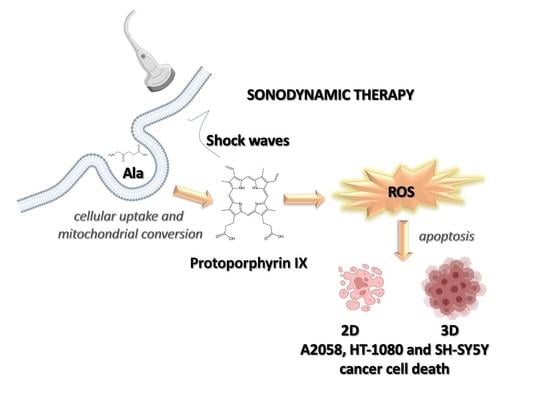
Exploiting Shock Waves to Trigger the Anticancer Sonodynamic Activity of 5-Aminolevulinc Acid-Derived Protoporphyrin IX on In Vitro 2D and 3D Cancer Models
Abstract
:1. Introduction
2. Materials and Methods
2.1. 2D and 3D Culture of Cell Lines
2.2. Glutathione Intracellular Concentration
2.3. Evaluation of Intracellular Ala-Derived PPIX
2.4. In Vitro Sonodynamic and Photodynamic Treatment
2.5. Cell Proliferation Assay
2.6. Flow Cytometric Analyses
2.7. Cytochrome c Analysis
2.8. Evaluation of DNA Damage
2.9. Real-Time RT-PCR
3. Results
3.1. Determination of Glutathione Intracellular Levels in A2058, HT-1080 and SH-SY5 Y Cells
3.2. Evaluation of Ala-Derived PPIX Intracellular Accumulation in A2058, HT-1080 and SH-SY5 Y Cells
3.3. Effect of SW-Mediated SDT and PDT on Cell Proliferation
3.4. Evaluation of Cell Death after SW-Mediated SDT and PDT
3.5. ROS Production after SW-Mediated SDT and PDT
3.6. Evaluation of Gene Expression and Cytochrome c Production after SW-Mediated SDT and PDT
3.7. Evaluation of SW-Mediated SDT Effect on DNA Damage
3.8. SW-Mediated SDT Effect on Spheroid Growth
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Choi, V.; Rajora, M.A.; Zheng, G. Activating Drugs with Sound: Mechanisms Behind Sonodynamic Therapy and the Role of Nanomedicine. Bioconjug. Chem. 2020, 31, 967–989. [Google Scholar] [CrossRef] [PubMed]
- Lafond, M.; Yoshizawa, S.; Umemura, S.-I. Sonodynamic Therapy: Advances and Challenges in Clinical Translation. J. Ultrasound Med. 2018, 38, 567–580. [Google Scholar] [CrossRef] [PubMed]
- Shibaguchi, H.; Tsuru, H.; Kuroki, M.; Kuroki, M. Sonodynamic cancer therapy: A non-invasive and repeatable approach using low-intensity ultrasound with a sonosensitizer. Anticancer Res. 2011, 31, 2425–2429. [Google Scholar] [PubMed]
- Villeneuve, L.; Alberti, L.; Steghens, J.-P.; Lancelin, J.-M.; Mestas, J.-L. Assay of hydroxyl radicals generated by focused ultrasound. Ultrason. Sonochem. 2009, 16, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Gielen, B.; Jordens, J.; Janssen, J.; Pfeiffer, H.; Wevers, M.; Thomassen, L.; Braeken, L.; Van Gerven, T. Characterization of stable and transient cavitation bubbles in a milliflow reactor using a multibubble sonoluminescence quenching technique. Ultrason. Sonochem. 2014, 25, 31–39. [Google Scholar] [CrossRef]
- Serpe, L.; Foglietta, F.; Canaparo, R. Nanosonotechnology: The next challenge in cancer sonodynamic therapy. Nanotechnol. Rev. 2012, 1, 173–182. [Google Scholar] [CrossRef]
- Wan, G.-Y.; Liu, Y.; Chen, B.-W.; Liu, Y.-Y.; Wang, Y.; Zhang, N. Recent advances of sonodynamic therapy in cancer treatment. Cancer Biol. Med. 2016, 13, 325–338. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Xu, H.; Shen, J.; Guo, S.; Shi, S.; Dan, J.; Tian, F.; Tian, Y.; Tian, Y. Real-time detection of intracellular reactive oxygen species and mitochondrial membrane potential in THP-1 macrophages during ultrasonic irradiation for optimal sonodynamic therapy. Ultrason. Sonochem. 2015, 22, 7–14. [Google Scholar] [CrossRef]
- Giuntini, F.; Foglietta, F.; Marucco, A.M.; Troia, A.; Dezhkunov, N.V.; Pozzoli, A.; Durando, G.; Fenoglio, I.; Serpe, L.; Canaparo, R. Insight into ultrasound-mediated reactive oxygen species generation by various metal-porphyrin complexes. Free Radic. Biol. Med. 2018, 121, 190–201. [Google Scholar] [CrossRef]
- Foglietta, F.; Canaparo, R.; Francovich, A.; Arena, F.; Civera, S.; Cravotto, G.; Frairia, R.; Serpe, L. Sonodynamic treatment as an innovative bimodal anticancer approach: Shock wave-mediated tumor growth inhibition in a syngeneic breast cancer model. Discov. Med. 2015, 20, 197–205. [Google Scholar]
- Maruyama, M.; Asano, T.; Uematsu, T.; Nakagohri, T.; Hasegawa, M.; Miyauchi, H.; Iwashita, C.; Isono, K. Enhancement of the Antitumor Effect by Combined Use of High-energy Shock Waves and ATX-70. Jpn. J. Cancer Res. 1995, 86, 800–801. [Google Scholar] [CrossRef] [PubMed]
- Wess, O.J. Physics and Technique of Shock Wave Lithotripsy (SWL). In Urolithiasis; Talati, J., Tiselius, H.G., Albala, D., Ye, Z., Eds.; Springer: London, UK, 2012. [Google Scholar]
- Serpe, L.; Canaparo, R.; Varchi, G.; Ballestri, M.; Foglietta, F.F.; Sotgiu, G.; Guerrini, A.; Francovich, A.; Civera, P.; Frairia, R. Polymeric nanoparticles enhance the sonodynamic activity of meso-tetrakis (4-sulfonatophenyl) porphyrin in an in vitro neuroblastoma model. Int. J. Nanomed. 2013, 8, 4247–4263. [Google Scholar] [CrossRef] [PubMed]
- Serpe, L.; Canaparo, R.; Berta, L.; Bargoni, A.; Zara, G.P.; Frairia, R. High Energy Shock Waves and 5-Aminolevulinic for Sonodynamic Therapy: Effects in a Syngeneic Model of Colon Cancer. Technol. Cancer Res. Treat. 2011, 10, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Catalano, M.G.; Costantino, L.; Fortunati, N.; Bosco, O.; Pugliese, M.; Boccuzzi, G.; Berta, L.; Frairia, R. High Energy Shock Waves Activate 5′-Aminolevulinic Acid and Increase Permeability to Paclitaxel: Antitumor Effects of a New Combined Treatment on Anaplastic Thyroid Cancer Cells. Thyroid 2007, 17, 91–99. [Google Scholar] [CrossRef]
- Kapałczyńska, M.; Kolenda, T.; Przybyła, W.; Zajączkowska, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Bliźniak, R.; Łuczewski, L.; Lamperska, K. 2D and 3D cell cultures—A comparison of different types of cancer cell cultures. Arch. Med. Sci. 2018, 14, 910–919. [Google Scholar] [CrossRef]
- Pape, J.; Emberton, M.; Cheema, U. 3D Cancer Models: The Need for a Complex Stroma, Compartmentalization and Stiffness. Front. Bioeng. Biotechnol. 2021, 9, 660502. [Google Scholar] [CrossRef]
- Foglietta, F.; Canaparo, R.; Muccioli, G.; Terreno, E.; Serpe, L. Methodological aspects and pharmacological applications of three-dimensional cancer cell cultures and organoids. Life Sci. 2020, 254, 117784. [Google Scholar] [CrossRef]
- Qin, S.; Jiang, J.; Lu, Y.; Nice, E.C.; Huang, C.; Zhang, J.; He, W. Emerging role of tumor cell plasticity in modifying therapeutic response. Signal Transduct. Target. Ther. 2020, 5, 228. [Google Scholar] [CrossRef]
- Wang, X.; Fang, H.; Huang, Z.; Shang, W.; Hou, T.; Cheng, A.; Cheng, H. Imaging ROS signaling in cells and animals. Klin. Wochenschr. 2013, 91, 917–927. [Google Scholar] [CrossRef] [Green Version]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [Green Version]
- Rosenthal, I.; Sostaric, J.Z.; Riesz, P. Sonodynamic therapy––a review of the synergistic effects of drugs and ultrasound. Ultrason. Sonochem. 2004, 11, 349–363. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Li, T.; Zhu, Y.; Hao, Y. Ultrasound-Activated Oxygen and ROS Generation Nanosystem Systematically Modulates Tumor Microenvironment and Sensitizes Sonodynamic Therapy for Hypoxic Solid Tumors. Adv. Funct. Mater. 2019, 29, 1906195. [Google Scholar] [CrossRef]
- Jin, Y.; Zhou, Q.; Geng, J.; Meng, Q.; Wei, Z.; Ding, M.; Zhou, J.; Zeng, Y.; Cao, W.; Liu, F.; et al. Sonodynamic Effects of a Novel Ether-Group Modified Porphyrin Derivative Combined with Pulsed Low-Intensity Ultrasound on PC-9 Cells. Front. Pharmacol. 2021, 12, 792360. [Google Scholar] [CrossRef] [PubMed]
- Castellano, A.G.; Díaz-Quintana, A.; Pérez-Mejías, G.; Elena-Real, C.A.; González-Arzola, K.; García-Mauriño, S.M.; De la Rosa, M.A.; Díaz-Moreno, I. Oxidative stress is tightly regulated by cytochrome c phosphorylation and respirasome factors in mitochondria. Proc. Natl. Acad. Sci. USA 2018, 115, 7955–7960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, X.; Wang, X. Cytochrome C-Mediated Apoptosis. Annu. Rev. Biochem. 2004, 73, 87–106. [Google Scholar] [CrossRef]
- Garrido, C.; Galluzzi, L.; Brunet, M.; Puig, P.E.; Didelot, C.; Kroemer, G. Mechanisms of cytochrome c release from mitochondria. Cell Death Differ. 2006, 13, 1423–1433. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Wang, H.; Zhang, K.; Liu, J.; Wang, P.; Wang, X.; Liu, Q. Correction: Sonodynamic therapy induces oxidative stress, DNA damage and apoptosis in glioma cells. RSC Adv. 2021, 11, 19569. [Google Scholar] [CrossRef]
- Noodt, B.B.; Berg, K.S.; Stokke, T.; Peng, Q.; Nesland, J.M. Apoptosis and necrosis induced with light and 5-aminolaevulinic acid-derived protoporphyrin IX. Br. J. Cancer 1996, 74, 22–29. [Google Scholar] [CrossRef] [Green Version]
- Cheing, G.L.Y.; Chang, H. Extracorporeal Shock Wave Therapy. J. Orthop. Sports Phys. Ther. 2003, 33, 337–343. [Google Scholar] [CrossRef] [Green Version]
- Kato, K.; Fujimura, M.; Nakagawa, A.; Saito, A.; Ohki, T.; Takayama, K.; Tominaga, T. Pressure-dependent effect of shock waves on rat brain: Induction of neuronal apoptosis mediated by a caspase-dependent pathway. J. Neurosurg. 2007, 106, 667–676. [Google Scholar] [CrossRef] [Green Version]
- Baghban, R.; Roshangar, L.; Jahanban-Esfahlan, R.; Seidi, K.; Ebrahimi-Kalan, A.; Jaymand, M.; Kolahian, S.; Javaheri, T.; Zare, P. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun. Signal. 2020, 18, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krieg, R.C.; Messmann, H.; Rauch, J.; Seeger, S.; Knuechel, R. Metabolic characterization of tumor cell-specific proto-porphyrin IX accumulation after exposure to 5-aminolevulinic acid in human colonic cells. Photochem. Photobiol. 2002, 76, 518–525. [Google Scholar] [CrossRef]
- Gibson, S.L.; Nguyen, M.L.; Havens, J.J.; Barbarin, A.; Hilf, R. Relationship of δ-Aminolevulinic Acid-Induced Protoporphyrin IX Levels to Mitochondrial Content in Neoplastic Cells in Vitro. Biochem. Biophys. Res. Commun. 1999, 265, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Wyld, L.; Burn, J.L.; Reed, M.; Brown, N. Factors affecting aminolaevulinic acid-induced generation of protoporphyrin IX. Br. J. Cancer 1997, 76, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, S.L.; Chen, B.; O’Hara, J.A.; Hoopes, P.J.; Hasan, T.; Pogue, B.W. Protoporphyrin IX Level Correlates with Number of Mitochondria, But Increase in Production Correlates with Tumor Cell Size. Photochem. Photobiol. 2006, 82, 1334–1341. [Google Scholar] [CrossRef]
- Moan, J.; Berg, K. The photodegradation of porphyrins in cells can be used to estimate the lifetime of singlet oxygen. Photochem. Photobiol. 1991, 53, 549–553. [Google Scholar] [CrossRef]
- Moor, A.C. Signaling pathways in cell death and survival after photodynamic therapy. J. Photochem. Photobiol. B Biol. 2000, 57, 1–13. [Google Scholar] [CrossRef]
- Beguin, E.; Shrivastava, S.; Dezhkunov, N.V.; McHale, A.P.; Callan, J.F.; Stride, E. Direct Evidence of Multibubble Sonoluminescence Using Therapeutic Ultrasound and Microbubbles. ACS Appl. Mater. Interfaces 2019, 11, 19913–19919. [Google Scholar] [CrossRef] [Green Version]
- Costley, D.; Mc Ewan, C.; Fowley, C.; McHale, A.P.; Atchison, J.; Nomikou, N.; Callan, J.F. Treating cancer with sonodynamic therapy: A review. Int. J. Hyperth. 2015, 31, 107–117. [Google Scholar] [CrossRef]
- Shen, Y.; Ou, J.; Chen, X.; Zeng, X.; Huang, L.; Pi, Z.; Hu, Y.; Chen, S.; Chen, T. An in vitro study on sonodynamic treatment of human colon cancer cells using sinoporphyrin sodium as sonosensitizer. Biomed. Eng. Online 2020, 19, 1–15. [Google Scholar] [CrossRef]
- Zong, W.-X.; Thompson, C.B. Necrotic death as a cell fate. Genes Dev. 2006, 20, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coupienne, I.; Fettweis, G.; Rubio, N.; Agostinis, P.; Piette, J. 5-ALA-PDT induces RIP3-dependent necrosis in glioblastoma. Photochem. Photobiol. Sci. 2011, 10, 1868–1878. [Google Scholar] [CrossRef] [PubMed]
- Rengeng, L.; Qianyu, Z.; Yuehong, L.; Zhongzhong, P.; Libo, L. Sonodynamic therapy, a treatment developing from photodynamic therapy. Photodiagnosis Photodyn. Ther. 2017, 19, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Donohoe, C.; Senge, M.O.; Arnaut, L.; da Silva, L.C.G. Cell death in photodynamic therapy: From oxidative stress to anti-tumor immunity. Biochim. Biophys. Acta Rev Cancer 2019, 1872, 188308. [Google Scholar] [CrossRef]
- Yang, L.; Li, X.; Gao, L.; Zheng, L.; Kou, J.; Zhu, X.; Jiang, Y.; Zhong, Z.; Dan, J.; Xu, H.; et al. The efficacy and mechanism of apoptosis induction by hypericin-mediated sonodynamic therapy in THP-1 macrophages. Int. J. Nanomed. 2015, 10, 821–838. [Google Scholar] [CrossRef] [Green Version]
- Rimann, M.; Graf-Hausner, U. Synthetic 3D multicellular systems for drug development. Curr. Opin. Biotechnol. 2012, 23, 803–809. [Google Scholar] [CrossRef]
- Griffith, L.G.; Swartz, M.A. Capturing complex 3D tissue physiology in vitro. Nat. Rev. Mol. Cell Biol. 2006, 7, 211–224. [Google Scholar] [CrossRef]
- Logan, K.; Foglietta, F.; Nesbitt, H.; Sheng, Y.; McKaig, T.; Kamila, S.; Gao, J.; Nomikou, N.; Callan, B.; McHale, A.P.; et al. Targeted chemo-sonodynamic therapy treatment of breast tumours using ultrasound responsive microbubbles loaded with paclitaxel, doxorubicin and Rose Bengal. Eur. J. Pharm. Biopharm. 2019, 139, 224–231. [Google Scholar] [CrossRef]








| A2058–Ala [mM] | PPIX at 12 h | |
| Cell Population (%) | iMFI Ratio | |
| 0.45 | 90.89 ± 0.16 | 57.71 ± 0.77 |
| 1.5 | 90.73 ± 1.03 | 57.39 ± 1.96 |
| 3.0 | 89.55 ± 0.64 | 45.92 ± 1.29 |
| HT-1080–Ala [mM] | PPIX at 4 h | |
| Cell Population (%) | iMFI ratio | |
| 0.45 | 85.43 ± 0.80 | 37.20 ± 1.13 |
| 1.5 | 84.37 ± 0.90 | 35.99 ± 0.72 |
| 3.0 | 83.54 ± 0.76 | 37.02 ± 1.44 |
| SH-SY5 Y–Ala [mM] | PPIX at 24 h | |
| Cell Population (%) | iMFI ratio | |
| 0.45 | 54.36 ± 0.19 | 8.28 ± 1.29 |
| 0.9 | 69.73 ± 0.99 | 38.77 ± 1.78 |
| 1.8 | 76.23 ± 0.92 | 53.89 ± 1.30 |
| 3.6 | 49.38 ± 0.16 | 10.90 ± 0.83 |
| A2058 Cells | |||
| Cell Treatment 48 h | Viable Cells (%) | Apoptotic Cells (%) | Necrotic Cells (%) |
| Untreated cells | 81.62 ± 0.54 | 8.66 ± 0.48 | 9.94 ± 0.09 |
| SW 1 | 81.73 ± 0.38 | 9.58 ± 0.60 | 8.55 ± 0.64 |
| LB | 80.26 ± 1.05 | 6.80 ± 1.00 | 14.59 ± 0.60 |
| Ala | 72.27 ± 0.38 | 10.91 ± 0.13 | 16.02 ± 0.02 |
| Ala + SW 1 | 74.58 ± 0.81 | 17.51 ± 1.21 * | 8.60 ± 0.57 |
| Ala + LB | 26.58 ± 0.59 *** | 7.56 ± 0.62 | 65.88 ± 0.18 *** |
| HT-1080 cells | |||
| Cell treatment 24 h | Viable cells (%) | Apoptotic cells (%) | Necrotic cells (%) |
| Untreated cells | 79.18 ± 1.17 | 6.76 ± 0.35 | 15.19 ± 1.15 |
| SW 2 | 74.44 ± 2.03 | 10.93 ± 0.11 | 13.13 ± 1.24 |
| LB | 77.22 ± 1.11 | 15.03 ± 0.67 * | 9.11 ± 0.55 |
| Ala | 81.89 ± 1.25 | 7.11 ± 0.16 | 8.79 ± 0.41 * |
| Ala + SW 2 | 65.90 ± 1.27 * | 21.46 ± 0.76 *** | 11.19 ± 0.26 |
| Ala + LB | 60.04 ± 1.36 * | 10.18 ± 1.17 | 27.94 ± 0.73 ** |
| SH-SY5 Y cells | |||
| Cell treatment 24 h | Viable cells (%) | Apoptotic cells (%) | Necrotic cells (%) |
| Untreated cells | 87.14 ± 1.22 | 7.89 ± 0.16 | 5.89 ± 0.16 |
| SW 2 | 85.31 ± 0.98 | 9.63 ± 0. 53 | 6.25 ± 0.36 |
| LB | 84.84 ± 1.18 | 7.12 ± 0.16 | 6.95 ± 0.08 |
| Ala | 89.22 ± 1.10 | 6.42 ± 0.59 | 5.04 ± 0.66 |
| Ala + SW 2 | 72.99 ± 1.40 | 15.97 ± 1.36 ** | 8.96 ± 0.06 |
| Ala + LB | 53.84 ± 0.23 *** | 10.69 ± 0.98 | 34.31 ± 0.43 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foglietta, F.; Panzanelli, P.; Serpe, L.; Canaparo, R. Exploiting Shock Waves to Trigger the Anticancer Sonodynamic Activity of 5-Aminolevulinc Acid-Derived Protoporphyrin IX on In Vitro 2D and 3D Cancer Models. Biomedicines 2022, 10, 615. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines10030615
Foglietta F, Panzanelli P, Serpe L, Canaparo R. Exploiting Shock Waves to Trigger the Anticancer Sonodynamic Activity of 5-Aminolevulinc Acid-Derived Protoporphyrin IX on In Vitro 2D and 3D Cancer Models. Biomedicines. 2022; 10(3):615. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines10030615
Chicago/Turabian StyleFoglietta, Federica, Patrizia Panzanelli, Loredana Serpe, and Roberto Canaparo. 2022. "Exploiting Shock Waves to Trigger the Anticancer Sonodynamic Activity of 5-Aminolevulinc Acid-Derived Protoporphyrin IX on In Vitro 2D and 3D Cancer Models" Biomedicines 10, no. 3: 615. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines10030615