Kasin Yadunandam, A.; Kim, S.-J.; Woo, H.-C.; Kim, H.-R.; Kim, G.-D. Dieckol, isolated from Ecklonia stolonifera, induces apoptosis in human hepatocellular carcinoma Hep3B cells. J. Nat. Med. 2013, 67, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Keusgen, M.; Glombitza, K.W. Pseudofuhalols from the brown alga Sargassum spinuligerum. Phytochemistry 1997, 46, 1403–1415. [Google Scholar] [CrossRef]
- Keusgen, M.; Glombitza, K.W. Phlorethols, fuhalols and their derivatives from the brown alga Sargassum spinuligerum. Phytochemistry 1995, 38, 975–985. [Google Scholar] [CrossRef]
- Choi, J.S.; Haulader, S.; Karki, S.; Jung, H.J.; Kim, H.R.; Jung, H.A. Acetyl- and butyryl-cholinesterase inhibitory activities of the edible brown alga Eisenia bicyclis. Arch. Pharm. Res. 2014, 38, 1477–1487. [Google Scholar] [CrossRef]
- Kim, K.B.W.R.; Jung, J.Y.; Cho, J.Y.; Ahn, D.H. Lipase inhibitory activity of ethyl acetate fraction from Ecklonia cava extracts. Biotechnol. Bioprocess Eng. 2012, 17, 739–745. [Google Scholar] [CrossRef]
- Choi, J.S.; Lee, J.H.; Jung, J.H. The screening of nitrite scavenging effect of marine algae and active principles of Ecklonia stolonifera. Korean J. Fish. Aquat. Sci. 1997, 30, 909–915. [Google Scholar]
- Kim, K.A.; Kim, S.M.; Kang, S.W.; Jeon, S.I.; Um, B.H.; Jung, S.H. Edible Seaweed, Eisenia bicyclis, Protects Retinal Ganglion Cells Death Caused by Oxidative Stress. Mar. Biotechnol. 2012, 14, 383–395. [Google Scholar] [CrossRef]
- Kim, J.M.; Lee, H.H.; Seo, M.J.; Kang, B.W.; Park, J.U.; Kim, K.S.; Kim, G.Y.; Joo, W.H.; Choi, Y.H.; Cho, Y.S.; et al. Identification of 5-Hydroxy-3,6,7,8,3,4-Hexamethoxyflavone from Hizikia fusiforme involved in the induction of the apoptosis mediators in human AGS carcinoma cells. J. Microbiol. Biotechnol. 2012, 22, 1665–1672. [Google Scholar] [CrossRef] [Green Version]
- Kwon, T.H.; Kim, T.W.; Kim, C.G.; Park, N.H. Antioxidant activity of various solvent fractions from edible brown alga, Eisenia bicyclis and its active compounds. J. Food Sci. 2013, 78. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Eom, S.; Kim, T.H.; Kim, B.; Kim, Y.; Kim, S. Deodorizing Effects of Phlorotannins from Edible Brown Alga Eisenia bicyclis on Methyl Mercaptan. J. Agric. Sci. 2013, 5, 95–103. [Google Scholar] [CrossRef]
- Li, Y.; Lee, S.H.; Le, Q.T.; Kim, M.M.; Kim, S.K. Anti-allergic effects of phlorotannins on histamine release via binding inhibition between IgE and FcεRI. J. Agric. Food Chem. 2008, 56, 12073–12080. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Lee, K.; Lee, B.B.; Kim, Y.C.; Kim, Y.D.; Hong, Y.K.; Cho, K.K.; Choi, I.S. Antibacterial activity of the phlorotannins dieckol and phlorofucofuroeckol-a from Ecklonia cava against Propionibacterium acnes. Bot. Sci. 2014, 92, 425–431. [Google Scholar] [CrossRef]
- Liu, H.; Gu, L. Phlorotannins from brown algae (Fucus vesiculosus) inhibited the formation of advanced glycation endproducts by scavenging reactive carbonyls. J. Agric. Food Chem. 2012, 60, 1326–1334. [Google Scholar] [CrossRef]
- Kang, M.C.; Kim, K.N.; Kang, S.M.; Yang, X.; Kim, E.A.; Song, C.B.; Nah, J.W.; Jang, M.K.; Lee, J.S.; Jung, W.K.; et al. Protective effect of dieckol isolated from Ecklonia cava against ethanol caused damage in vitro and in zebrafish model. Environ. Toxicol. Pharmacol. 2013, 36, 1217–1226. [Google Scholar] [CrossRef]
- Bélanger, J.M.R.; Jocelyn Paré, J.R.; Sigouin, M. High performance liquid chromatography (HPLC): Principles and applications. In Instrumental Methods in Food Analysis; Paré, J.R.J., Bélanger, J.M.R., Eds.; Elsevier: Berlin/Heidelberg, Germany, 1997; Volume 18, pp. 37–59. ISBN 0167-9244. [Google Scholar]
- Sailler, B.; Glombitza, K.W. Phlorethols and fucophlorethols from the brown alga Cystophora retroflexa. Phytochemistry 1999, 50, 869–881. [Google Scholar] [CrossRef]
- Glombitza, K.W.; Schmidt, A. Trihydroxyphlorethols from the brown alga Carpophyllum angustifolium. Phytochemistry 1999, 51, 1095–1100. [Google Scholar] [CrossRef]
- Mwangi, H.M.; Van Der Westhuizen, J.; Marnewick, J.; Mabusela, W.T.; Kabanda, M.M.; Ebenso, E.E. Isolation, identification and radical scavenging activity of phlorotannin derivatives from brown algae, Ecklonia maxima: An experimental and theoretical study. Free Radic. Antioxid. 2013, 3, S1–S10. [Google Scholar] [CrossRef] [Green Version]
- Bangoura, I.; Chowdhury, M.T.H.; Kang, J.Y.; Cho, J.Y.; Jun, J.C.; Hong, Y.K. Accumulation of phlorotannins in the abalone Haliotis discus hannai after feeding the brown seaweed Ecklonia cava. J. Appl. Phycol. 2014, 26, 967–972. [Google Scholar] [CrossRef]
- Chowdhury, M.T.H.; Cho, J.Y.; Ahn, D.H.; Hong, Y.K. Methyl jasmonate enhances phlorotannin production in the brown seaweed Ecklonia cava. J. Appl. Phycol. 2015, 27, 1651–1656. [Google Scholar] [CrossRef]
- Karthik, R.; Manigandan, V.; Sheeba, R.; Saravanan, R.; Rajesh, P.R. Structural characterization and comparative biomedical properties of phloroglucinol from Indian brown seaweeds. J. Appl. Phycol. 2016, 28, 3561–3573. [Google Scholar] [CrossRef]
- Sun, Y.-Y.; Wang, H.; Guo, G.-L.; Pu, Y.-F.; Yan, B.-L.; Wang, C.-H. Green alga Ulva pertusa-a new source of bioactive compounds with antialgal activity. Environ. Sci. Pollut. Res. Int. 2015, 22, 10351–10359. [Google Scholar]
- Kagan, I.A.; Flythe, M.D. Thin-layer Chromatographic (TLC) Separations and Bioassays of Plant Extracts to Identify Antimicrobial Compounds. J. Vis. Exp. 2014, 85, e51411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isaza Martínez, J.H.; Torres Castañeda, H.G. Preparation and Chromatographic Analysis of Phlorotannins. J. Chromatogr. Sci. 2013, 51, 825–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souza, B.W.S.; Cerqueira, M.A.; Martins, J.T.; Quintas, M.A.C.; Ferreira, A.C.S.; Teixeira, J.A.; Vicente, A.A. Antioxidant potential of two red seaweeds from the Brazilian coasts. J. Agric. Food Chem. 2011, 59, 5589–5594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Camargo, A.P.; Montero, L.; Cifuentes, A.; Herrero, M.; Ibáñez, E. Application of Hansen solubility approach for the subcritical and supercritical selective extraction of phlorotannins from Cystoseira abies-marina. RSC Adv. 2016, 6, 94884–94895. [Google Scholar] [CrossRef]
- Ye, H.; Zhou, C.; Sun, Y.; Zhang, X.; Liu, J.; Hu, Q.; Zeng, X. Antioxidant activities in vitro of ethanol extract from brown seaweed Sargassum pallidum. Eur. Food Res. Technol. 2009, 230, 101–109. [Google Scholar] [CrossRef]
- Palma, P.; Famiglini, G.; Trufelli, H.; Pierini, E.; Termopoli, V.; Cappiello, A. Electron ionization in LC-MS: Recent developments and applications of the direct-EI LC-MS interface. Anal. Bioanal. Chem. 2011, 399, 2683–2693. [Google Scholar] [CrossRef]
- Kellogg, J.; Grace, M.H.; Lila, M.A. Phlorotannins from alaskan seaweed inhibit carbolytic enzyme activity. Mar. Drugs 2014, 12, 5277–5294. [Google Scholar] [CrossRef]
- Iwai, K. Antidiabetic and antioxidant effects of polyphenols in brown alga Ecklonia stolonifera in genetically diabetic KK-Ay mice. Plant Foods Hum. Nutr. 2008, 63, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Goo, H.R.; Choi, J.S.; Na, D.H. Quantitative determination of major phlorotannins in Ecklonia stolonifera. Arch. Pharm. Res. 2010, 33, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Kazłowska, K.; Hsu, T.; Hou, C.C.; Yang, W.C.; Tsai, G.J. Anti-inflammatory properties of phenolic compounds and crude extract from Porphyra dentata. J. Ethnopharmacol. 2010, 128, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Bernaldo de Quirós, A.; Lage-Yusty, M.A.; López-Hernández, J. Determination of phenolic compounds in macroalgae for human consumption. Food Chem. 2010, 121, 634–638. [Google Scholar] [CrossRef]
- Shibata, T.; Nagayama, K.; Sugiura, S.; Makino, S.; Ueda, M.; Tamaru, Y. Analysis on Composition and Antioxidative Properties of Phlorotannins Isolated from Japanese Eisenia and Ecklonia Species. Am. J. Plant Sci. 2015, 06, 2510–2521. [Google Scholar] [CrossRef] [Green Version]
- Corona, G.; Ji, Y.; Anegboonlap, P.; Hotchkiss, S.; Gill, C.; Yaqoob, P.; Spencer, J.P.E.; Rowland, I. Gastrointestinal modifications and bioavailability of brown seaweed phlorotannins and effects on inflammatory markers. Br. J. Nutr. 2016, 115, 1240–1253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konermann, L.; Ahadi, E.; Rodriguez, A.D.; Vahidi, S. Unraveling the Mechanism of Electrospray Ionization. Anal. Chem. 2013, 85, 2–9. [Google Scholar] [CrossRef]
- Moilanen, J.; Sinkkonen, J.; Salminen, J.-P. Characterization of bioactive plant ellagitannins by chromatographic, spectroscopic and mass spectrometric methods. Chemoecology 2013, 23, 165–179. [Google Scholar] [CrossRef]
- Glombitza, K.W.; Zieprath, G. Phlorotannins from the Brown Alga Analipus japonicus. Planta Med. 1989, 55, 171–175. [Google Scholar] [CrossRef]
- Glombitza, K.W.; Pauli, K. Fucols and phlorethols from the brown alga Scytothamnus australis Hook. et Harv. (Chnoosporaceae). Bot. Mar. 2003, 46, 315–320. [Google Scholar] [CrossRef]
- Wünsche, C.; Sachs, A.; Einwiller, A.; Mayer, W. Electron-impact induced alkyl and hydrogen migration in diphenic acid derivatives. Tetrahedron 1968, 24, 3407–3416. [Google Scholar] [CrossRef]
- Glombitza, K.W.; Rauwald, H.W.; Eckhardt, G. Fucole, polyhydroxyoligophenyle aus Fucus vesiculosus. Phytochemistry 1975, 14, 1403–1405. [Google Scholar] [CrossRef]
- Li, Y.; Fu, X.; Duan, D.; Liu, X.; Xu, J.; Gao, X. Extraction and Identification of Phlorotannins from the Brown Alga, Sargassum fusiforme (Harvey) Setchell. Mar. Drugs 2017, 15, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glombitza, K.-W.; Rösener, H.-U. Bifuhalol: Ein diphenyläther aus Bifurcaria bifurcata. Phytochemistry 1974, 13, 1245–1247. [Google Scholar] [CrossRef]
- Glombitza, K.W.; Rosener, H.U.; Müller, D. Bifuhalol and diphlorethol of Cystoseira tamariscifolia. Phytochemistry 1975, 14, 1115–1116. [Google Scholar] [CrossRef]
- Kiehlmann, E.; Lauener, R.W. Bromophloroglucinols and their methyl ethers. Can. J. Chem. 1989, 67, 335–344. [Google Scholar] [CrossRef]
- Peterkatalinic, J.; Egge, H.; Deutscher, B.; Knoss, W.; Glombitza, K.W. A Rapid Method for the Structural Characterization of Sulfated Phlorotannins by Negative-Ion Fast Atom Bombardment Mass-Spectrometry and Acetylation on the Target. Biomed. Environ. Mass Spectrom. 1988, 15, 595–602. [Google Scholar] [CrossRef]
- Cui, Y.; Amarsanaa, K.; Lee, J.H.; Rhim, J.K.; Kwon, J.M.; Kim, S.H.; Park, J.M.; Jung, S.C.; Eun, S.Y. Neuroprotective mechanisms of dieckol against glutamate toxicity through reactive oxygen species scavenging and nuclear factor-like 2/heme oxygenase-1 pathway. Korean J. Physiol. Pharmacol. 2019, 23, 121–130. [Google Scholar] [CrossRef] [Green Version]
- Yoon, M.; Cho, S. Triphlorethol A, a dietary polyphenol from seaweed, decreases sleep latency and increases non-rapid eye movement sleep in mice. Mar. Drugs 2018, 16, 139. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Wang, C.; Shen, L.; Shin, H.C.; Lee, K.B.; Ji, B. Comparative analysis of oxidative mechanisms of phloroglucinol and dieckol by electrochemical, spectroscopic, cellular and computational methods. RSC Adv. 2018, 8, 1963–1972. [Google Scholar] [CrossRef] [Green Version]






| Solvent Mixture | SLr (w/v) | t (h) | T (°C) | Species | Phenolic Compounds Extracted | Type of Characterization b | Reference |
|---|---|---|---|---|---|---|---|
| Ethyl acetate, acetone, ethanol, methanol, acetone:H2O (70:30), ethanol:H2O (80:20), methanol:H2O (80:20), H2O | 1:50 | 1 (×4) | r.t. | Fucus vesiculosus | n.i. d | TPC | [79] |
| Acetone:water (70:30) + 0.3% ascorbic acid | 1:50 | 1 (×4) | r.t. | Fucus vesiculosus | Phlorotannins | TPC, HPLC-MS | [34] |
| Ethanol | 1:1.6 | 2 | On ice | Fucus vesiculosus | Phlorotannins | HPLC-DAD, HPLC-ESI-MS, UV–vis, FT-IR, NMR | [62] |
| Methanol, acetonitrile:water (50:50) + 0.2% formic acid | 1:10 | overnight | r.t. | Ascophyllum nodosum, Ulva lactuca, Palmaria palmata, Alaria esculenta | Phlorotannins | TPC, AA, HPLC-MS | [77] |
| Acetone:water (70:30) | 1:20 | 1 (×4) | r.t. | Cystoseira nodicaulis, Cystoseira tamariscifolia, Cystoseira usneoides, Fucus spiralis | Phlorotannins | AA, HPLC-MS | [35] |
| Acetone:water (70:30) | 1:20 | 1 (×4) | r.t. | Cladostephus spongiosus, Cystoseira nodicaulis, Cystoseira tamariscifolia, Cystoseira usneoides, Fucus spiralis, Halopteris filicina, Padina pavonica, Saccorhiza polyschides, Sargassum vulgare, Stypocaulon scoparium | n.i. | TPhC | [70] |
| Methanol:water (80:20) | 1:7.5–13.2 followed by 1:3.4–6.6 | 0.5 + 3 (×3) | r.t. | Fucus vesiculosus, Pelvetia canaliculata, Fucus spiralis, Ascophyllum nodosum, Saccharina longicruris | Phlorotannins | HPLC-MS | [10] |
| Methanol: water (80:20), ethyl acetate: water (80:20), acetone: water (70:30), ethanol:water (80:20 and 50:50) | 1:25 | 24 | r.t. | Fucus vesiculosus | Phlorotannins | TPC, AA, HPLC-MS | [80] |
| Water | 1:20 | 24 | r.t. | Ascophyllum nodosum, Pelvetia canaliculata, Fucus spiralis, Ulva intestinalis | n.i. | TPC, AA | [87] |
| Ethanol:water (80:20) | 1:10 | ||||||
| Acetone:water (80:20) | 1:10 | ||||||
| Ethanol:water | 1:10 | 24 | r.t. | Fucus spiralis, Pelvetica canaliculata, Ascophyllum nodosum | Phlorotannins | TPC, AA, MS | [78] |
| Water | 1:20 | 24 | r.t. | ||||
| Water | 1:20 | 24 | 60 | ||||
| Ethanol | 1:10 | 12 | r.t. | Eisenia bicyclis | Phlorotannins | HPLC-DAD | [38] |
| Water, water + HCl 5 mM, water + 0.2% formic acid, acetonitrile: water (50:50) + 0.2% formic acid | 1:10 | 1 | r.t. | Ascophyllum nodosum | Phlorotannins | HPLC-MS | [88] |
| Water | 1:100 | 5 min | 100 | Ecklonia cava, Ecklonia stolonifera, Eisenia bicyclis | Phlorotannins | AA, HPLC-UV, NMR | [86] |
| Methanol | 1:4 | 2 | r.t. | ||||
| Ethanol | 1:10 | overnight | r.t. | Fucus serratus, Fucus vesiculosus, Fucus distichus, Fucus spiralis, Sargassum muticum, Saccharina latissima, Laminaria digitata, Dictyota dichotoma, Enteromorpha intestinalis, Ulva lactuca, Palmaria palmata, Porphyra purpurea, Chondrus crispus, Mastocarpus stellatus, Polysiphonia fucoides, Gracilaria vermiculophylla | Phenolic acids | TPC, AA, HPLC-DAD | [12] |
| Water | 1:20 | ||||||
| Methanol:water (80:20) | 1:10 | 2 | r.t. | Kappaphycus alvarezii | n.i. | TPC, TFC, TAC, AA | [66] |
| Ethanol:water (80:20) | 1:10 | 24 | r.t. | Fucus, serratus, Fucus vesiculosus, Himanthalia elongata, Cystoseira nodicaulis | Phlorotannins | TPC, AA, HPLC-MS | [28] |
| Water | 1:15 | 4 | 55 | Macrocystis pyrifera | Phlorotannins | TPC, AA, HPLC-MS | [69] |
| Methanol:water (60:40) | 1:10 | 2 | 40 | Himanthalia elongata | Phloroglucinol, phenolic acids, flavonoids and phenolic terpenes | TPC, AA, HPLC-DAD-MS | [17] |
| H2O, acetone, ethanol | 1:2 | 19 | r.t. | Fucus vesiculosus | Phlorotannins | TPC, UV–vis, FT-IR, NMR | [91] |
| Methanol:water (80:20) | 1:40 | 1.5 | 20 | Laminaria digitata | Phlorotannins | TPC, TPhC, NMR, UHPLC-UV-MS, MALDI-TOF-MS | [44] |
| Acetone | - | - | - e | Padina pavonica | n.i. | AA, TPC, TFC, TTC | [92] |
| Ethanol:water (80:20) | 1:20 | 24 | r.t. | Fucus vesiculosus | Phlorotannins | UHPLC-DAD-MS | [59] |
| Ethanol | 1:1.4 | 30 days | r.t. | Costaria costata, Fucus evanescens | Phlorotannins | NMR, UV–vis, ESI-MS | [61] |
| Methanol | 1:10 | 72 | r.t. | Padina tetrastromatica | Coumarin and flavones | TPC, AA, GC-MS | [20] |
| Ethanol:water (80:20) | - | 2 × 72 | 55 | Ecklonia cava | Phlorotannins | NMR | [101] |
| Methanol:water (70:30) | 1:50 | - | - | Caulerpa spp. | n.i. | TPC, TFC, AA | [102] |
| Ethyl acetate | 1:10 | 6 | 60–80 c | Sargassum wightii | Phlorotannins | AA, FT-IR, HPLC-MS | [56] |
| Ethanol:water(20:80, 40:60, 60:40, 80:20, 100:0) | 1:25 | 12 | r.t. | Cladophora glomerata | Phenolic acids and flavonoids | TPC, AA, HPLC-DAD-MS | [14] |
| Methanol:water (60:40) | 1:10 | 2 | 40 | Himanthalia elongata | Phenolic acids and flavonoids | TPC, AA, HPLC-DAD-ESI-MS | [63] |
| Laminaria digitata, Laminaria saccharina, Himamthalia elongata | n.i. | TPC, TFC, TTC, AA | [63] | ||||
| Ethanol:water (50:50) | 1:10 | 24 | 25–35 | Laurencia caspica | n.i. | TPC, AA | [89] |
| Acetone:water (70:30) | 1:70 | 3 | 25 | Fucus vesiculosus | Phlorotannins | TPhC, UHPLC-DAD-ESI-MS | [9] |
| Ethanol:water (50:50) | 1:2.5 | 24 | r.t. | Durvillaea antarctica, Lessonia spicata, Macrocystis integrifolia | Miscellaneous phenolic compounds | TPC, AA, HPLC-UV-ESI-MS | [19] |
| Macroalgae | Type of Enzyme | t (h)/pH/T (°C) | Phenolic Compounds Extracted | Type of Characterization a | Extraction Methodology Associated | Reference |
|---|---|---|---|---|---|---|
| Hizikia fusiformis | Endo-peptidase + β-glucanase | 24/7.0–8.0/50–60 | n.i.b | TPC, AA | Conventional SLE | [99] |
| Palmaria palmata | Endo- and exo-peptidase complex | 24/7.0/50 | n.i. | TPC, AA | - | [97] |
| Endo-peptidase | 24/8.0/50 | |||||
| Protease complex | 24/6.0/40 | |||||
| Amino- and carboxy-peptidase | 24/6.0/40 | |||||
| Metallo-endo-protease | 24/6.0/50 | |||||
| Endo-protease and exo-peptidase | 24/7.0/50 | |||||
| Multi-enzyme complex | 24/4.5/50 | |||||
| β-Glucanase | 24/7.0/60 | |||||
| Exo-1,4-α-glucosidase | 24/4.5/60 | |||||
| Cellulase | 24/4.5/50 | |||||
| α-Amylase | 24/6.0/60 | |||||
| Sargassum muticum, Osmundea pinnatifida, Codium tomentosum | Endo-peptidase | 24/8.0/50 | Flavan-3-ols | HPLC-UV-ESI-MS | - | [95] |
| Endo-protease and exo-peptidase | 24/7.0/50 | |||||
| Cellulase | 24/4.5/50 | |||||
| Multi-enzyme complex | 24/4.5/50 | |||||
| Lessonia nigrescens, Macrocystis pyrifera, Durvillaea antarctica | Cellulase | 17/4.5/50 | n.i. | TPC | - | [98] |
| α-Amylase | 17/6.0/60 | |||||
| Ecklonia radiata | Multi-enzyme complex | 24/4.5/50 | n.i. | TPC, AA | Microwave-assisted enzymatic extraction | [100] |
| Cellulase | 24/4.5/50 | |||||
| β-Glucanase | 24/7.0/50 | |||||
| Endo- peptidase | 24/8.0/50 | |||||
| Metallo-endo-protease | 24/6.0/50 | |||||
| Endo-protease and exo-peptidase | 24/7.0/50 | |||||
| Sargassum muticum | Endo-peptidase | 2 and 4/7.0/50 | Hydroxytetrafuhalol, triphlorethol and dihydroxypentafuhalol | TPC, TPhC, AA, HPLC-DAD-MS | ASE applied to the biomass residue | [96] |
| Multi-enzyme complex | 2 and 4/4.5/50 | |||||
| Macrocystis pyrifera | Alginate lyase + fucoidanase + 1,3-β-d-glucanase | 36/7.0/25 | Phloroeckol and 1 tetramer of phloroglucinol | TPC, AA, HPLC-UV-ESI-MS | Conventional SLE | [71] |
| Solvent of Extraction (v:v) | P (psi) | T (°C) | t (min) | Macroalgae Species | Compounds Extracted | Type of Characterization a | Reference |
|---|---|---|---|---|---|---|---|
| Ethanol:water (25:75; 75:25) | 1500 | 120 | 20 | Sargassum muticum | n.i. b | TPC, AA, NMR | [8] |
| Water | 1500 | 120 | 25 | Fucus serratus, Laminaria digitata, Gracilaria gracilis, Codium fragile | n.i. | TPC, AA | [90] |
| Ethanol:water (80:20) | 1000 | 100 | |||||
| Methanol:water (70:30) | 1000 | 90 | |||||
| Ethanol:water (95:5) | 1500 | 160 | 20 | Sargassum muticum | Phlorotannins | TPC, TPhC, AA, LCxLC-DAD-MS | [104] |
| Ethanol:water (72:25) | 1500 | 120 | 20 | Sargassum muticum | Phlorotannins | TPC, TPhC, AA, HPLC-DAD-MS | [96] |
| Dichloromethane + Methanol b | - | - | 5 + 5 c | Fucus vesiculosus | Phlorotannins | AA, HPLC-DAD-MS | [106] |
| Hexane, ethyl acetate, ethanol, ethanol:water (50:50) | 1500 | 80, 120, 160 | 10 | Laminaria ochroleuca | n.i. | TPC | [105] |
| Extraction Solvent | SLr | t (min) | T (°C)/Power (w) | Macroalgae | Phenolic Compounds Extracted | Type of Characterization a | Reference |
|---|---|---|---|---|---|---|---|
| Ethanol:water (20–100%) | 1:10–1:50 | 5–60 | 20–70/100–600 | Caulerpa racemosa | n.i. b | TPC, AA, FT-IR, HPLC-UV | [109] |
| Ethanol:water (50:50; 60:40; 70:30) | 1:4–1:12 | 5–25 | 45–65/400–600 | Saccharina japonica | n.i. | TPC | [108] |
| Solvent free followed by water addition | - | 5–35 (×1–5) | -/200–900 | Sargassum muticum | n.i. | TPC, AA, HPLC-DAD | [112] |
| Ethanol | - | 10 × 2 | -/400 | ||||
| Ethanol:water (50:50) | - | 10 × 3 | -/400 | ||||
| Water c | - | 180 | 50/- | Ecklonia radiata | n.i. | TPC, AA | [100] |
| Water | 1:3 | 30 | 25,40,60/1000 | Polysiphonia, Ulva and Cladophora (mixed) | n.i. | TPC | [111] |
| Acetone:water (100:0; 30:70; 50:50; 70:30; 0:100) | 1:20 | 4–12 | -/200–600 | Chaetomorpha sp. | n.i. | TPC, AA | [54] |
| Methanol:water (70:30) | 1:10 | 15 | 110/- | Ascophyllum nodosum, Laminaria japonica, Lessonia trabeculate, Lessonia nigrecens | Phenolic acid, flavonoids, phlorotannins | TPC, AA, HPLC-DAD-MS | [110] |
| Extraction Solvent | Frequency/Power (kHz/W) | Conditions | Macroalgae | Phenolic Compounds Extracted | Type of Characterization a | Reference |
|---|---|---|---|---|---|---|
| Water and water + HCl (0.03M) | 20/750 | 10 min; amplitude between 22.8 and 114 µm | Ascophyllum nodosum | n.i. b | TPC | [114] |
| Water + HCl (0–0.06 M) | 20/750 | 5–25 min; amplitude between 22.8 and 114 µm | Ascophyllum nodosum | Phlorotannins | TPC, ESI-MS | [55] |
| Water | 50–60/400 | 24h c + 60 min (pauses of 2 min after each 10 min of sonication); 50 °C | Sargassum muticum, Osmundea pinnatifida and Codium tomentosum | n.i. | TPC, AA | [95] |
| Ethanol | 40/250 | 30–60 min; 30–50°C | Laurencia obtusa | n.i. | TPC, AA | [117] |
| Water | -/- | 5 minc + 10 min | Ascophyllum nodosum, Bifurcaria bifurcata, Fucus vesiculosus | Phenolic acids, flavonoids and phlorotannins | HPLC-DAD-ESI-MS | [118] |
| Methanol | -/- | 5 × 15 min | Dasycladus vermicularis | Coumarins | ESI-MS, 1H NMR, 13C-NMR | [21] |
| Methanol:water (50:50) | Coumarins and phenolic acids | |||||
| Ethanol:water (50:50) | -/- | 30 min, r.t. d +2 h, 70 °C c | Macrocystis pyrifera, Ecklonia arborea, Silvetia compressa, Ulva intestinalis, Solieria filiformis | Flavonoid glycosides and phlorotannins | TPC, HPLC-ESI-MS | [119] |
| Ethanol:water (55:45) | 45/- | 90 min, 60 °C | Enteromorpha prolifera | Naphtalenone, flavone and flavanone glycosides | UHPLC-ESI-MS | [120] |
| Methanol:water (50:50) | -/- | 1 h + 24 h c + 1 h r.t. | Fucus guiryi, Fucus serratus, Fucus spiralis, Fucus vesiculosus | Phlorotannins | TPhC, HPLC-ESI-MS | [58] |
| Acetone:water (70:30) | 35/- | 5.75 h, 30 °C | Agarum cribrosum | Trifuhalol | TPC, AA, HPLC-DAD-ESI-MS | [60] |
| Methanol:water (80:20) | -/- | 2 days, r.t. | Ecklonia cava | Phlorotannins | 1H NMR, 13C-NMR, FT-IR, HPLC-ESI-MS | [116] |
| Methanol | -/- | 2 h, 20 °C | Ulva intestinalis, Codium tomentosum, Dictyota dichotoma, Halopteris scoparia | Flavone | TPC, | [57] |
| Ethanol:water (80:20) | -/- | 90 min; | Ecklonia maxima | Eckmaxol | HR-ESI UHPLC-ESI-MS-MS, 1H NMR, 13C-NMR | [115] |
| SFE Modifier | Pressure (MPa)/Temperature (°C)/FlowCO2 (kg CO2 h−1) | Extraction Time (h:min) | Macroalgae | Phenolic Compounds Extracted | Type of Characterization a | Reference |
|---|---|---|---|---|---|---|
| Ethanol (3%) | 8–30/30–60/1.69 | 1:00 | Undaria pinnatifida | n.i. b | TPC | [126] |
| Methanol:water (10:90) (3%) | 35/40/- | 1:00 | Sargassum muticum, Sargassum vulgare, Hypnea spinella, Porphyra sp., Undaria pinnatifida, Chondrus crispus and Halopytis incurvus (mixed) | Isoflavones | Fast LC-MS | [18] |
| Ethanol (12%) | 15.2/60/- | 1:30 | Sargassum muticum | n.i. | TPC, AA, NMR | [8] |
| Ethanol (0.5–10%) | 10–30/30–50/1.5 | 1:00 | Sargassum muticum | n.i. | TPC, AA | [125] |
| - | 50/40/200 | 5:17–13:28 | Cladophora glomerata, Ulva flexuosa and Ulva clathrata (mixed) | n.i. | TPC | [127] |
| - | 37.9/50/34 | - | Laminaria digitata, Undaria pinnatifida, Porphyra umbilicalis, Eucheuma denticulatum, Gelidium pusillum | Phlorotanninsc | HPLC-DAD | [42] |
| Compounds | Stationary Phase | Mobile Phase a | Reference |
|---|---|---|---|
| Normal-Phase | |||
| Phlorotannins | Silica gel | TCM:MeOH 9:1 | [45] |
| TCM to TCM:MeOH 4:1 | [46] | ||
| Hex to Hex:EtOAc 1:1 to DCM to DCM:MeOH 1:1 | [153] | ||
| DCM:MeOH 6:1–1:6 | [169] | ||
| TCM:MeOH 99:1 to MeOH | [143] | ||
| TCM:Hex 1:1–4:1, TCM-AcetO 49:1–4:1, MeOH | [43,154,162,170,171] | ||
| EtOAc–MeOH 50:1–5:1 | [32,76,151,172] | ||
| DCM, MeOH | [173] | ||
| TCM:MeOH 1:1 | [174] | ||
| TCM:MeOH 100:1 to 1:1 | [157] | ||
| EtOAc:MeOH 50:1−5:1 | [32] | ||
| EtOAc, MeOH, Water | [148] | ||
| TCM:MeOH:Water 80:18:2–50:49:1 | [175] | ||
| Hex:EtOAc 1:1 | [32,76] | ||
| Hex to AcetO to MeOH | [44] | ||
| Benz to Benz:EtOAc(10:1–1:1) to EtOAc to TCM to TCM:EtOH(30:1–1:1) to EtOH | [61] | ||
| Hex:EtOAc 9.5:0.5–7.5:2.5 | [58] | ||
| Diatomaceous earth | Hex, DCM, EtOEt, MeOH | [152] | |
| Miscellaneous | Silica gel | EtOAc:MeOH 1:0 to 0:1 | [17] |
| Phenolic acids | Silica gel | Hex–TCM 1:1, TCM:MeOH 1:1 | [13] |
| Flavones | Silica gel | TCM:MeOH 3:2 | [176] |
| Aurones | Silica gel | Hex:TCM:MeOH 1:0:0–0:1:0–0:0:1 | [23] |
| Coumarins | Silica gel | DCM, EtOAc, MeOH, water | [21] |
| Reversed-Phase | |||
| Phlorotannins | Octadecyl (unspecified) | MeOH (aq.) 10–100% | [177] |
| MeOH (aq.) 20–100% | [32,76,151] | ||
| Cosmosil 75C 18-OPN | MeOH (aq.) 0–100% | [159] | |
| LiChroprep RP-18 | MeOH (aq.) 20–100% | [172,178] | |
| Coumarins and phenolic acids | Reveleris RP-18 | MeOH (aq.), 0.25% FA | [21] |
| Size-Exclusion | |||
| Phlorotannins | Sephadex LH-20 | MeOH | [76,116,151,174,175,177,178,179,180] |
| EtOH (aq.) 50–80%, AcetO (aq.) 50–80% | [77] | ||
| MeOH (aq.) 80% | [157] | ||
| MeOH (aq.) 60–100%, AcetO (aq.) 70% | [181] | ||
| MeOH:EtOAc (7:3) | [156] | ||
| TCM:MeOH 2:1–1:1–0:1 | [182] | ||
| EtOH (aq.) 50–0%, AcetO (aq.) 0–70% | [88] | ||
| MeOH:water 1:1–1:3–0:1, MeOH:AcetO 5:1–3:1–1:1 | [60] | ||
| DCM:MeOH (1:1) | [115] | ||
| Coumarins | Sephadex LH-20 | MeOH (aq.) 50% | [21] |
| Column (a/b/c) a | Mobile Phase b | Flow-Rate (ml/min) | UV Detection (nm) | Species Outcome [Reference] |
|---|---|---|---|---|
| Normal Phase | ||||
| LiChrosorb Si-60 (250/16/7) (250/8/5) | A: TCM B: EtOH B: 0.5–3.0% | - | 275 | Sargassum spinuligerum Isol. of 20 fuhalols and deshydroxufuhalols [154]. |
| A: TCM B: EtOH B: 0–2.5% | - | 275 | Sargassum spinuligerum Sep. of pseudofuhalols [170] Isol. of 14 new (hydroxy)phlorethols and [(des)hydroxy]fuhalols [171]. | |
| Sargassum spinuligerum and Cystophora torulosa. Isol. of 9 new fucophlorethols and 8 known ones from NP-CC fractions [43]. | ||||
| Cystophora retroflexa Isol. of 18 phlorethols and fucophlorethols (to isomer lever) [184] Isol. of 12 halogenated phlorethols and fucophlorethols (partially co-eluted) [41]. | ||||
| LiChrosorb Si-60 (250/16/7) | TCM-Hex (+0.3% MeOH) TCM-MeOH (higher MW) | - | - | Cystophora congesta Isol. of 11 brominated and non-halogenated phlorotannins [46]. |
| LiChrosorb Si-60 (250/8/5) | TCM-Hex TCM-MeOH (higher MW). | - | 250–275 | Carpophyllum angustifolium Isol. of 3 trihidroxyphlorethols (2 isomers) [40] Sep. of NP-CC fractions into 4 trihidroxyphlorethols [185]. |
| LiChrosorb Si-60 (250/7.5/10) Partisil 10 (500/5/10) | A: TCM B: EtOH B: 0.3–2.0% | - | 275 | Eisenia arborea. Sep. of extract into 17 fractions, containing a total of 21 compounds (19 new eckols) [45]. |
| Pharmprep60CC SiO2 (250/2.1/-) | 0.1% AcAc (aq.) | 5.0 | - | Fucus vesiculosus Enrichment of crude extract to further preparative RP-HPLC [91]. |
| LiChrosorb Si-60 (250/10/5) | A: TCM:water (5:95) B: EtOH %B: 15–100 | 1.0 | 270 | Laminaria digitata, Undaria pinnatifida, Porphyra umbilicalis, Eucheuma denticulatum, Gelidium pusillum [42]. |
| Reversed-Phase | ||||
| µBondapak C18 (300/7.8/10) | 36% ACN | 1.0 | 245 | Ecklonia cava Isolation of dieckol (Rt~6 min) from NP-CC fraction [173]. |
| Luna-C18 (-) | A: 0.1% FA (aq.) B: 0.1% FA in ACN 20–100% B | 3.0 | 245 | Ecklonia stolonifera Isolation of 2-phloroeckol, eckol, phlorofucofuroeckol B and bieckol from previous RP-HPLC fractions [32]. |
| Shim-pack PREP-ODS (250/20/5) | A: 0.1% FA (aq.) B: 0.1% FA in ACN 20–100% B | 7.0 | 245 | Ecklonia stolonifera Separation of SEC-HPLC fraction into 6 subfractions. SF1, SF4, SF5, SF6 further purified by RP-HPLC [32,169]. |
| Develosil ODS5 (-) | A: 0.1% TFA (aq.) B: 0.1% TFA in ACN 0–30% B | - | - | Eisenia arborea Isolation of active fraction at TR 130–150 min; Repetition with 47% methanol 0.1% TFA for isolation of phlorofucofuroeckol B at tR = 41 min [73]. |
| C18 (150/4.5/5) | 18% ACN (aq.) (+0.1% formic acid) | 0.7 | - | Ecklonia maxima Isolation of eckol, 7-phloroecckol and 2-phloroeckol [186]. |
| J’sphere ODS-H80 (150/20/4) | MeOH (aq., increasing % v/v) | 0.8 | 230 | Ecklonia cava Purification of dieckol with purity > 95% from SEC-CC fraction [157,182]. |
| Alltima C18 column (250/10/5) | MeOH 30–100% | 4.0 | 290 | Ecklonia cava Purification of dieckol and phlorofucofuroeckol A from SEC-CC fraction [180]. |
| Mightysil RP-18 GP II (250/10/5) | MeOH 40%(1.0% FA aq.) | 0.5 | DAD 190–500 (rec = 204–208) | Ecklonia kurome Separation of dieckol, 974-B, 974-A, phlorofucofuroeckol B and phlorofucofuroeckol A from RP-CC fraction [159]. |
| C18 (250/10/-) | 30–100% MeOH | 1.0 | 290 | Ecklonia cava Isolation of 7-phloroeckol and eckol from ethyl ether fraction of crude extract [187]. Isolation of dieckol, phlorotannin 974B, phlorotannin 974A, and phlorofucofuroeckol-A from ethyl ether fraction of crude extract [188]. |
| Phenomenex aqua C18 (250/10/5) | A: 1% AcAc B: 1% AcAc in ACN %B: 1–100% | 2.5 | - | Fucus vesiculosus 10 fractions. Repetition with isocratic A:B 90:10 isolated six subfractions, from which 3 fucophlorethols were isolated [62]. |
| Amberlite XAD7HP (250/21/-) | A: 0.1% AcAc (aq.) B: ACN %B: 0–100% | 5.0 | - | Fucus vesiculosus 67 fractions. Bioassay-guided fractionation [91]. |
| LiChrosorb RP-18 (250/8/5) (250/10/7) | 0.015 M K2SO4 | - | 235 | Pleurophycus gardneri Isolation of sulphated phlorotanin from PVP adsorbate of crude extract [39]. |
| 2 to 50% MeOH (aq.) | 1.0 | 350 | Dasycladus vermicularis Isolationf of a sulphated coumarin from a RP-CC fraction [21]. | |
| C18 (50/2.1/1.8) | A: 0.5% TFA (aq.) B: 0.5% TFA in ACN %B: +4%/min | 1.0 | 280 | Sargassum wightii, S. tenerrimum and Turbinaria conoides Isolation of a phlorotannins-enriched fraction from crude extract [189]. |
| Optimapack C18 (250/10/10) | A: ACN (aq.)/0.1% FA B: ACN %B: 20 (65 min)–100 (65 min)/30 (60 min)–100 (65 min) | 2 | - | Ecklonia cava Isolation of phlorofucofuroeckol, dioxinodehydroeckol and fucofuroeckol [116]. |
| Size-Exclusion | ||||
| Asahipak GS-310 (500/20/13) | MeOH | 5.0 | 245 | Ecklonia stolonifera Separation of NP-CC fraction in 5 subfractions; SF4 further purified by RP-HPLC [32,169]. |
| Superose 6 colum (300/10/13) | 100 mM sodium acetate buffer (pH 6.3) 20 mM EDTA, 250 mM NaCl | 0.25 | 210 | Padina gymnospora Recovery of 2-[1′-Oxo-hexadecyl]-1,3,5-trihydroxybenzene (a phloroglucinol derivative) from RP-CC fraction [164]. |
| Species [Reference] | Outcome | Configuration a | MS Mode b | HPLC Column | Mobile Phase c |
|---|---|---|---|---|---|
| Fucus vesiculosus [34] | Detection of 16 phlorotanins. | NP-HPLC-ESI-QqQ | (-) FULL SCAN | LiChrospher Si 60 250 × 4 mm, 5 µm LiChrospher Si 60 guard cartridge 4 × 4 mm, 5 µm | A: 2% water, 2% AcAc, 14% MeOH in DCM B: 2% water, 2% AcAc in MeOH %B: 0–87.8 (50 min), 87.5−0 (80 min) 1.0 mL/min |
| Sargassum muticum, Sargassum vulgare, Hypnea spinella, Porphyra sp., Undaria pinnatifida, Chondrus crispus, Halopytis incurvus [18] | Sep. and ident. of 8 isoflavones | NP-HPLC-ESI-QqQ | (-) MRM | Zorbax SB-CN 100 × 2.1 mm, 3.5 μm | A: 0.2% AcAc (aq.) B: ACN %B: 30–80 (6 min), 80−30 (10 min) 0.4 mL/min |
| Fucus distichus, Saccharina latissima, Saccharina groenlandica, Alaria marginata, Porphyra fallax, Ulva lactuca, [197] | Sep. and ident. of 24 phlorotannins (DP-level) | SEC-HPLC-ESI-IT-TOF | (+)/(-) FULL SCAN | Develosil Diol 250 × 4.6 mm, 5 μm | A: 0.2% AcAc in ACN B: 0.2% AcAc, 3% water in MeOH %B: 0–100 (40 min), 100–0 (50 min) 0.8 mL/min |
| Ecklonia stolonifera [198] | Sep. and tentative ident. of 18 phlorotannins | RP-HPLC-ESI-IT | (+) FULL SCAN | Capcell Pak C18 UG120 2.0 × 150 mm, - | A: 1% FA in MeOH:water (5:95) B: 1% FA in MeOH:water (70:30) %B: 0–100 (60 min) 0.2 mL/min |
| Ecklonia stolonifera [199] | Separation and identification of 3 phlorotanins. | RP-HPLC-ESI-IT | (+) FULL SCAN | Hypersil Gold C-18 250 × 4.6 mm, 5 µm Gemini C-18 guard column 30 × 4.6 mm, 5 µm | A: 0.1% FA (aq.) B: 0.1% FA (in ACN) %B: 13–60 (48 min),60−13 (60 min) 1.0 mL/min |
| Porphyra tenera, Undaria pinnatifida [72] | Sep., ident. and quant. of 12 misc. phenolics | RP-HPLC-ESI-Q | (-) SIM | Zorbax SB-C18 150 × 4.6 mm, 3.5 μm | A: 0.2% AcAc (aq.) B: ACN %B: 4–30 (10 min), 30−4 (15 min) 1.1 mL/min |
| Porphyra dentata [200] | Sep. and ident. of 1 catechol and 2 flavonoid glycosides | RP-HPLC-ESI-IT | (+) FULL SCAN | Luna C18 150 × 2 mm, 3 µm | A: 0.05% TFA in ACN B: 0.05% TFA (aq.) %B: 15–100 (50 min) 0.2 mL/min |
| Palmaria spp., Porphyra spp., Himanthalia elongata, Laminaria ochroleuca, Undaria pinnatifida [201] | Sep. and detection of 7 phlorotannins | RP-HPLC-ESI-TOF | (-) FULL SCAN | Mediterranean Sea18 150 × 4 mm, 3 μm | A: 1% AcAc (aq.) B: 1% AcAc in ACN:water (32:68) %B: 0–100 (25 min) 0.5 mL/min |
| Gracilaria birdiae, Gracilaria córnea [193] | Detection and tentative ident. of 2 misc. phenolics | RP-HPLC-ESI-? | (+)/(-) MRM | Supelcosil LC-18 250 × 5 mm, - | A: MeOH B: 0.1% FA (aq.) %B: 50 (isocratic) 0.4 mL/min |
| Ascophyllum nodosum [77] | Sep. and ident. of 2 phlorotannins (DP-level) | RP-HPLC-ESI-IT | (+)/(-) FULL SCAN | Synergi Hydro C18 with polar end capping 150 × 2 mm, - | A: Water B: ACN %B: 5–100 (30 min) 0.2 mL/min |
| Cystoseira nodicaulis, Cystoseira tamariscifolia, Cystoseira usneoides, Fucus spiralis [35] | Sep. and ident. 22 of phlorotannins | RP-HPLC-ESI-IT | (+)/(-) FULL SCAN | Luna C18 250 × 4.6 mm, 5 µm | A: 1% FA (aq.) B: ACN %B: 0–80 (40), 80−0 (52) 1.0 mL/min |
| Ascophyllum nodosum [88] | Sep. and ident. of 6 phlorotannins (DP-level) | RP-HPLC-ESI-IT | (+)/(-) FULL SCAN/DDA | Synergi Hydro C18 w/polar end-capping 2.0 × 150 mm, - | A: 0.1% FA in ACN:water (5:95) B: 0.1% FA in ACN:water (40:60) %B: 0–100 (30 min) 0.2 mL/min |
| Ecklonia bicyclis, Ecklonia kurome, Ecklonia arborea, Ecklonia cava [202] | Sep. and ident. of 12 phlorotannins (DP-level) | RP-HPLC-ESI-Q | (-) FULL SCAN | Inertsil ODS-3 250 × 4.6 mm, 4 µm | A: water B: MeOH %B: 20–100 (40 min) - |
| Ascophyllum nodosum, Laminaria digitata [163] | Detection of phlorotannins | RP-HPLC-ESI-IT | (-) FULL SCAN | Nova-Pak C18 - | A: Formate:methanol 95:5 v/v B: MeOH %B: 0–100 (30 min) 1.0 mL/min |
| Macrocystis pyrifera [69,71] | Sep. and tentative ident. of 2 phlorotannins | RP-HPLC-ESI-IT | (+)/(-) FULL SCAN | LunaC18 150 × 4.6 mm, 5 μm | A: 1% FA (aq.) B: ACN %B: 5 (0–15 min), 5−100 (75–85 min), 100−5 (90 min) 1.0 mL/min |
| Ascophyllum nodosum [203] | Sep. and tentative ident. of 6 phlorotannins | RP-HPLC-ESI-IT | (-) FULL SCAN | Zorbax SB C18 100 × 2.1 mm, 1.8 µm | A: 0.1% FA (aq.) B: 0.1% FA in ACN %B: 10–70 (50), 70−10 (65) 2.0 mL/min |
| Sargassum muticum [96] | Detection and tentative identification of 3 phlorotanins. | RP-HPLC-ESI-IT | (+)/(-) FULL SCAN | Zorbax Eclipse XDB-C18 150 × 4.6 mm, 5 μm | A: 0.1% FA (aq.) B: ACN %B: 2–20 (35 min) 0.2 mL/min |
| Ulva intestinales, Porphyra umbilicalis, Palmaria palmata, Fucus sechees, Himanthalia elongata, Laminaria japonica, Saccorhiza polyschides, Ascophyllum nodosum, Fucus vesiculosus, Laminaria digitata, Undaria pinnatifida [146] | Sep. and detection of 22 phlorotannins | RP HPLC-ESI-QqTOF | (-) FULL SCAN | Luna C18 150 × 4.6 mm, 3 μm | A: 0.1% FA, 5% ACN (aq.) B: 0.1% FA in ACN:water (95:5) %B: 40–100 (65 min), 100−40 (67 min) 0.5 mL/min |
| Himanthalia elongata [17] | Sep. and ident. of 8 misc. phenolics | RP-HPLC-ESI-QqQ | (-) FULL SCAN | Atlantis C-18 250 × 4.6 mm, 5 μm C-18 guard cartridge 4.0 × 3.0 mm, - | A: 0.25% aqueous AcAc B: 0.25% AcAc in ACN:water (80/20) %B: 10 (20 min), 10−20 (30 min) 1.0 mL/min |
| Achophyllum nodosum, Bifurcaria bifurcata, Fucus vesiculosus [118] | Sep and ident. of 41 misc. phenolics | RP-HPLC-ESI-QqQ | (-) FULL SCAN | Zorbax SB C18 150 × 3 mm, 3.5 μm | A:2.5% AcAc (aq.) B: 2.5%AcAc in ACN %B: 5–15(0–15 min), 15−30 (35 min), 30−40 (40 min) –60 (50 min) − 90 (55 min) − 100 (55.01–75 min) 1.0 mL/min |
| Dasycladus vermicularis [21] | Sep and ident. of 4 coumarins and 2 phenolic acids | RP-HPLC-ESI-IT | (-) FULL SCAN | Gemini C18 150 × 4.6 mm, 3 μm | A: 20 mM ammonium acetate/1.5% AcAc (aq.) B: MeOH:water (90:10) %B: 2–15 (5 min), 15−60 (20 min), 60−98 (25 min) 0.3 mL/min |
| Agarum cribrosum [60] | Identification of 1 trifuhalol | RP HPLC-DAD-ESI-Q | (+) FULL SACN | Zorbax Analytical Rx-C18 250 × 4.6 mm, 5 μm | A: 0.1%FA (aq.) B: 0.1% FA in ACN %B: 5–15 (0–8 min), 15−30 (10 min), 30−35 (18 min), 35−100 (23–35 min), 100−5 (40 min) 1.0 mL/min |
| Ecklonia arborea, Solieria filiformis [119] | Sep and ident. of 1chromone, 5 flavonoid glycosides and 3 phlorotannins | RP-HPLC-ESI-TOF- | (+) FULL SCAN | SunFire C18 150 × 4.6 mm, 5 μm | A: 3% AcAc (aq.) B: 3% AcAc in ACN %B: 10 (0–3.5 min), 10−50 (5 min), 50−0 (10–20 min) 1.5 mL/min |
| Ascophyllum nodosum, Laminaria japonica, Lessonia trabeculate, Lessonia nigrecens [110] | Sep., ident. and quant. of 12 misc. phenolics | RP-HPLC-ESI-Q | (-) FULL SCAN | Hypersil Gold C-18 150 × 2.1 mm | A: 0.25% AcAc (aq.) B: CAN:water(80:20) %B: 40 (20 min), 40−75 (35 min), 75−90 (46 min), 90−40 (50 min) 0.2 mL/min |
| Cladophora glomerata [14] | Sep., ident. and quant. of 10 phenolic acids and flavonoids | RP-HPLC-ESI-IT | (-) MRM | LichroCART C-18 150 × 4.6 mm, 5 µm | A: 10mM ammonium formate/FA (pH4) B: ACN %B: 0 (0–5 min). 0−20 (10 min), 20 (20 min), 20−40 (60 min) 1.0 mL/min |
| Himanthalia elongata [64] | Sep, ident. and quant. of 7 phenolic acids and flavonoids | RP-HPLC-ESI-QqQ | (-) FULL SCAN | Atlantis C-18 250 × 4.6 mm, 5 µm | A: 0.25% AcAc (aq.) B: 0.25%AcAc in ACN:water(80:20) %B: 10 (0–20 min), 10−20 (30 min), 20−30 (35 min), 30−0(45 min) 1.0 mL/min |
| Fucus guiryi, Fucus serratus, Fucus spiralis, Fucus vesiculosus [58] | Sep and ident. of 22 phlorotannins (DP-level) | RP-HPLC-ESI-IT | (-) FULL SCAN | Kinetex 150 × 4.6 mm, 5 µm | A: 1% FA (aq.) B: ACN %B: 1–10 (12 min), 10−30 (25 min), 30−50 (27 min), 50−1 (29 min) 0.8 mL/min |
| RP-UHPLC-ESI-QTOF | (-) FULL SCAN | Luna Omega 50 × 2.1 mm, 1.6 µm | A: 0.1% FA(aq.) B: 0.1% FA in ACN %B: 1–10 (8 min), 10−30 (14 min), 30−50 (16 min), 50–1 (20 min) 0.5 mL/min | ||
| Ecklonia cava [116] | Sep and ident. of 15 phlorotannins | RP-HPLC-ESI-qTOF | (+)/(-) FULL SCAN | YMC-Triart C18 150 × 3.0 mm, 5 µm | A: 0.01% FA (aq.) B: 0.01% FA in ACN %B: 10–60 (40 min), 60 (45 min)/10–100 (40 min), 100(45 min) 0.3 mL/min |
| Ulva intestinalis, Dictyota dichotoma [57] | Sep and ident. of 1 flavone | RP-HPLC-ESI-TOF | (+)/(-) FULL SCAN | Zorbax SB-C18 100 × 2.1 mm, 3.5 μm | A: 0.05 FA (aq.) B:ACN %B: 10–90 (60 min), 90−10 (65 min) 0.3 mL/min |
| RP-HPLC-ESI-Q | (-) SIM | Zorbax SB-C18 100 × 2.1 mm, 3.5 μm | A: 0.05 FA (aq.) B:ACN %B: 10–90 (90 min), 90−10 (95 min) 0.3 mL/min | ||
| Sargassum pallidum [195] | Sep. and ident. of 6 phlorotannins (DP-level) | RP-UHPLC-ESI-QqQ | (-) FULL SCAN | Bridged Ethyl Hybrid (BEH) C18 100 × 2.1 mm, 1.7 μm | A: MeOH B: water %B: 30–100 (10 min),100–30 (20 min) 0.3 mL/min |
| Ecklonia cava [152] | Sep. and ident. of 4 phlorotannins | RP-UHPLC-ESI-? | (+) FULL SCAN | Bridged Ethyl Hybrid (BEH) C18 100 × 2.1 mm, 1.7 μm | A: 0.1% FA (aq.) B: 0.1% FA in ACN %B: 10–45 (5 min) 0.4 mL/min |
| Ascophyllum nodosum, Pelvetia canaliculata, Fucus spiralis [165] | Sep. and ident. of >100 phlorotannins (DP-level, isomers) | RP-UHPLC-ESI-QqQ | (-) MRM | HSS PFP 100 × 2.1 mm, 1.8 μm | A: 0.1% FA (aq.) B: 0.1% FA in ACN %B: 0.5–90 (28 min), 90−0.5 (30 min) 0.5 mL/min |
| Fucus serratus, Fucus vesiculosus, Himanthalia elongata, Cystoseira nodicaulis [28] | Sep. and ident. of >100 phlorotannins (DP-level, isomers) | RP-UHPLC-ESI-QqQ | (-) MRM | HSS PFP 100 × 2.1 mm, 1.8 μm | A: 0.1% FA (aq.) B: 0.1% FA in ACN %B: 0.5–90 (28 min), 90−0.5 (30 min) 0.5 mL/min |
| Fucus vesiculosus [166] | Sep. and ident. of >100 phlorotannins (DP-level, isomers) | RP-UHPLC-ESI-QqQ | (-) MRM | HSS PFP 100 × 2.1 mm, 1.8 μm | A: 0.1% FA (aq.) B: 0.1% FA in ACN %B: 0.5–90 (28 min), 90−0.5 (30 min) - |
| Laminaria digitata [44] | Sep. and ident. of 34 phlorotannins (Class and DP-level) | RP-UHPLC-ESI-IT | (-) FULL SCAN | Bridged Ethyl Hybid (BEH) C18 150 × 2.1 mm, 1.7 μm | A: 0.1% FA (aq.) B: 0.1% FA in ACN %B: 1 (1 min), 1−8 (1.5 min), 8−55 (24 min), 55−100 (25–28 min), 100−1 (29 min) 0.4 mL/min |
| Fucus vesiculosus [59] | Sep and ident. of 13 phlorotannins (Class and DP-level) | RP-UHPLC-QTOF | (+)/(-) FULL SCAN | Prodigy ODS 3 150 × 2 mm, 3 μm | A: Ammonium formate buffer/20 mM FA, pH3 B: 20 mM FA in ACN %B: 0 (0–2 min), 0−40 (16 min), 40−100 (18 min) |
| Fucus vesiculosus [106] | Sep and ident. of 1 phlorotannin | RP-UHPLC-ESI-QTOF-MS | (+) FULL SCAN, DDA | HSS T3 100 × 2.1 mm, - | A: 0.1% FA (aq.) B: ACN %B 1–40 (2.5 min), 40−100 (13.5–14.5 min), 100−1 (14.6 min) 0.6 mL/min |
| Pelvetia canaliculata, Fucus spiralis, Fucus vesiculosus, Ascophyllum nodosum, Saccharina longicruris [10] | Sep. and ident. of 21 phlorotannins (DP-level) | HILIC-UHPLC-ESI-Orbitrap | (-) FULL SCAN | UHPLC W BEH Amide 100 × 2.1 mm, 1.7 μm | A: 10.0mM ammonium acetate pH 9.0 B: ACN. %B: 95–65 (16 min), 95 (21 min) 0.4 mL/min |
| Enteromorpha prolifera [120] | Sep and ident. of 1 naphtalenone, 1 flavone and 1 flavanone | RP-UHPLC-ESI-QuanTOF | (+) FULL SCAN | Unspecified C18 100 × 2.1 mm, 1.7 µm | A: 0.1% FA (aq.) B: 0.1% FA in ACN %B: 1 (0–0.25 min), 1−99 (16.25 min), 99 (17 min) 0.45 mL/min |
| Fucus vesiculosus [9] | Sep. and tentative ident. of 17 phlorotannins | RP-UHPLC-ESI-IT | (-) FULL SCAN | Hypersil Gold-C18 100 × 2.1 mm, 1.9 µm | A: 0.1 % FA (aq.) B: ACN %B: 5–40% (14.72 min), 40–100% (1.91 min), 100 (2.19 min). 0.2 mL min−1 |
| Durvillaea antarctica, Lessonia spicata, Macrocystis integrifolia [19] | Sep and ident. of 84 miscellaneous phenolics | RP-HPLC-ESI-IT/n | Unspecified C18 250 × 4.6 mm, 5 µm | A: 1% FA (aq.) B: ACN %B: 5 (0–5 min), 5−30 (60 min), 30−60 (70–80 min), 60−5 (90 min) 1.0 mL/min | |
| Cystoseira abies-marina [36] | Sep. [52 + spots] and ident. of phlorotannins (DP-level) | HILICxRP-2D-HPLC-ESI-IT | (-) FULL SCAN | D1 precolumn Lichrospher diol-5 Lichrospher diol-5 150 × 1.0 mm, 5 µm D2 precolumn C18 Ascentis Express C18 50 ×4.6 mm, 2.7 μm OR Kinetex pentafluorophenyl (PFP) 50 × 4.6 mm, 2.6 μm | D1 A: 2% AcAc in ACN B: 2% AcAc, 3% water in MeOH %B: 0–25 (85 min) 15 μL/min D2 A: 0.1% FA (aq.) B: ACN %B: 0–90 (0.9 min), 90−0( 1.3 min) 3 mL/min |
| Sargassum muticum [104] | Sep. [73+ spots] and tentative ident. of phlorotannins (Class-level) | HILICxRP-2D-HPLC-ESI-IT | (-) FULL SCAN | D1 Lichrospher diol-5 150 × 1.0 mm, 5 μm D2 C18 precolumn Ascentis Express C18 50 × 4.6 mm, 2.7 μm | D1 A: 2% AcAc in ACN B: 2% AcAc, 3% water in MeOH %B: 0–25 (85 min) 15 μL/min D2 A: 0.1% FA (aq.) B: ACN %B: 0–90 (0.9 min) − 0 (1.3 min) 3 mL/min |
| Cystoseira abies-marina [194] | Sep. [35 spots] and ident. of 29 phlorotannins (DP-level) | HILICxRP-2D-HPLC-ESI-IT | (-) FULL SCAN | D1 precolumn Lichrospher diol-5 Lichrospher diol-5 150 × 1.0 mm, 5 µm D2 C18 precolumn Partially porous C18 50 × 4.6 mm, 2.7 µm | D1 A: ACN/AcAc (98:2, v/v) B: MeOH/water/AcAc (95:3:2, v/v/v) %B: 3–35 (70 min) 15 µL/min D2 A: 0.1% FA (aq.) B: ACN %B: 0–90 (1 min), 90−0 (1.01 min), − repeat 3 mL min−1 |
| DP | Class | Compound IUPAC Name | Formula | Mass Spectrometry | Reference |
|---|---|---|---|---|---|
| 1 | Phloroglucinol | Phloroglucinol 1,3,5-trihydroxybenzene | C6H6O3 | Native EI: 126 ([M]+, 100), 111 (9), 97 (20), 85 (30), 69 (41), 52 (26) ESI: 125.05 ([M-H]−, 100), 97.0 (-), 80.0 (-) | [17,153,186] |
| Phloroglucinol (H) | 2-Bromo-phloroglucinol 2-bromo-1,3,5-trihydroxybenzene | C6H6BrO3 | Native EI: 206/204 ([M]+, 100/91), 188/186 (4.7/5.3), 177/175 (4.2/4.0), 97 (19), 85 (11), 79 (11), 78 (14), 69 (88) Tri-Ac EI: 332/330 ([M]+, -/-), 290/288 (-/-), 248/246 (-/-), 206/204 (-/-) | [45,213] | |
| 2-Chloro-phloroglucinol 2-chloro-1,3,5-trihydroxybenzene | C6H6ClO3 | Tri-Ac EI: 288/286 ([M]+, -/-), 246/244 (-/-), 204/202 (-/-), 162/160 (-/-) | [40] | ||
| 2-Iodo-phloroglucinol 2-iodo-1,3,5-trihydroxybenzene | C6H6IO3 | Tri-Ac EI: 378 (-), 336 (-), 294 (-), 252 (-), 210 (-), 168 (-), 126 (-) | [45] | ||
| 2 | Fucol | Difucol 2,2′,4,4′,6,6′-Hexahydroxybiphenyl | C12H10O6 | Hexa-Met EI: 334 (100), 181 (9.5) Hex-Ac EI: 502 ([M]+, -), 460 (-), 418 (-), 376 (-), 334 (-), 292 (-), 250 (-) | [208,209] |
| Difucol-4,4′-disulphate (dipotassium salt) 2,2′,6,6′-Tetrahydroxybiphenyl-4,4′-disulphate | C12H10S2O12 | Native FAB: 485 ([M]+, 25), 447 (100),409 (15), 367 (30), 351 (40), 329 (50), 281 (35), 243 (100), 227 (35), 205 (15) Tetra-Ac FAB: 615 ([M]+, 70), 599 (17), 577 (15), 573 (40), 557 (5), 535/531 (20), 497 (25), 493 (15), 481 (4), 469 (12), 455 (20), 289 (100), 273 (31), 255 (31), 249 (56), 243 (51), 230 (31), 215 (51) | [39,214] | ||
| Fucol (H) | 3-Chloro-difucol 3[A4]-Chloro-difucol 3-Chloro-2,2′,4,4′,6,6′-biphenylhexol | C12H9ClO6 | Hex-Ac FAB: 559 ([M+Na]+, -), 537 ([M+H]+, -), 495 (-), 453 (-), 411 (-), 369 (-), 327 (-), 285 (-) EI: 538/536 ([M]+, -/-), 496/494 (-/-), 454/452 (-/-), 412/410 (-/-),…,370/368 (-/-), 328/326 (-/-), 286/284 (-/-) | [40] | |
| Fuhalol | Bifuhalol 5-(2,4,6-Trihydroxyphenoxy)-1,2,3-benzenetriol | C12H10O7 | Native ESI: 265 ([M-H]-, -), 247 (-), 141 (-), 139 (-), 125 (-), 123 (-), 111 (-) Hex-Ac EI: 518 (-), 476 (-), 434 (-), 392 (-), 350(-), 308 (-), 266 (-), 142 (-), 126 (-) | [210,211] | |
| Fuhalol (H) | 3′-Chloro-bifuhalol 3[A]-Chloro-bifuhalol 5-(3-chloro-2,4,6-Trihydroxyphenoxy)-benzene-1,2,3-triol | C12H9ClO7 | Hex-Ac FAB: 591 ([M+K]+, -) 575 ([M+Na]+, -), 553 ([M+H]+, -), 511 (-), 469 (-), 427 (-), 385 (-), 343 (-), 301 (-) EI: 554/552 (-/-), 512/510 (-/-), 470/468 (-/-), 428/426 (-/-), 386/384 (-/-), 344/342 (-/-), 302/300 (-/-) | [40] | |
| Phlorethol | Diphlorethol 2-(3,5-dihydroxyphenoxy)benzene-1,3,5-triol | C12H10O6 | Native ESI: 251 ([M+H]+, -), 233 (-), 139 (-), 123 (-), 109 (-), 93 (-) Penta-Ac EI: 460 ([M]+, -), 418 (-), 376 (-), 334 (-), 292 (-), 250 (-), 142 (-), 126 (-), 110 (-) | [210,212] | |
| Phlorethol (H) | 3-Bromo-diphlorethol 3[A]-Bromo-diphlorethol 3[A1]-Bromo-diphlorethol 2-(3,5-Dihydroxyphenoxy)-3-bromo-1,5-benzenediol | C12H9BrO6 | Penta-Ac EI: 540/538 ([M]+, 3/3), 498/496 (21/20), 456/454 (40/40), 414/412 (65/65), 372/370 (45/44), 330/328 (28/29), 418 (1), 376 (1), 334 (3), 292 (6), 250 (2), 248 (12), 231 (9), 69 (18), 43 (100) | [41,46] | |
| 2′-Bromo-diphlorethol 2[D]-Bromo-diphlorethol 2[D′]-Bromo-diphlorethol 2-(2′-bromo-3′,5′-Dihydroxyphenoxy)-1,3,5-benzenetriol | C12H9BrO6 | Pent-Ac EI: 540/538 ([M]+, 3/3), 498/496 (21/20), 456/454 (40/40), 414/412 (65/65), 372/370 (45/44), 330/328 (28/29), 418 (1), 376 (1), 334 (3), 292 (6), 250 (2), 248 (12), 231 (9), 69 (18), 43 (100) | [41,46] | ||
| 4′-Bromo-diphlorethol 4[D]-Bromo-diphlorethol 4[D′]-Bromo-diphlorethol 2-(4-bromo-3,5-Dihydroxyphenoxy)-1,3,5-benzenetriol | C12H9BrO6 | Pent-Ac EI: 540/538 ([M]+, 3/3), 498/496 (21/20), 456/454 (40/40), 414/412 (65/65), 372/370 (45/44), 330/328 (28/29), 418 (1), 376 (1), 334 (3), 292 (6), 250 (2), 248 (12), 231 (9), 69 (18), 43 (100) | [41,46] | ||
| Phlorethol (H)(Cont.) | 4′-Chloro-diphlorethol 4[D]-Chloro-diphlorethol 4[D′]-Chloro-diphlorethol 2-(4-bromo-3,5-Dihydroxyphenoxy)-1,3,5-benzenetriol | C12H9ClO6 | Pent-Ac EI: 496/494 (-/-), 454/452 (-/-), 412/410 (-/-), 370/368 (-/-), 328/326 (-/-), 286/284 (-/-) | [41] | |
| 2′-Iodo-diphlorethol 2[D′]-Iodo-diphlorethol 2′-iodo-2-(3,5-Dihydroxyphenoxy)-1,3,5-benzenetriol | C12H9IO6 | Pent-Ac EI: 586 (-), 544 (-), 502 (-), 460 (-), 418 (-), 376 (-) | [40] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, S.A.O.; Félix, R.; Pais, A.C.S.; Rocha, S.M.; Silvestre, A.J.D. The Quest for Phenolic Compounds from Macroalgae: A Review of Extraction and Identification Methodologies. Biomolecules 2019, 9, 847. https://0-doi-org.brum.beds.ac.uk/10.3390/biom9120847
Santos SAO, Félix R, Pais ACS, Rocha SM, Silvestre AJD. The Quest for Phenolic Compounds from Macroalgae: A Review of Extraction and Identification Methodologies. Biomolecules. 2019; 9(12):847. https://0-doi-org.brum.beds.ac.uk/10.3390/biom9120847
Chicago/Turabian StyleSantos, Sónia A. O., Rafael Félix, Adriana C. S. Pais, Sílvia M. Rocha, and Armando J. D. Silvestre. 2019. "The Quest for Phenolic Compounds from Macroalgae: A Review of Extraction and Identification Methodologies" Biomolecules 9, no. 12: 847. https://0-doi-org.brum.beds.ac.uk/10.3390/biom9120847