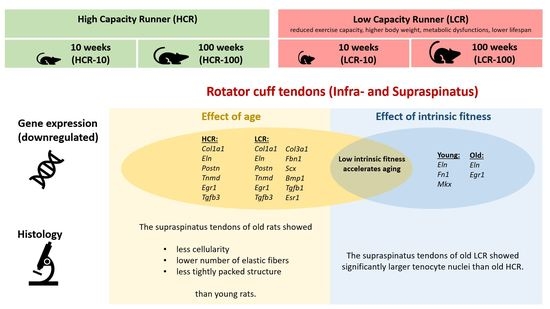
Age and Intrinsic Fitness Affect the Female Rotator Cuff Tendon Tissue
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Model
2.2. Sample Collection
2.3. Histology
2.4. Image Analysis
2.5. qRT-PCR
2.6. Statistics
3. Results
3.1. Histology of the Rotator Cuff
3.2. Molecular Analysis of the Rotator Cuff Tendons
4. Discussion
4.1. The Effect of Age on the Rat Rotator Cuff Tendon
4.2. The Effect of Low Intrinsic Fitness on the Rat Rotator Cuff Tendon
- -
- influences the RC tendons at least as much as age;
- -
- may result in pronounced changes in the expression of ECM proteins, tendon-related genes and transcription factors that are essential for tendon formation, maturation, maintenance, remodeling, repair and function;
- -
- affects especially aged tendons (the tendons of old LCR rats were more affected than the tendons of young LCR);
- -
- may accelerate unhealthy aging with the pathogenesis of tendinopathy via repression of gene expression.
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Animal Model Availability
Acknowledgments
Conflicts of Interest
References
- Teunis, T.; Lubberts, B.; Reilly, B.T.; Ring, D. A systematic review and pooled analysis of the prevalence of rotator cuff disease with increasing age. J. Shoulder Elbow Surg. 2014, 23, 1913–1921. [Google Scholar] [CrossRef]
- Park, J.S.; Park, H.J.; Kim, S.H.; Oh, J.H. Prognostic factors affecting rotator cuff healing after arthroscopic repair in small to medium-sized tears. Am. J. Sports Med. 2015, 43, 2386–2392. [Google Scholar] [CrossRef] [PubMed]
- Klatte-Schulz, F.; Aleyt, T.; Pauly, S.; Geissler, S.; Gerhardt, C.; Scheibel, M.; Wildemann, B. Do matrix metalloproteases and tissue inhibitors of metalloproteases in tenocytes of the rotator cuff differ with varying donor characteristics? Int. J. Mol. Sci. 2015, 16, 13141–13157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klatte-Schulz, F.; Pauly, S.; Scheibel, M.; Greiner, S.; Gerhardt, C.; Schmidmaier, G.; Wildemann, B. Influence of age on the cell biological characteristics and the stimulation potential of male human tenocyte-like cells. Eur. Cell Mater. 2012, 24, 74–89. [Google Scholar] [CrossRef] [PubMed]
- Lebaschi, A.; Deng, X.H.; Zong, J.; Cong, G.T.; Carballo, C.B.; Album, Z.M.; Camp, C.; Rodeo, S.A. Animal models for rotator cuff repair. Ann. N. Y. Acad. Sci. 2016, 1383, 43–57. [Google Scholar] [CrossRef]
- Koch, L.G.; Britton, S.L. Artificial selection for intrinsic aerobic endurance running capacity in rats. Physiol. Genom. 2001, 5, 45–52. [Google Scholar] [CrossRef] [Green Version]
- Koch, L.G.; Britton, S.L.; Wisløff, U. A rat model system to study complex disease risks, fitness, aging, and longevity. Trends Cardiovasc. Med. 2012, 22, 29–34. [Google Scholar] [CrossRef] [Green Version]
- Wisloff, U.; Najjar, S.M.; Ellingsen, O.; Haram, P.M.; Swoap, S.; Al-Share, Q.; Fernstrom, M.; Rezaei, K.; Lee, S.J.; Koch, L.G.; et al. Cardiovascular risk factors emerge after artificial selection for low aerobic capacity. Science 2005, 307, 418–420. [Google Scholar] [CrossRef] [Green Version]
- Dirks, R.C.; Galley, M.R.; Childress, P.J.; Fearon, A.M.; Scott, A.; Koch, L.G.; Britton, S.L.; Warden, S.J. Uphill running does not exacerbate collagenase-induced pathological changes in the achilles tendon of rats selectively bred for high-capacity running. Connect. Tissue Res. 2013, 54, 386–393. [Google Scholar] [CrossRef]
- Dirks, R.C.; Richard, J.S.; Fearon, A.M.; Scott, A.; Koch, L.G.; Britton, S.L.; Warden, S.J. Uphill treadmill running does not induce histopathological changes in the rat achilles tendon. BMC Musculoskelet. Disord. 2013, 14, 90. [Google Scholar] [CrossRef] [Green Version]
- Simon, P. Q-Gene: Processing quantitative real-time RT-PCR data. Bioinformatics 2003, 19, 1439–1440. [Google Scholar] [CrossRef] [Green Version]
- Ippolioto, E.; Natali, P.G.; Postacchini, F.; Accinni, L.; De Martino, C. Morphological, immunochemical, and biochemical study of rabbit achilles tendon at various ages. JBJS 1980, 62, 583–598. [Google Scholar] [CrossRef]
- Dunkman, A.A.; Buckley, M.R.; Mienaltowski, M.J.; Adams, S.M.; Thomas, S.J.; Satchell, L.; Kumar, A.; Pathmanathan, L.; Beason, D.P.; Iozzo, R.V.; et al. Decorin Expression Is Important for Age-Related Changes in Tendon Structure and Mechanical Properties. Matrix Biol. 2013, 32, 3–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuskov, A.; Freedman, B.R.; Gordon, J.A.; Sarver, J.J.; Buckley, M.R.; Soslowsky, L.J. Tendon Biomechanics and Crimp Properties Following Fatigue Loading Are Influenced by Tendon Type and Age in Mice. J. Orthop. Res. 2020, 38, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Laitinen, O. The metabolism of collagen and its hormonal control in the rat with special emphasis on the interaction of collagen and calcium in the bones. Acta Endocrinol. 1967, 120 (Suppl. S1967), S17–S86. [Google Scholar] [CrossRef]
- Ribitsch, I.; Gueltekin, S.; Keith, M.F.; Minichmair, K.; Peham, C.; Jenner, F.; Egerbacher, M. Age-related changes of tendon fibril micro-morphology and gene expression. J. Anat. 2020, 236, 688–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jozsa, L.; Kannus, P. Human Tendons. Anatomy, Physiology, and Pathology. Champaign IL Hum. Kinet. 1997, 33, 185. [Google Scholar]
- Green, E.M.; Mansfield, J.C.; Bell, J.S.; Winlove, C.P. The structure and micromechanics of elastic tissue. Interface Focus 2014, 4, 20130058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Godinho, M.S.C.; Thorpe, C.T.; Greenwald, S.E.; Screen, H.R.C. Elastin is Localised to the Interfascicular Matrix of Energy Storing Tendons and Becomes Increasingly Disorganised With Ageing. Sci. Rep. 2017, 7, 9713. [Google Scholar] [CrossRef]
- Duchamp de Lageneste, O.; Julien, A.; Abou-Khalil, R.; Frangi, G.; Carvalho, C.; Cagnard, N.; Cordier, C.; Conway, S.J.; Colnot, C. Periosteum contains skeletal stem cells with high bone regenerative potential controlled by Periostin. Nat. Commun. 2018, 9, 773. [Google Scholar] [CrossRef] [Green Version]
- Norris, R.A.; Damon, B.; Mironov, V.; Kasyanov, V.; Ramamurthi, A.; Moreno-Rodriguez, R.; Trusk, T.; Potts, J.D.; Goodwin, R.L.; Davis, J.; et al. Periostin regulates collagen fibrillogenesis and the biomechanical properties of connective tissues. J. Cell Biochem. 2007, 101, 695–711. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Jin, S.; Luo, D.; He, D.; Shi, C.; Zhu, L.; Guan, B.; Li, Z.; Zhang, T.; Zhou, Y.; et al. Functional regeneration and repair of tendons using biomimetic scaffolds loaded with recombinant periostin. Nat. Commun. 2021, 12, 1293. [Google Scholar] [CrossRef] [PubMed]
- Dasheng, L.; Alberton, P.; Caceres, M.D.; Prein, C.; Clausen-Schaumann, H.; Dong, J.; Aszodi, A.; Shukunami, C.; CIatridis, J.; Docheva, D. Loss of tenomodulin expression is a risk factor for age-related intervertebral disc degeneration. Aging Cell 2020, 19, e13091. [Google Scholar]
- Manning, C.N.; Kim, H.M.; Sakiyama–Elbert, S.; Galatz, L.M.; Havlioglu, N.; Thomopoulos, S. Sustained delivery of transforming growth factor beta three enhances tendon-to-bone healing in a rat model. J. Orthop. Res. 2011, 29, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Kaji, D.A.; Howell, K.L.; Balic, Z.; Hubmacher, D.; Huang, A.H. Tgfβ signaling is required for tenocyte recruitment and functional neonatal tendon regeneration. eLife 2020, 9, e51779. [Google Scholar] [CrossRef] [PubMed]
- Lejard, V.; Blais, F.; Guerquin, M.-J.; Bonnet, A.; Bonnin, M.-A.; Havis, E.; Malbouyres, M.; Bonod Bidaud, C.; Maro, G.; Pascale Gilardi-Hebenstreit, P.; et al. EGR1 and EGR2 involvement in vertebrate tendon differentiation. J. Biol. Chem. 2011, 286, 5855–5867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, X.; Liu, J.; Chen, L.; Zhou, Y.; Tang, K. EGR1 induces tenogenic differentiation of tendon stem cells and promotes rabbit rotator cuff repair. Cell Physiol. Biochem. 2015, 35, 699–709. [Google Scholar] [CrossRef]
- Guerquin, M.J.; Charvet, B.; Nourissat, G.; Havis, E.; Ronsin, O.; Bonnin, M.A.; Ruggiu, M.; Olivera-Martinez, I.; Robert, N.; Lu, Y.; et al. Transcription factor EGR1 directs tendon differentiation and promotes tendon repair. J. Clin. Investig. 2013, 123, 3564–3576. [Google Scholar] [CrossRef] [Green Version]
- Kosaka, H.; Sairyo, K.; Biyani, A.; Leaman, D.; Yeasting, R.; Higashino, K.; Sakai, T.; Katoh, S.; Sano, T.; Goel, V.K.; et al. Pathomechanism of loss of elasticity and hypertrophy of lumbar ligamentum flavum in elderly patients with lumbar spinal canal stenosis. Spine 2007, 32, 2805–2811. [Google Scholar] [CrossRef]
- Sindram, D.; Martin, K.; Meadows, J.P.; Prabhu, A.S.; Heath, J.J.; McKillop, I.H.; Iannitti, D.A. Collagen-elastin ratio predicts burst pressure of arterial seals created using a bipolar vessel sealing device in a porcine model. Surg. Endosc. 2011, 25, 2604–2612. [Google Scholar] [CrossRef]
- Akturk, M.; Karaahmetoglu, S.; Kacar, M.; Muftuoglu, O. Thickness of the Supraspinatus and Biceps Tendons in Diabetic Patients. Diabetes Care 2002, 25, 408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Oliveira, R.R.; Martins, C.S.; Rocha, Y.R.; Braga, A.B.; Mattos, R.M.; Hecht, F.; Brito, G.A.; Nasciutti, L.E. Experimental diabetes induces structural, inflammatory and vascular changes of Achilles tendons. PLoS ONE 2013, 8, e74942. [Google Scholar]
- Boivin, G.P.; Elenes, E.Y.; Schultze, A.K.; Chodavarapu, H.; Hunter, S.A.; Elased, K.M. Biomechanical properties and histology of db/db diabetic mouse Achilles tendon. Muscles Ligaments Tendons J. 2014, 17, 280–284. [Google Scholar] [CrossRef]
- Guney, A.; Vatansever, F.; Karaman, I.; Kafadar, I.H.; Oner, M.; Turk, C.Y. Biomechanical Properties of Achilles Tendon in Diabetic vs. Non-diabetic Patients. Exp. Clin. Endocrinol. Diabetes 2015, 123, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Kadler, K.E.; Hill, A.; Canty-Laird, E.G. Collagen fibrillogenesis: Fibronectin, integrins, and minor collagens as organizers and nucleators. Curr. Opin. Cell Biol. 2008, 20, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Toriuchi, N.; Yoshitaka, T.; Ueno-Kudoh, H.; Sato, T.; Yokoyama, S.; Nishida, K.; Akimoto, T.; Takahashi, M.; Miyaki, S.; et al. The Mohawk homeobox gene is a critical regulator of tendon differentiation. Proc. Natl. Acad. Sci. USA 2010, 107, 10538–10542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murchison, N.D.; Price, B.A.; Conner, D.A.; Keene, D.R.; Olson, E.N.; Tabin, C.J.; Schweitzer, R. Regulation of tendon differentiation by scleraxis distinguishes force-transmitting tendons from muscle-anchoring tendons. Development 2007, 134, 2697–2708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kessler, E.; Takahara, K.; Biniaminov, L.; Brusel, M.; Greenspan, D.S. Bone morphogenetic protein-1: The type I procollagen C-proteinase. Science 1996, 271, 360–362. [Google Scholar] [CrossRef]
- Sharir, A.; Zelzer, E. Tendon homeostasis: The right pull. Curr. Biol. 2011, 21, R472–R474. [Google Scholar] [CrossRef] [Green Version]
- Burner, T.; Gohr, C.; Mitton-Fitzgerald, E.; Rosenthal, A.K. Hyperglycemia reduces proteoglycan levels in tendons. Connect. Tissue Res. 2012, 53, 535–541. [Google Scholar] [CrossRef]
- Robinson, K.A.; Sun, M.; Barnum, C.E.; Weiss, S.N.; Huegel, J.; Shetye, S.S.; Lin, L.; Saez, D.; Adams, S.M.; Iozzo, R.V.; et al. Decorin and biglycan are necessary for maintaining collagen fibril structure, fiber realignment, and mechanical properties of mature tendons. Matrix Biol. 2017, 64, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Raspanti, M.; Congiu, T.; Guizzardi, S. Structural aspects of the extracellular matrix of the tendon: An atomic force and scanning electron microscopy study. Arch. Histol. Cytol. 2002, 65, 37–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, G.; Ezura, Y.; Chervoneva, I.; Robinson, P.S.; Beason, D.P.; Carine, E.T.; Soslowsky, L.J.; Iozzo, R.V.; Birk, D.E. Decorin regulates assembly of collagen fibrils and acquisition of biomechanical properties during tendon development. J. Cell. Biochem. 2006, 98, 1436–1449. [Google Scholar] [CrossRef] [PubMed]
- Eliasson, P.; Andersson, T.; Aspenberg, P. Rat Achilles tendon healing: Mechanical loading and gene expression. J. Appl. Physiol. 2009, 107, 399–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, J.A.; Mehr, D.; Pardubsky, P.D.; Buckwalter, J.A. The role of tenascin-C in adaptation of tendons to compressive loading. Biorheology 2003, 40, 321–329. [Google Scholar] [PubMed]
- Bryzgalova, G.; Gao, H.; Ahren, B.; Zierath, J.R.; Galuska, D.; Steiler, T.L.; Dahlman-Wright, K.; Nilsson, S.; Gustafsson, J.A.; Efendic, S.; et al. Evidence that oestrogen receptor-alpha plays an important role in the regulation of glucose homeostasis in mice: Insulin sensitivity in the liver. Diabetologia 2006, 49, 588–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manrique, C.; Lastra, G.; Habibi, J.; Mugerfeld, I.; Garro, M.; Sowers, J.R. Loss of estrogen receptor α signaling leads to insulin resistance and obesity in young and adult female mice. Cardiorenal. Med. 2012, 2, 200–210. [Google Scholar] [CrossRef] [Green Version]
- Ribas, V.; Nguyen, M.T.; Henstridge, D.C.; Nguyen, A.K.; Beaven, S.W.; Watt, M.J.; Hevener, A.L. Impaired oxidative metabolism and inflammation are associated with insulin resistance in ERalpha-deficient mice. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E304–E319. [Google Scholar] [CrossRef] [Green Version]
- Barros, R.P.; Gabbi, C.; Morani, A.; Warner, M.; Gustafsson, J.A. Participation of ERalpha and ERbeta in glucose homeostasis in skeletal muscle and white adipose tissue. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E124–E133. [Google Scholar] [CrossRef]
- Barros, R.P.; Machado, U.F.; Warner, M.; Gustafsson, J.A. Muscle GLUT4 regulation by estrogen receptors ERbeta and ERalpha. Proc. Natl. Acad. Sci. USA 2006, 103, 1605–1608, Erratum in Proc. Natl. Acad. Sci. USA 2006, 103, 8298–8299. [Google Scholar] [CrossRef] [Green Version]
- Ogawa, S.; Chan, J.; Gustafsson, J.A.; Korach, K.S.; Pfaff, D.W. Estrogen increases locomotor activity in mice through estrogen receptor alpha: Specificity for the type of activity. Endocrinology 2003, 144, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Gorres, B.K.; Bomhoff, G.L.; Morris, J.K.; Geiger, P.C. In vivo stimulation of oestrogen receptor α increases insulin-stimulated skeletal muscle glucose uptake. J. Physiol. 2011, 589, 2041–2054. [Google Scholar] [CrossRef]
- Heine, P.A.; Taylor, J.A.; Iwamoto, G.A.; Lubahn, D.B.; Cooke, P.S. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc. Natl. Acad. Sci. USA 2000, 97, 12729–12734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hevener, A.L.; Clegg, D.J.; Mauvais-Jarvis, F. Impaired estrogen receptor action in the pathogenesis of the metabolic syndrome. Mol. Cell Endocrinol. 2015, 418, 306–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matic, M.; Bryzgalova, G.; Gao, H.; Antonson, P.; Humire, P.; Omoto, Y.; Portwood, N.; Pramfalk, C.; Efendic, S.; Berggren, P.O.; et al. Estrogen signalling and the metabolic syndrome: Targeting the hepatic estrogen receptor alpha action. PLoS ONE 2013, 8, e57458. [Google Scholar] [CrossRef] [Green Version]
- Kinitz, R.; Heyne, E.; Koch, L.G.; Britton, S.L.; Thierbach, M.; Wildemann, B. The Effect of Age and Intrinsic Aerobic Exercise Capacity on the Expression of Inflammation and Remodeling Markers in Rat Achilles Tendons. Int. J. Mol. Sci. 2022, 23, 79. [Google Scholar] [CrossRef]







| Gene | Accession Number | Primer Sequence | Amplicon Size (bp) |
|---|---|---|---|
| 18S rRNA | NM_213557.1 | forward: 5′tgtggtgttgaggaaagcag3′ reverse: 5′cctctatgggctcggatttt3′ | 240 |
| Ar | NM_012502.1 | forward: 5′tatcccagtcccagttgtgtta3′ reverse: 5′ccacagatcaggcaggtcttc3′ | 152 |
| Bgn | NM_017087.1 | forward: 5′gattgagaatgggagcctga3′ reverse: 5′ccttggtgatgttgttggag3′ | 143 |
| Bmp1 | NM_031323.1 | forward: 5′caattaccccgacgattacc3′ reverse: 5′tacccacaatatcgcccaat3′ | 189 |
| Col1a1 | NM_053304.1 | forward: 5′tgactggaagagcggagagt3′ reverse: 5′gatagcgacatcggcaggat3′ | 250 |
| Col3a1 | NM_032085.1 | forward: 5′tgggatccaatgagggaga3′ reverse: 5′tcatggccttgcgtgttt3′ | 135 |
| Dcn | NM_024129.1 | forward: 5′gcagggaatgaagggtctc3′ reverse: 5′tccacaacggtgatgctatt3′ | 195 |
| Egr1 | NM_012551 | forward:5′cacctgaccacagagtcctttt3′ reverse: 5′aaagtgttgccactgttggg3′ | 152 |
| Eln | NM_012722.1 | forward: 5′gtgtcggtcttccaggtgta3′ reverse: 5′gaaccttggccttgactcct3′ | 117 |
| Esr1 | NM_012689.1 | forward: 5′gccttctacaggtccaattctga3′ reverse: 5′acagcacagtagcgagtctcc3′ | 119 |
| Fbn1 | NM_031825.1 | forward: 5′gtgtgaactgagcgcgaac3′ reverse: 5′cactggccaccatcacagata3′ | 288 |
| Fn1 | NM_019143.2 | forward: 5′tcccacgatccgatgatgt3′ reverse: 5′tccacacggtatccagtcac3′ | 118 |
| Mkx | XM_017600733.1 | forward: 5′gctctaggctcgcagatgac3′ reverse: 5′gcgttgccctgaacatactt3′ | 143 |
| Postn | NM_001108550 | forward: 5′tagggtgtgagggagacagc3′ reverse: 5′caggtccgtgaaagtggttt3′ | 170 |
| Scx | NM_001130508.1 | Qiagen (QT01596028) | |
| Tgfb1 | NM_021578.2 | forward: 5′aactgtggagcaacacgtagaa3′ reverse: 5′tattccgtctccttggttcag3′ | 157 |
| Tgfb3 | NM_013174.2 | forward: 5′gagggtggaagccattagg3′ reverse: 5′gcagactgccagttcattgtg3′ | 256 |
| Tnc | NM_053861.1 | forward: 5′atgttccaaagagccagcaa3′ reverse: 5′aggctgtagttgaggcggta3′ | 247 |
| Tnmd | NM_022290.1 | forward:5′ggcccgaggtatccaagaag3′ reverse: 5′agatgccagtgtatccgttttt3′ | 177 |
| Group | HCR-10 | HCR-100 | LCR-10 | LCR-100 |
|---|---|---|---|---|
| Cell number | 149 (113–189) | 60 (43–61) a | 99 (79–118) | 47 (39–56) b |
| Area nucleus µm2 | 30.9 (28.5–32.8) | 28.4 (27.8–29.5) | 37.8 (36.7–38.5) | 33.3 (31.8–34.8) c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thierbach, M.; Heyne, E.; Schwarzer, M.; Koch, L.G.; Britton, S.L.; Wildemann, B. Age and Intrinsic Fitness Affect the Female Rotator Cuff Tendon Tissue. Biomedicines 2022, 10, 509. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines10020509
Thierbach M, Heyne E, Schwarzer M, Koch LG, Britton SL, Wildemann B. Age and Intrinsic Fitness Affect the Female Rotator Cuff Tendon Tissue. Biomedicines. 2022; 10(2):509. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines10020509
Chicago/Turabian StyleThierbach, Manuela, Estelle Heyne, Michael Schwarzer, Lauren G. Koch, Steven L. Britton, and Britt Wildemann. 2022. "Age and Intrinsic Fitness Affect the Female Rotator Cuff Tendon Tissue" Biomedicines 10, no. 2: 509. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines10020509