Recent Advances in Non-Conventional Antimicrobial Approaches for Chronic Wound Biofilms: Have We Found the ‘Chink in the Armor’?
Abstract
:1. Introduction
1.1. Chronic Wounds and Biofilms
1.2. The Chronic Wound Biofilm Microenvironment
1.3. Status Quorum in the Treatment of Chronic Wound Biofilms
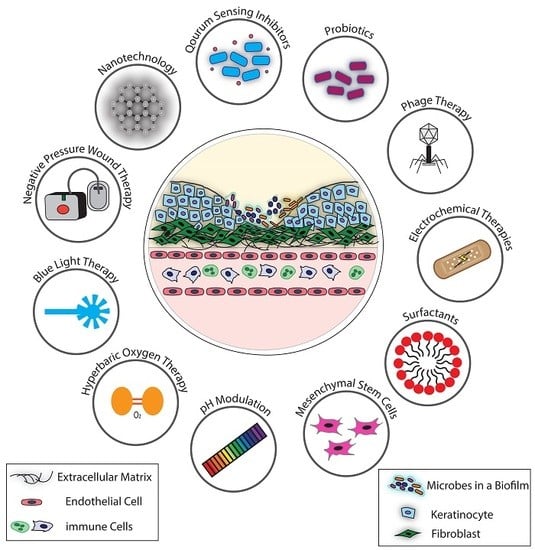
2. Recent Advances in Non-Conventional Antimicrobial Approaches for Chronic Wound Biofilms
2.1. Antimicrobial Therapies that Directly Target Microbial Processes
2.1.1. Phage Therapy
2.1.2. Nano-Based Technologies
2.1.3. Blue Light Therapy
2.1.4. Quorum Sensing Inhibitors
2.2. Antimicrobial Therapies that Target the Chronic Wound Biofilm Microenvironment, Indirectly Affecting Microbial Growth and Survival
2.2.1. pH Modulation
2.2.2. Negative Pressure Wound Therapy
2.2.3. Hyperbaric Oxygen Therapy
2.2.4. Surfactants
2.2.5. Electrical and Electrochemical Approaches
2.3. Antimicrobial Therapies that Target Bacteria and the Chronic Wound Biofilm Microenvironment, Both Directly and Indirectly Impacting Microbial Growth and Survival
2.3.1. Probiotics
2.3.2. Mesenchymal Stem Cells
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mustoe, T. Understanding chronic wounds: A unifying hypothesis on their pathogenesis and implications for therapy. Am. J. Surg. 2004, 187, S65–S70. [Google Scholar] [CrossRef]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef]
- Attinger, C.; Wolcott, R. Clinically Addressing Biofilm in Chronic Wounds. Adv. Wound Care 2012, 1, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, A.R.; Bernstein, J.M. Chronic wound infection: Facts and controversies. Clin. Dermatol. 2010, 28, 519–526. [Google Scholar] [CrossRef]
- MacLeod, A.S.; Mansbridge, J.N. The Innate Immune System in Acute and Chronic Wounds. Adv. Wound Care 2016, 5, 65–78. [Google Scholar] [CrossRef] [PubMed]
- James, G.A.; Swogger, E.; Wolcott, R.; Pulcini, E.d.L.; Secor, P.; Sestrich, J.; Costerton, J.W.; Stewart, P.S. Biofilms in chronic wounds. Wound Repair Regen. 2008, 16, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Malone, M.; Bjarnsholt, T.; McBain, A.J.; James, G.A.; Stoodley, P.; Leaper, D.; Tachi, M.; Schultz, G.; Swanson, T.; Wolcott, R.D. The prevalence of biofilms in chronic wounds: A systematic review and meta-analysis of published data. J. Wound Care 2017, 26, 20–25. [Google Scholar] [CrossRef]
- Bowler, P.G.; Duerden, B.I.; Armstrong, D.G. Wound microbiology and associated approaches to wound management. Clin. Microbiol. Rev. 2001, 14, 244–269. [Google Scholar] [CrossRef] [PubMed]
- Kalan, L.; Loesche, M.; Hodkinson, B.P.; Heilmann, K.; Ruthel, G.; Gardner, S.E.; Grice, E.A. Redefining the Chronic-Wound Microbiome: Fungal Communities Are Prevalent, Dynamic, and Associated with Delayed Healing. MBio 2016, 7, e01058-16. [Google Scholar] [CrossRef]
- Kalan, L.; Grice, E.A. Fungi in the Wound Microbiome. Adv. Wound Care 2018, 7, 247–255. [Google Scholar] [CrossRef]
- Zhao, G.; Hochwalt, P.C.; Usui, M.L.; Underwood, R.A.; Singh, P.K.; James, G.A.; Stewart, P.S.; Fleckman, P.; Olerud, J.E. Delayed wound healing in diabetic (db/db) mice with Pseudomonas aeruginosa biofilm challenge: A model for the study of chronic wounds. Wound Repair Regen. 2010, 18, 467–477. [Google Scholar] [CrossRef]
- Kirketerp-Møller, K.; Jensen, P.Ø.; Fazli, M.; Madsen, K.G.; Pedersen, J.; Moser, C.; Tolker-Nielsen, T.; Høiby, N.; Givskov, M.; Bjarnsholt, T. Distribution, organization, and ecology of bacteria in chronic wounds. J. Clin. Microbiol. 2008, 46, 2717–2722. [Google Scholar] [CrossRef]
- Metcalf, D.; Bowler, P. Biofilm delays wound healing: A review of the evidence. Burn. Trauma 2013, 1, 5. [Google Scholar] [CrossRef]
- Demidova-Rice, T.N.; Hamblin, M.R.; Herman, I.M. Acute and impaired wound healing: Pathophysiology and current methods for drug delivery, part 1: Normal and chronic wounds: biology, causes, and approaches to care. Adv. Skin Wound Care 2012, 25, 304–314. [Google Scholar] [CrossRef]
- Grice, E.A.; Segre, J.A. Interaction of the microbiome with the innate immune response in chronic wounds. Adv. Exp. Med. Biol. 2012, 946, 55–68. [Google Scholar] [CrossRef]
- Zhao, G.; Usui, M.L.; Lippman, S.I.; James, G.A.; Stewart, P.S.; Fleckman, P.; Olerud, J.E. Biofilms and Inflammation in Chronic Wounds. Adv. Wound Care 2013, 2, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Dhall, S.; Do, D.; Garcia, M.; Wijesinghe, D.S.; Brandon, A.; Kim, J.; Sanchez, A.; Lyubovitsky, J.; Gallagher, S.; Nothnagel, E.A.; et al. A Novel Model of Chronic Wounds: Importance of Redox Imbalance and Biofilm-Forming Bacteria for Establishment of Chronicity. PLoS ONE 2014, 9, e109848. [Google Scholar] [CrossRef]
- Vatansever, F.; de Melo, W.C.M.A.; Avci, P.; Vecchio, D.; Sadasivam, M.; Gupta, A.; Chandran, R.; Karimi, M.; Parizotto, N.A.; Yin, R.; et al. Antimicrobial strategies centered around reactive oxygen species—Bactericidal antibiotics, photodynamic therapy, and beyond. FEMS Microbiol. Rev. 2013, 37, 955–989. [Google Scholar] [CrossRef]
- André-Lévigne, D.; Modarressi, A.; Pepper, M.S.; Pittet-Cuénod, B. Reactive oxygen species and NOX enzymes are emerging as key players in cutaneous wound repair. Int. J. Mol. Sci. 2017, 18, 2149. [Google Scholar] [CrossRef]
- James, G.A.; Ge Zhao, A.; Usui, M.; Underwood, R.A.; Nguyen, H.; Beyenal, H.; deLancey Pulcini, E.; Agostinho Hunt, A.; Bernstein, H.C.; Fleckman, P.; et al. Microsensor and transcriptomic signatures of oxygen depletion in biofilms associated with chronic wounds. Wound Repair Regen. 2016, 24, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Castilla, D.M.; Liu, Z.-J.; Velazquez, O.C. Oxygen: Implications for Wound Healing. Adv. Wound Care 2012, 1, 225–230. [Google Scholar] [CrossRef]
- Mashruwala, A.A.; van de Guchte, A.; Boyd, J.M. Impaired respiration elicits SrrAB-dependent programmed cell lysis and biofilm formation in Staphylococcus aureus. eLife 2017, 6. [Google Scholar] [CrossRef]
- Hall, C.W.; Mah, T.-F. Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol. Rev. 2017, 41, 276–301. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.M.; Cochrane, C.A.; Percival, S.L. The Effect of pH on the Extracellular Matrix and Biofilms. Adv. Wound Care 2015, 4, 431–439. [Google Scholar] [CrossRef]
- Widgerow, A.D. Chronic wound fluid-thinking outside the box. Wound Repair Regen. 2011, 19, 287–291. [Google Scholar] [CrossRef]
- Wolcott, R.D.; Rhoads, D.D.; Dowd, S.E. Biofilms and chronic wound inflammation. J. Wound Care 2008, 17, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, R. The use of systemic antibiotics in the treatment of chronic wounds. Dermatol. Ther. 2006, 19, 326–337. [Google Scholar] [CrossRef]
- Lipsky, B.A.; Hoey, C. Topical Antimicrobial Therapy for Treating Chronic Wounds. Clin. Infect. Dis. 2009, 49, 1541–1549. [Google Scholar] [CrossRef] [PubMed]
- Omar, A.; Wright, J.; Schultz, G.; Burrell, R.; Nadworny, P. Microbial Biofilms and Chronic Wounds. Microorganisms 2017, 5, 9. [Google Scholar] [CrossRef]
- Høiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 2010, 35, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.S. Mechanisms of antibiotic resistance in bacterial biofilms. Int. J. Med. Microbiol. 2002, 292, 107–113. [Google Scholar] [CrossRef]
- Percival, S.L.; McCarty, S.; Hunt, J.A.; Woods, E.J. The effects of pH on wound healing, biofilms, and antimicrobial efficacy. Wound Repair Regen. 2014, 22, 174–186. [Google Scholar] [CrossRef]
- Gupta, S.; Laskar, N.; Kadouri, D.E. Evaluating the Effect of Oxygen Concentrations on Antibiotic Sensitivity, Growth, and Biofilm Formation of Human Pathogens. Microbiol. Insights 2016, 9, MBI.S40767. [Google Scholar] [CrossRef]
- Ansaldi, M. Cell biology perspectives in phage biology. Front. Biosci. (Elite Ed.) 2012, 4, 1823–1829. [Google Scholar] [CrossRef] [PubMed]
- Sulakvelidze, A.; Alavidze, Z.; Morris, J.G. Bacteriophage therapy. Antimicrob. Agents Chemother. 2001, 45, 649–659. [Google Scholar] [CrossRef]
- Alisky, J.; Iczkowski, K.; Rapoport, A.; Troitsky, N. Bacteriophages show promise as antimicrobial agents. J. Infect. 1998, 36, 5–15. [Google Scholar] [CrossRef]
- Pires, D.P.; Melo, L.D.R.; Vilas Boas, D.; Sillankorva, S.; Azeredo, J. Phage therapy as an alternative or complementary strategy to prevent and control biofilm-related infections. Curr. Opin. Microbiol. 2017, 39, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.M. Preventing biofilms of clinically relevant organisms using bacteriophage. Trends Microbiol. 2009, 17, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Rose, T.; Verbeken, G.; De Vos, D.; Merabishvili, M.; Vaneechoutte, M.; Lavigne, R.; Jennes, S.; Zizi, M.; Pirnay, J.-P. Experimental phage therapy of burn wound infection: Difficult first steps. Int. J. Burns Trauma 2014, 4, 66–73. [Google Scholar]
- Kumari, S.; Harjai, K.; Chhibber, S. Bacteriophage versus antimicrobial agents for the treatment of murine burn wound infection caused by Klebsiella pneumoniae B5055. J. Med. Microbiol. 2011, 60, 205–210. [Google Scholar] [CrossRef]
- Jault, P.; Leclerc, T.; Jennes, S.; Pirnay, J.P.; Que, Y.-A.; Resch, G.; Rousseau, A.F.; Ravat, F.; Carsin, H.; Le Floch, R.; et al. Efficacy and tolerability of a cocktail of bacteriophages to treat burn wounds infected by Pseudomonas aeruginosa (PhagoBurn): A randomised, controlled, double-blind phase 1/2 trial. Lancet Infect. Dis. 2019, 19, 35–45. [Google Scholar] [CrossRef]
- Merabishvili, M.; Monserez, R.; Van Belleghem, J.; Rose, T.; Jennes, S.; De Vos, D.; Verbeken, G.; Vaneechoutte, M.; Pirnay, J.P. Stability of bacteriophages in burn wound care products. PLoS ONE 2017, 12, e0182121. [Google Scholar] [CrossRef]
- Furfaro, L.L.; Payne, M.S.; Chang, B.J. Bacteriophage Therapy: Clinical Trials and Regulatory Hurdles. Front. Cell. Infect. Microbiol. 2017, 12, e0182121. [Google Scholar] [CrossRef] [PubMed]
- Morozova, V.V.; Vlassov, V.V.; Tikunova, N.V. Applications of Bacteriophages in the Treatment of Localized Infections in Humans. Front. Microbiol. 2018, 9, 1696. [Google Scholar] [CrossRef] [PubMed]
- Seth, A.K.; Geringer, M.R.; Nguyen, K.T.; Agnew, S.P.; Dumanian, Z.; Galiano, R.D.; Leung, K.P.; Mustoe, T.A.; Hong, S.J. Bacteriophage therapy for staphylococcus aureus biofilm-infected wounds: A new approach to chronic wound care. Plast. Reconstr. Surg. 2013, 131, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Bertozzi Silva, J.; Storms, Z.; Sauvageau, D. Host receptors for bacteriophage adsorption. FEMS Microbiol. Lett. 2016, 363. [Google Scholar] [CrossRef] [PubMed]
- Moye, Z.; Woolston, J.; Sulakvelidze, A.; Moye, Z.D.; Woolston, J.; Sulakvelidze, A. Bacteriophage Applications for Food Production and Processing. Viruses 2018, 10, 205. [Google Scholar] [CrossRef]
- Harper, D.; Parracho, H.; Walker, J.; Sharp, R.; Hughes, G.; Werthén, M.; Lehman, S.; Morales, S.; Harper, D.R.; Parracho, H.M.R.T.; et al. Bacteriophages and Biofilms. Antibiotics 2014, 3, 270–284. [Google Scholar] [CrossRef]
- Chan, B.K.; Abedon, S.T. Bacteriophages and their enzymes in biofilm control. Curr. Pharm. Des. 2015, 21, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Alves, D.R.; Booth, S.P.; Scavone, P.; Schellenberger, P.; Salvage, J.; Dedi, C.; Thet, N.-T.; Jenkins, A.T.A.; Waters, R.; Ng, K.W.; et al. Development of a High-Throughput ex-Vivo Burn Wound Model Using Porcine Skin, and Its Application to Evaluate New Approaches to Control Wound Infection. Front. Cell. Infect. Microbiol. 2018, 8. [Google Scholar] [CrossRef]
- Mendes, J.J.; Leandro, C.; Mottola, C.; Barbosa, R.; Silva, F.A.; Oliveira, M.; Vilela, C.L.; Melo-Cristino, J.; Górski, A.; Pimentel, M.; et al. In vitro design of a novel lytic bacteriophage cocktail with therapeutic potential against organisms causing diabetic foot infections. J. Med. Microbiol. 2014, 63, 1055–1065. [Google Scholar] [CrossRef]
- Mendes, J.J.; Leandro, C.; Corte-Real, S.; Barbosa, R.; Cavaco-Silva, P.; Melo-Cristino, J.; Górski, A.; Garcia, M. Wound healing potential of topical bacteriophage therapy on diabetic cutaneous wounds. Wound Repair Regen. 2013, 21, 595–603. [Google Scholar] [CrossRef]
- Oliveira, A.; Sousa, J.C.; Silva, A.C.; Melo, L.D.R.; Sillankorva, S. Chestnut Honey and Bacteriophage Application to Control Pseudomonas aeruginosa and Escherichia coli Biofilms: Evaluation in an ex vivo Wound Model. Front. Microbiol. 2018, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Reindel, R.; Fiore, C.R. Phage Therapy: Considerations and Challenges for Development. Clin. Infect. Dis. 2017, 64, 1589–1590. [Google Scholar] [CrossRef]
- Negut, I.; Grumezescu, V.; Grumezescu, A.M. Treatment strategies for infected wounds. Molecules 2018, 23, 2392. [Google Scholar] [CrossRef] [PubMed]
- Hamdan, S.; Pastar, I.; Drakulich, S.; Dikici, E.; Tomic-Canic, M.; Deo, S.; Daunert, S. Nanotechnology-Driven Therapeutic Interventions in Wound Healing: Potential Uses and Applications. ACS Cent. Sci. 2017, 3, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Paladini, F.; Pollini, M.; Sannino, A.; Ambrosio, L. Progress and Perspectives in the Management of Wound Infections. In Wound Healing—New Insights into Ancient Challenges; IntechOpen: London, UK, 2016. [Google Scholar]
- Chaw, K.C.; Manimaran, M.; Tay, F.E.H. Role of silver ions in destabilization of intermolecular adhesion forces measured by atomic force microscopy in Staphylococcus epidermidis biofilms. Antimicrob. Agents Chemother. 2005, 49, 4853–4859. [Google Scholar] [CrossRef]
- Kostenko, V.; Lyczak, J.; Turner, K.; Martinuzzi, R.J. Impact of silver-containing wound dressings on bacterial biofilm viability and susceptibility to antibiotics during prolonged treatment. Antimicrob. Agents Chemother. 2010, 54, 5120–5131. [Google Scholar] [CrossRef] [PubMed]
- Kalishwaralal, K.; BarathManiKanth, S.; Pandian, S.R.K.; Deepak, V.; Gurunathan, S. Silver nanoparticles impede the biofilm formation by Pseudomonas aeruginosa and Staphylococcus epidermidis. Colloids Surfa. B Biointerfaces 2010, 79, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Habash, M.B.; Park, A.J.; Vis, E.C.; Harris, R.J.; Khursigara, C.M. Synergy of Silver nanoparticles and aztreonam against pseudomonas aeruginosa PAO1 Biofilms. Antimicrob. Agents Chemother. 2014, 58, 5818–5830. [Google Scholar] [CrossRef]
- Mekkawy, A.I.; El-Mokhtar, M.A.; Nafady, N.A.; Yousef, N.; Hamad, M.; El-Shanawany, S.M.; Ibrahim, E.H.; Elsabahy, M. In vitro and in vivo evaluation of biologically synthesized silver nanoparticles for topical applications: Effect of surface coating and loading into hydrogels. Int. J. Nanomed. 2017, 12, 759–777. [Google Scholar] [CrossRef]
- You, C.; Li, Q.; Wang, X.; Wu, P.; Ho, J.K.; Jin, R.; Zhang, L.; Shao, H.; Han, C. Silver nanoparticle loaded collagen/chitosan scaffolds promote wound healing via regulating fibroblast migration and macrophage activation. Sci. Rep. 2017, 7, 10489. [Google Scholar] [CrossRef] [PubMed]
- Daghdari, S.G.; Ahmadi, M.; Saei, H.D.; Tehrani, A.A. The effect of ZnO nanoparticles on bacterial load of experimental infectious wounds contaminated with Staphylococcus aureus in mice. Nanomed. J. 2017, 4, 232–236. [Google Scholar] [CrossRef]
- Babushkina, I.V.; Mamontova, I.A.; Gladkova, E.V. Metal Nanoparticles Reduce Bacterial Contamination of Experimental Purulent Wounds. Bull. Exp. Biol. Med. 2015, 158, 692–694. [Google Scholar] [CrossRef]
- Loh, J.V.; Percival, S.L.; Woods, E.J.; Williams, N.J.; Cochrane, C.A. Silver resistance in MRSA isolated from wound and nasal sources in humans and animals. Int. Wound J. 2009, 6, 32–38. [Google Scholar] [CrossRef]
- Panáček, A.; Kvítek, L.; Smékalová, M.; Večeřová, R.; Kolář, M.; Röderová, M.; Dyčka, F.; Šebela, M.; Prucek, R.; Tomanec, O.; et al. Bacterial resistance to silver nanoparticles and how to overcome it. Nat. Nanotechnol. 2018, 13, 65–71. [Google Scholar] [CrossRef]
- Park, H.; Park, H.J.; Kim, J.A.; Lee, S.H.; Kim, J.H.; Yoon, J.; Park, T.H. Inactivation of Pseudomonas aeruginosa PA01 biofilms by hyperthermia using superparamagnetic nanoparticles. J. Microbiol. Methods 2011, 84, 41–45. [Google Scholar] [CrossRef]
- Kim, M.H.; Yamayoshi, I.; Mathew, S.; Lin, H.; Nayfach, J.; Simon, S.I. Magnetic nanoparticle targeted hyperthermia of cutaneous staphylococcus aureus infection. Ann. Biomed. Eng. 2013, 41, 598–609. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, D.; Bañobre-López, M.; Espiña, B.; Rivas, J.; Azeredo, J. Effect of magnetic hyperthermia on the structure of biofilm and cellular viability of a food spoilage bacterium. Biofouling 2013, 29, 1225–1232. [Google Scholar] [CrossRef]
- Nguyen, T.K.; Duong, H.T.T.; Selvanayagam, R.; Boyer, C.; Barraud, N. Iron oxide nanoparticle-mediated hyperthermia stimulates dispersal in bacterial biofilms and enhances antibiotic efficacy. Sci. Rep. 2015, 5, 18385. [Google Scholar] [CrossRef]
- Abenojar, E.C.; Wickramasinghe, S.; Ju, M.; Uppaluri, S.; Klika, A.; George, J.; Barsoum, W.; Frangiamore, S.J.; Higuera-rueda, C.A.; Samia, A.C.S. Magnetic Glycol Chitin-Based Hydrogel Nanocomposite for Combined Thermal and d-Amino-Acid-Assisted Biofilm Disruption. ACS Infect. Dis. 2018, 4, 1246–1256. [Google Scholar] [CrossRef]
- Wang, Z.; Dong, K.; Liu, Z.; Zhang, Y.; Chen, Z.; Sun, H.; Ren, J.; Qu, X. Activation of biologically relevant levels of reactive oxygen species by Au/g-C3N4hybrid nanozyme for bacteria killing and wound disinfection. Biomaterials 2017, 113, 145–157. [Google Scholar] [CrossRef]
- Memar, M.Y.; Ghotaslou, R.; Samiei, M.; Adibkia, K. Antimicrobial use of reactive oxygen therapy: Current insights. Infect. Drug Resist. 2018, 11, 567–576. [Google Scholar] [CrossRef]
- Villa, F.; Villa, S.; Gelain, A.; Cappitelli, F. Sub-lethal Activity of Small Molecules from Natural Sources and their Synthetic Derivatives Against Biofilm Forming Nosocomial Pathogens. Curr. Top. Med. Chem. 2013, 13, 3184–3204. [Google Scholar] [CrossRef]
- Cattò, C.; Villa, F.; Cappitelli, F. Recent progress in bio-inspired biofilm-resistant polymeric surfaces. Crit. Rev. Microbiol. 2018, 44, 633–652. [Google Scholar] [CrossRef]
- Panwar, R.; Pemmaraju, S.C.; Sharma, A.K.; Pruthi, V. Efficacy of ferulic acid encapsulated chitosan nanoparticles against Candida albicans biofilm. Microb. Pathog. 2016, 95, 21–31. [Google Scholar] [CrossRef]
- Alavarce, R.A.S.; Saldanha, L.L.; Almeida, N.L.M.; Porto, V.C.; Dokkedal, A.L.; Lara, V.S. The Beneficial Effect of Equisetum giganteum L. against Candida Biofilm Formation: New Approaches to Denture Stomatitis. Evidence-Based Complement. Altern. Med. 2015, 2015, 1–9. [Google Scholar] [CrossRef]
- Adamskaya, N.; Dungel, P.; Mittermayr, R.; Hartinger, J.; Feichtinger, G.; Wassermann, K.; Redl, H.; van Griensven, M. Light therapy by blue LED improves wound healing in an excision model in rats. Injury 2011, 42, 917–921. [Google Scholar] [CrossRef]
- Chaves, M.E.d.A.; Araújo, A.R.d.; Piancastelli, A.C.C.; Pinotti, M. Effects of low-power light therapy on wound healing: LASER x LED. An. Bras. Dermatol. 2014, 89, 616–623. [Google Scholar] [CrossRef]
- Percival, S.L.; Francolini, I.; Donelli, G. Low-level laser therapy as an antimicrobial and antibiofilm technology and its relevance to wound healing. Future Microbiol. 2015, 10, 255–272. [Google Scholar] [CrossRef]
- Enwemeka, C.S. Antimicrobial Blue Light: An Emerging Alternative to Antibiotics. Photomed. Laser Surg. 2013, 31, 509–511. [Google Scholar] [CrossRef]
- Hughes, G.; Webber, M.A. Novel approaches to the treatment of bacterial biofilm infections. Br. J. Pharmacol. 2017, 174, 2237–2246. [Google Scholar] [CrossRef]
- Halstead, F.D.; Thwaite, J.E.; Burt, R.; Laws, T.R.; Raguse, M.; Moeller, R.; Webber, M.A.; Oppenheim, B.A.; Besser, T.E. Antibacterial Activity of Blue Light against Nosocomial Wound Pathogens Growing Planktonically and as Mature Biofilms. Appl. Environ. Microbiol. 2016, 82, 4006–4016. [Google Scholar] [CrossRef]
- Hu, X.; Huang, Y.-Y.; Wang, Y.; Wang, X.; Hamblin, M.R. Antimicrobial Photodynamic Therapy to Control Clinically Relevant Biofilm Infections. Front. Microbiol. 2018, 9, 1299. [Google Scholar] [CrossRef] [PubMed]
- Marinic, K.; Manoil, D.; Filieri, A.; Wataha, J.C.; Schrenzel, J.; Lange, N.; Bouillaguet, S. Repeated exposures to blue light-activated eosin Y enhance inactivation of E. faecalis biofilms, in vitro. Photodiagnosis Photodyn. Ther. 2015, 12, 393–400. [Google Scholar] [CrossRef] [PubMed]
- De Melo, W.C.M.A.; Avci, P.; De Oliveira, M.N.; Gupta, A.; Vecchio, D.; Sadasivam, M.; Chandran, R.; Huang, Y.Y.; Yin, R.; Perussi, L.R.; et al. Photodynamic inactivation of biofilm: Taking a lightly colored approach to stubborn infection. Expert Rev. Anti. Infect. Ther. 2013, 11, 669–693. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yang, Z.; Peng, Y.; Guo, Y.; Yao, M.; Dong, J. Application of 460 nm visible light for the elimination of Candida albicans in vitro and in vivo. Mol. Med. Rep. 2018, 18, 2017–2026. [Google Scholar] [CrossRef]
- Dai, T.; Gupta, A.; Huang, Y.-Y.; Yin, R.; Murray, C.K.; Vrahas, M.S.; Sherwood, M.E.; Tegos, G.P.; Hamblin, M.R. Blue light rescues mice from potentially fatal Pseudomonas aeruginosa burn infection: Efficacy, safety, and mechanism of action. Antimicrob. Agents Chemother. 2013, 57, 1238–1245. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, X.; Chen, J.; Amin, R.; Lu, M.; Bhayana, B.; Zhao, J.; Murray, C.K.; Hamblin, M.R.; Hooper, D.C.; et al. Antimicrobial Blue Light Inactivation of Gram-Negative Pathogens in Biofilms: In Vitro and in Vivo Studies. J. Infect. Dis. 2016, 213, 1380–1387. [Google Scholar] [CrossRef] [PubMed]
- Amin, R.M.; Bhayana, B.; Hamblin, M.R.; Dai, T. Antimicrobial blue light inactivation of Pseudomonas aeruginosa by photo-excitation of endogenous porphyrins: In vitro and in vivo studies. Lasers Surg. Med. 2016. [Google Scholar] [CrossRef] [PubMed]
- Tomb, R.M.; Maclean, M.; Coia, J.E.; MacGregor, S.J.; Anderson, J.G. Assessment of the potential for resistance to antimicrobial violet-blue light in Staphylococcus aureus. Antimicrob. Resist. Infect. Control 2017, 6, 100. [Google Scholar] [CrossRef] [PubMed]
- Lins de Sousa, D.; Araújo Lima, R.; Zanin, I.C.; Klein, M.I.; Janal, M.N.; Duarte, S. Effect of Twice-Daily Blue Light Treatment on Matrix-Rich Biofilm Development. PLoS ONE 2015, 10, e0131941. [Google Scholar] [CrossRef]
- Parsek, M.R.; Greenberg, E.P. Sociomicrobiology: The connections between quorum sensing and biofilms. Trends Microbiol. 2005, 13, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Whiteley, M.; Diggle, S.P.; Greenberg, E.P. Progress in and promise of bacterial quorum sensing research. Nature 2017, 48, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Bjarnsholt, T.; Tolker-Nielsen, T.; Høiby, N.; Givskov, M. Interference of Pseudomonas aeruginosa signalling and biofilm formation for infection control. Expert Rev. Mol. Med. 2010, 12, e11. [Google Scholar] [CrossRef]
- Le, K.Y.; Otto, M. Quorum-sensing regulation in staphylococci-an overview. Front. Microbiol. 2015, 6, 1174. [Google Scholar] [CrossRef] [PubMed]
- Asfour, H.Z. Anti-Quorum Sensing Natural Compounds. J. Microsc. Ultrastruct. 2018, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Manner, S.; Fallarero, A. Screening of Natural Product Derivatives Identifies Two Structurally Related Flavonoids as Potent Quorum Sensing Inhibitors against Gram-Negative Bacteria. Int. J. Mol. Sci. 2018, 19, 1346. [Google Scholar] [CrossRef] [PubMed]
- Borlee, B.R.; Geske, G.D.; Blackwell, H.E.; Handelsman, J. Identification of synthetic inducers and inhibitors of the quorum-sensing regulator LasR in Pseudomonas aeruginosa by high-throughput screening. Appl. Environ. Microbiol. 2010, 76, 8255–8258. [Google Scholar] [CrossRef] [PubMed]
- Vermote, A.; Brackman, G.; Risseeuw, M.D.P.; Coenye, T.; Van Calenbergh, S. Novel hamamelitannin analogues for the treatment of biofilm related MRSA infections–A scaffold hopping approach. Eur. J. Med. Chem. 2017, 127, 757–770. [Google Scholar] [CrossRef] [PubMed]
- Brackman, G.; Coenye, T. Inhibition of Quorum Sensing in Staphylococcus spp. Curr. Pharm. Des. 2015, 21, 2101–2108. [Google Scholar] [CrossRef] [PubMed]
- Reuter, K.; Steinbach, A.; Helms, V. Interfering with bacterial quorum sensing. Perspect. Medicin. Chem. 2016, 8, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Rémy, B.; Mion, S.; Plener, L.; Elias, M.; Chabrière, E.; Daudé, D. Interference in Bacterial Quorum Sensing: A Biopharmaceutical Perspective. Front. Pharmacol. 2018, 9, 203. [Google Scholar] [CrossRef]
- Wang, H.; Chu, W.; Ye, C.; Gaeta, B.; Tao, H.; Wang, M.; Qiu, Z.; Wang, M. Chlorogenic acid attenuates virulence factors and pathogenicity of Pseudomonas aeruginosa by regulating quorum sensing. Appl. Microbiol. Biotechnol. 2018, 103, 903–915. [Google Scholar] [CrossRef]
- Dell’Acqua, G.; Giacometti, A.; Cirioni, O.; Ghiselli, R.; Saba, V.; Scalise, G.; Gov, Y.; Balaban, N. Suppression of Drug-Resistant Staphylococcal Infections by the Quorum-Sensing Inhibitor RNAIII-Inhibiting Peptide. J. Infect. Dis. 2004, 190, 318–320. [Google Scholar] [CrossRef]
- Gov, Y.; Bitler, A.; Dell’Acqua, G.; Torres, J.V.; Balaban, N. RNAIII inhibiting peptide (RIP), a global inhibitor of Staphylococcus aureus pathogenesis: Structure and function analysis. Peptides 2001, 22, 1609–1620. [Google Scholar] [CrossRef]
- Baldassarre, L.; Fornasari, E.; Cornacchia, C.; Cirioni, O.; Silvestri, C.; Castelli, P.; Giocometti, A.; Cacciatore, I. Discovery of novel RIP derivatives by alanine scanning for the treatment of S. aureus infections. Med. Chem. Commun. 2013, 4, 1114. [Google Scholar] [CrossRef]
- Simonetti, O.; Cirioni, O.; Cacciatore, I.; Baldassarre, L.; Orlando, F.; Pierpaoli, E.; Lucarini, G.; Orsetti, E.; Provinciali, M.; Fornasari, E.; et al. Efficacy of the Quorum Sensing Inhibitor FS10 Alone and in Combination with Tigecycline in an Animal Model of Staphylococcal Infected Wound. PLoS ONE 2016, 11, e0151956. [Google Scholar] [CrossRef]
- Brackman, G.; Cos, P.; Maes, L.; Nelis, H.J.; Coenye, T. Quorum Sensing Inhibitors Increase the Susceptibility of Bacterial Biofilms to Antibiotics In Vitro and In Vivo. Antimicrob. Agents Chemother. 2011, 55, 2655–2661. [Google Scholar] [CrossRef] [PubMed]
- Fong, J.; Mortensen, K.T.; Nørskov, A.; Qvortrup, K.; Yang, L.; Tan, C.H.; Nielsen, T.E.; Givskov, M. Itaconimides as Novel Quorum Sensing Inhibitors of Pseudomonas aeruginosa. Front. Cell. Infect. Microbiol. 2019, 8, 443. [Google Scholar] [CrossRef]
- Paes, C.; Nakagami, G.; Minematsu, T.; Nagase, T.; Huang, L.; Sari, Y.; Sanada, H. The Pseudomonas aeruginosa quorum sensing signal molecule N-(3-oxododecanoyl) homoserine lactone enhances keratinocyte migration and induces Mmp13 gene expression in vitro. Biochem. Biophys. Res. Commun. 2012, 427, 273–279. [Google Scholar] [CrossRef]
- Nakagami, G.; Minematsu, T.; Morohoshi, T.; Yamane, T.; Kanazawa, T.; Huang, L.; Asada, M.; Nagase, T.; Ikeda, S.I.; Ikeda, T.; et al. Pseudomonas aeruginosa quorum-sensing signaling molecule N-3-oxododecanoyl homoserine lactone induces matrix metalloproteinase 9 expression via the AP1 pathway in rat fibroblasts. Biosci. Biotechnol. Biochem. 2015, 79, 1–5. [Google Scholar] [CrossRef]
- Zhang, Y.; Sass, A.; Van Acker, H.; Wille, J.; Verhasselt, B.; Van Nieuwerburgh, F.; Kaever, V.; Crabbé, A.; Coenye, T. Coumarin reduces virulence and biofilm formation in Pseudomonas aeruginosa by affecting quorum sensing, type III secretion and C-di-GMP levels. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef]
- Gethin, G. The significance of surface pH in chronic wounds. Wounds UK 2007, 3, 52. [Google Scholar]
- Rumbaugh, K.; Watters, C.; Yuan, T. Beneficial and deleterious bacterial-host interactions in chronic wound pathophysiology. Chronic Wound Care Manag. Res. 2015, 2, 53. [Google Scholar] [CrossRef]
- Percival, S.L.; Hill, K.E.; Williams, D.W.; Hooper, S.J.; Thomas, D.W.; Costerton, J.W. A review of the scientific evidence for biofilms in wounds. Wound Repair Regen. 2012, 20, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Gethin, G.T.; Cowman, S.; Conroy, R.M. The impact of Manuka honey dressings on the surface pH of chronic wounds. Int. Wound J. 2008, 5, 185–194. [Google Scholar] [CrossRef]
- Madhusudhan, V.L. Efficacy of 1% acetic acid in the treatment of chronic wounds infected with Pseudomonas aeruginosa: Prospective randomised controlled clinical trial. Int. Wound J. 2016, 13, 1129–1136. [Google Scholar] [CrossRef]
- Agrawal, K.; Sarda, A.; Shrotriya, R.; Bachhav, M.; Puri, V.; Nataraj, G. Acetic acid dressings: Finding the Holy Grail for infected wound management. Indian J. Plast. Surg. 2017, 50, 273–280. [Google Scholar] [PubMed]
- Nagoba, B.S.; Selkar, S.P.; Wadher, B.J.; Gandhi, R.C. Acetic acid treatment of pseudomonal wound infections—A review. J. Infect. Public Health 2013, 6, 410–415. [Google Scholar] [CrossRef]
- Nagoba, B.S.; Suryawanshi, N.M.; Wadher, B.; Selkar, S. Acidic Environment and Wound Healing: A Review. Wounds 2015, 27, 5–11. [Google Scholar] [CrossRef]
- Kaushik, K.S.; Ratnayeke, N.; Katira, P.; Gordon, V.D. The spatial profiles and metabolic capabilities of microbial populations impact the growth of antibiotic-resistant mutants. J. R. Soc. Interface 2015, 12. [Google Scholar] [CrossRef] [PubMed]
- Sandoz, H. Negative pressure wound therapy: Clinical utility. Chronic Wound Care Manag. Res. 2015, 71. [Google Scholar] [CrossRef]
- Matiasek, J.; Assadian, O.; Domig, K.J.; Djedovic, G.; Babeluk, R. The spatial profiles and metabolic capabilities of microbial populations impact the growth of antibiotic-resistant mutants. J. Wound Care 2017. [Google Scholar] [CrossRef]
- Wang, G.; Li, Z.; Li, T.; Wang, S.; Zheng, L.; Zhang, L.; Tang, P. Negative-Pressure Wound Therapy in a Pseudomonas aeruginosa Infection Model. Biomed Res. Int. 2018, 2018, 1–11. [Google Scholar] [CrossRef]
- Ludolph, I.; Fried, F.W.; Kneppe, K.; Arkudas, A.; Schmitz, M.; Horch, R.E. Negative pressure wound treatment with computer-controlled irrigation/instillation decreases bacterial load in contaminated wounds and facilitates wound closure. Int. Wound J. 2018, 15, 978–984. [Google Scholar] [CrossRef]
- Bjarnsholt, T.; Alhede, M.; Jensen, P.Ø.; Nielsen, A.K.; Johansen, H.K.; Homøe, P.; Høiby, N.; Givskov, M.; Kirketerp-Møller, K. Antibiofilm Properties of Acetic Acid. Adv. Wound Care 2014. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.S.; Lee, B.H.; Lee, H.K.; Kim, H.S.; Moon, M.S.; Suh, I.S. Negative pressure wound therapy of chronically infected wounds using 1% acetic acid irrigation. Arch. Plast. Surg. 2015. [Google Scholar] [CrossRef]
- Goldstein, L.J. Hyperbaric oxygen for chronic wounds. Dermatol. Ther. 2013, 26, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Dissemond, J.; Kröger, K.; Storck, M.; Risse, A.; Engels, P. Topical oxygen wound therapies for chronic wounds: A review. J. Wound Care 2015, 24, 53–63. [Google Scholar] [CrossRef]
- Memar, M.Y.; Yekani, M.; Alizadeh, N.; Baghi, H.B. Hyperbaric oxygen therapy: Antimicrobial mechanisms and clinical application for infections. Biomed. Pharmacother. 2019, 109, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Lam, G.; Fontaine, R.; Ross, F.L.; Chiu, E.S. Hyperbaric Oxygen Therapy. Adv. Skin Wound Care 2017, 30, 181–190. [Google Scholar] [CrossRef]
- Sanford, N.E.; Wilkinson, J.E.; Nguyen, H.; Diaz, G.; Wolcott, R. Efficacy of hyperbaric oxygen therapy in bacterial biofilm eradication. J. Wound Care 2018, 27, S20–S28. [Google Scholar] [CrossRef]
- Kolpen, M.; Lerche, C.J.; Kragh, K.N.; Sams, T.; Koren, K.; Jensen, A.S.; Line, L.; Bjarnsholt, T.; Ciofu, O.; Moser, C.; et al. Hyperbaric Oxygen Sensitizes Anoxic Pseudomonas aeruginosa Biofilm to Ciprofloxacin. Antimicrob. Agents Chemother. 2017, 61, e01024-17. [Google Scholar] [CrossRef]
- Gade, P.A.V.; Olsen, T.B.; Jensen, P.Ø.; Kolpen, M.; Høiby, N.; Henneberg, K.-Å.; Sams, T. Modelling of ciprofloxacin killing enhanced by hyperbaric oxygen treatment in Pseudomonas aeruginosa PAO1 biofilms. PLoS ONE 2018, 13, e0198909. [Google Scholar] [CrossRef]
- Lerche, C.J.; Christophersen, L.J.; Kolpen, M.; Nielsen, P.R.; Trøstrup, H.; Thomsen, K.; Hyldegaard, O.; Bundgaard, H.; Jensen, P.Ø.; Høiby, N.; et al. Hyperbaric oxygen therapy augments tobramycin efficacy in experimental Staphylococcus aureus endocarditis. Int. J. Antimicrob. Agents 2017, 50, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, N.P.; Hansen, K.; Andreasen, C.M.; Pedersen, M.; Fuursted, K.; Meyer, R.L.; Petersen, E. Hyperbaric Oxygen Therapy is Ineffective as an Adjuvant to Daptomycin with Rifampicin Treatment in a Murine Model of Staphylococcus aureus in Implant-Associated Osteomyelitis. Microorganisms 2017, 5, 21. [Google Scholar] [CrossRef] [PubMed]
- Percival, S.L.; Mayer, D.; Malone, M.; Swanson, T.; Gibson, D.; Schultz, G. Surfactants and their role in wound cleansing and biofilm management. J. Wound Care 2017, 26, 680–690. [Google Scholar] [CrossRef]
- Das Ghatak, P.; Math, S.S.; Pandey, P.; Roy, S. OPEN A surfactant polymer dressing potentiates antimicrobial efficacy in biofilm disruption. Sci. Rep. 2018, 8, 873. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Larose, C.; Della Porta, A.C.; Schultz, G.S.; Gibson, D.J. A surfactant-based wound dressing can reduce bacterial biofilms in a porcine skin explant model. Int. Wound J. 2016, 14, 408–413. [Google Scholar] [CrossRef]
- Hunckler, J.; de Mel, A. A current affair: Electrotherapy in wound healing. J. Multidiscip. Healthc. 2017, 10, 179–194. [Google Scholar] [CrossRef]
- Thakral, G.; Lafontaine, J.; Najafi, B.; Talal, T.K.; Kim, P.; Lavery, L.A. Electrical stimulation to accelerate wound healing. Diabet. Foot Ankle 2013, 4, 660. [Google Scholar] [CrossRef]
- Banerjee, J.; Das Ghatak, P.; Roy, S.; Khanna, S.; Hemann, C. Silver-Zinc Redox-Coupled Electroceutical Wound Dressing Disrupts Bacterial Biofilm. PLoS ONE 2015, 10, e0119531. [Google Scholar] [CrossRef]
- Barki, K.G.; Das, A.; Dixith, S.; Ghatak, P.D.; Mathew-Steiner, S.; Schwab, E.; Khanna, S.; Wozniak, D.J.; Roy, S.; Sen, C.K. Electric Field Based Dressing Disrupts Mixed-Species Bacterial Biofilm Infection and Restores Functional Wound Healing. Ann. Surg. 2019, 269, 756–766. [Google Scholar] [CrossRef]
- Sultana, S.T.; Atci, E.; Babauta, J.T.; Falghoush, A.M.; Snekvik, K.R.; Call, D.R.; Beyenal, H. Electrochemical scaffold generates localized, low concentration of hydrogen peroxide that inhibits bacterial pathogens and biofilms. Nat. Publ. Gr. 2015, 5, 14908. [Google Scholar] [CrossRef]
- Sultana, S.T.; Call, D.R.; Beyenal, H. Maltodextrin enhances biofilm elimination by electrochemical scaffold. Nat. Publ. Gr. 2016, 6, 36003. [Google Scholar] [CrossRef]
- Markowiak, P.; Śliżewska, K.; Markowiak, P.; Śliżewska, K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef]
- Vuotto, C.; Longo, F.; Donelli, G. Probiotics to counteract biofilm-associated infections: Promising and conflicting data. Int. J. Oral Sci. 2014, 6, 189–194. [Google Scholar] [CrossRef]
- Scales, B.S.; Huffnagle, G.B. The microbiome in wound repair and tissue fibrosis. J. Pathol. 2013, 229, 323–331. [Google Scholar] [CrossRef]
- Wong, V.W.; Martindale, R.G.; Longaker, M.T.; Gurtner, G.C. From germ theory to germ therapy: Skin microbiota, chronic wounds, and probiotics. Plast. Reconstr. Surg. 2013, 132, 854–861. [Google Scholar] [CrossRef]
- Sikorska, H.; Smoragiewicz, W. Role of probiotics in the prevention and treatment of meticillin-resistant Staphylococcus aureus infections. Int. J. Antimicrob. Agents 2013, 42, 475–481. [Google Scholar] [CrossRef]
- Valdéz, J.C.; Peral, M.C.; Rachid, M.; Santana, M.; Perdigón, G. Interference of Lactobacillus plantarum with Pseudomonas aeruginosa in vitro and in infected burns: The potential use of probiotics in wound treatment. Clin. Microbiol. Infect. 2005, 11, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.N.; Sesto Cabral, M.E.; Noseda, D.; Bosch, A.; Yantorno, O.M.; Valdez, J.C. Antipathogenic properties of Lactobacillus plantarum on Pseudomonas aeruginosa: The potential use of its supernatants in the treatment of infected chronic wounds. Wound Repair Regen. 2012, 20, 552–562. [Google Scholar] [CrossRef]
- Ramos, A.N.; Sesto Cabral, M.E.; Arena, M.E.; Arrighi, C.F.; Arroyo Aguilar, A.A.; Valdéz, J.C. Compounds from Lactobacillus plantarum culture supernatants with potential pro-healing and anti-pathogenic properties in skin chronic wounds. Pharm. Biol. 2015, 53, 350–358. [Google Scholar] [CrossRef]
- Gan, B.S.; Kim, J.; Reid, G.; Cadieux, P.; Howard, J.C. Lactobacillus fermentum RC-14 Inhibits Staphylococcus aureus Infection of Surgical Implants in Rats. J. Infect. Dis. 2002, 185, 1369–1372. [Google Scholar] [CrossRef] [PubMed]
- Vahedi Shahandashti, R.; Kasra Kermanshahi, R.; Ghadam, P. The inhibitory effect of bacteriocin produced by Lactobacillus acidophilus ATCC 4356 and Lactobacillus plantarum ATCC 8014 on planktonic cells and biofilms of Serratia marcescens. Turkish J. Med. Sci. 2016, 46, 1188–1196. [Google Scholar] [CrossRef]
- Onbas, T.; Osmanagaoglu, O.; Kiran, F. Potential Properties of Lactobacillus plantarum F-10 as a Bio-control Strategy for Wound Infections. Probiotics Antimicrob. Proteins 2018. [Google Scholar] [CrossRef] [PubMed]
- Lopes, E.G.; Moreira, D.A.; Gullón, P.; Gullón, B.; Cardelle-Cobas, A.; Tavaria, F.K. Topical application of probiotics in skin: Adhesion, antimicrobial and antibiofilm in vitro assays. J. Appl. Microbiol. 2017, 122, 450–461. [Google Scholar] [CrossRef]
- Shokri, D.; Khorasgani, M.R.; Mohkam, M.; Fatemi, S.M.; Ghasemi, Y.; Taheri-Kafrani, A. The Inhibition Effect of Lactobacilli Against Growth and Biofilm Formation of Pseudomonas aeruginosa. Probiotics Antimicrob. Proteins 2018, 10, 34–42. [Google Scholar] [CrossRef]
- Argenta, A.; Satish, L.; Gallo, P.; Liu, F.; Kathju, S. Local application of probiotic bacteria prophylaxes against sepsis and death resulting from burn wound infection. PLoS ONE 2016, 11, e0165294. [Google Scholar] [CrossRef] [PubMed]
- Peral, M.C.; Huaman Martinez, M.A.; Valdez, J.C. Bacteriotherapy with Lactobacillus plantarum in burns. Int. Wound J. 2009, 6, 73–81. [Google Scholar] [CrossRef]
- Brachkova, M.I.; Marques, P.; Rocha, J.; Sepodes, B.; Duarte, M.A.; Pinto, J.F. Alginate films containing Lactobacillus plantarum as wound dressing for prevention of burn infection. J. Hosp. Infect. 2011, 79, 375–377. [Google Scholar] [CrossRef] [PubMed]
- Satish, L.; Gallo, P.H.; Johnson, S.; Yates, C.C.; Kathju, S. Local Probiotic Therapy with Lactobacillus plantarum Mitigates Scar Formation in Rabbits after Burn Injury and Infection. Surg. Infect. (Larchmt). 2017, 18, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Mohammedsaeed, W.; Cruickshank, S.; McBain, A.J.; O’Neill, C.A. Lactobacillus rhamnosus GG Lysate Increases Re-Epithelialization of Keratinocyte Scratch Assays by Promoting Migration. Sci. Rep. 2015, 5, 16147. [Google Scholar] [CrossRef]
- Vågesjö, E.; Öhnstedt, E.; Mortier, A.; Lofton, H.; Huss, F.; Proost, P.; Roos, S.; Phillipson, M. Accelerated wound healing in mice by on-site production and delivery of CXCL12 by transformed lactic acid bacteria. Proc. Natl. Acad. Sci. USA 2018, 115, 1895–1900. [Google Scholar] [CrossRef]
- Bianco, P.; Robey, P.G.; Simmons, P.J. Mesenchymal stem cells: Revisiting history, concepts, and assays. Cell Stem Cell 2008, 2, 313–319. [Google Scholar] [CrossRef]
- Cortés-Araya, Y.; Amilon, K.; Rink, B.E.; Black, G.; Lisowski, Z.; Donadeu, F.X.; Esteves, C.L. Comparison of Antibacterial and Immunological Properties of Mesenchymal Stem/Stromal Cells from Equine Bone Marrow, Endometrium, and Adipose Tissue. Stem Cells Dev. 2018, 27, 1518–1525. [Google Scholar] [CrossRef]
- Wang, M.; Yuan, Q.; Xie, L. Mesenchymal Stem Cell-Based Immunomodulation: Properties and Clinical Application. Stem Cells Int. 2018, 2018, 3057624. [Google Scholar] [CrossRef] [PubMed]
- Glenn, J.D.; Whartenby, K.A. Mesenchymal stem cells: Emerging mechanisms of immunomodulation and therapy. World J. Stem Cells 2014, 6, 526–539. [Google Scholar] [CrossRef] [PubMed]
- Mezey, É.; Nemeth, K. Mesenchymal stem cells and infectious diseases: Smarter than drugs. Immunol. Lett. 2015, 168, 208–214. [Google Scholar] [CrossRef]
- Sutton, M.T.; Fletcher, D.; Ghosh, S.K.; Weinberg, A.; van Heeckeren, R.; Kaur, S.; Sadeghi, Z.; Hijaz, A.; Reese, J.; Lazarus, H.M.; et al. Antimicrobial Properties of Mesenchymal Stem Cells: Therapeutic Potential for Cystic Fibrosis Infection, and Treatment. Stem Cells Int. 2016, 2016, 1–12. [Google Scholar] [CrossRef]
- Alcayaga-Miranda, F.; Cuenca, J.; Khoury, M. Antimicrobial Activity of Mesenchymal Stem Cells: Current Status and New Perspectives of Antimicrobial Peptide-Based Therapies. Front. Immunol. 2017, 8, 339. [Google Scholar] [CrossRef] [PubMed]
- Krasnodembskaya, A.; Song, Y.; Fang, X.; Gupta, N.; Serikov, V.; Lee, J.-W.; Matthay, M.A. Antibacterial Effect of Human Mesenchymal Stem Cells Is Mediated in Part from Secretion of the Antimicrobial Peptide LL-37. Stem Cells 2010, 28, 2229–2238. [Google Scholar] [CrossRef]
- Otero-Viñas, M.; Falanga, V. Mesenchymal Stem Cells in Chronic Wounds: The Spectrum from Basic to Advanced Therapy. Adv. Wound Care 2016, 5, 149–163. [Google Scholar] [CrossRef]
- Johnson, V.; Webb, T.; Norman, A.; Coy, J.; Kurihara, J.; Regan, D.; Dow, S. Activated Mesenchymal Stem Cells Interact with Antibiotics and Host Innate Immune Responses to Control Chronic Bacterial Infections. Sci. Rep. 2017, 7, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Sung, D.K.; Chang, Y.S.; Sung, S.I.; Yoo, H.S.; Ahn, S.Y.; Park, W.S. Antibacterial effect of mesenchymal stem cells against Escherichia coli is mediated by secretion of beta-defensin-2 via toll-like receptor 4 signalling. Cell. Microbiol. 2016, 18, 424–436. [Google Scholar] [CrossRef] [PubMed]
- Mei, S.H.J.; Haitsma, J.J.; Dos Santos, C.C.; Deng, Y.; Lai, P.F.H.; Slutsky, A.S.; Liles, W.C.; Stewart, D.J. Mesenchymal Stem Cells Reduce Inflammation while Enhancing Bacterial Clearance and Improving Survival in Sepsis. Am. J. Respir. Crit. Care Med. 2010, 182, 1047–1057. [Google Scholar] [CrossRef]
- Gupta, N.; Krasnodembskaya, A.; Kapetanaki, M.; Mouded, M.; Tan, X.; Serikov, V.; Matthay, M.A. Mesenchymal stem cells enhance survival and bacterial clearance in murine Escherichia coli pneumonia. Thorax 2012, 67, 533–539. [Google Scholar] [CrossRef]
- Harman, R.M.; Yang, S.; He, M.K.; Van de Walle, G.R. Antimicrobial peptides secreted by equine mesenchymal stromal cells inhibit the growth of bacteria commonly found in skin wounds. Stem Cell Res. Ther. 2017, 8, 157. [Google Scholar] [CrossRef]
- Wood, C.R.; Al Dhahri, D.; Al Delfi, I.; Pickles, N.A.; Sammons, R.L.; Worthington, T.; Wright, K.T.; Johnson, W.E.B. Human adipose tissue-derived mesenchymal stem/stromal cells adhere to and inhibit the growth of Staphylococcus aureus and Pseudomonas aeruginosa. J. Med. Microbiol. 2018, 67, 1789–1795. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, P.; Halabian, R.; Fooladi, A.A.I. Antimicrobial effects of mesenchymal stem cells primed by modified LPS on bacterial clearance in sepsis. J. Cell. Physiol. 2019, 234, 4970–4986. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, K.S.; Stolhandske, J.; Shindell, O.; Smyth, H.D.; Gordon, V.D. Tobramycin and bicarbonate synergise to kill planktonic Pseudomonas aeruginosa, but antagonise to promote biofilm survival. NPJ Biofilms Microbiomes 2016, 2, 16006. [Google Scholar] [CrossRef] [PubMed]
| Therapy | Advantages | Limitations | Current Status of the Therapeutic in Wound Infection Management | References |
|---|---|---|---|---|
| Antimicrobial therapies that directly target microbial processes | ||||
| Phage therapy | -Highly-specificity for bacterial strains -High-density biofilms could enable -Efficient propagation of phage -Less likelihood of resistance development -Able to infect dormant cells and persister variants | -Maintaining phage viability in the delivery vehicle is a concern -Phage therapy to gain a foothold in infection management | -Several clinical trials conducted for usage and safety in burns and post-surgical infections | [34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54] |
| Nano-based technologies | -A wide-range of formulations and combinations available -Physical parameters enable penetration into dense biofilm matrix -Can be coated onto dressings, bandages, sutures, drains -Reduced likelihood of resistance development | -Often effective only in combination with conventional antibiotics but not as stand-alone therapy | -Several commercial products based on nanomaterials available and in commercial use | [55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78] |
| Blue light therapy | -Effective against a wide range of pathogens -Reduced likelihood of resistance development -Ease of administration -Observable adverse effects to host cells minimal -In use for skin ailments such as acne | -Less effective against Gram positive pathogens; important given the polymicrobial nature of wound biofilms | -In vivo preclinical evidence supporting its use -No reports of clinical trials for use in chronic wound biofilms | [79,80,81,82,83,84,85,86,87,88,89,90,91,92,93] |
| Quorum sensing inhibitors | -Potential to prevent early stage biofilm formation -A wide-range of potential therapeutic molecules available | -Highly strain/species-specific -Toxicity to host cells -Efficacy in complex, in vivo models is reduced -Yet to gain a foothold in infection management | -In vivo preclinical evidence with mixed results -No reports of clinical trials for use in chronic wound biofilms | [94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114] |
| Antimicrobial therapies that target the chronic wound biofilm microenvironment, indirectly affecting microbial growth and survival | ||||
| Modulation of pH | -In principle, pH modifying agents are easy to administer onto the wound surface -Less likelihood of resistance development | -Fine-tuning pH in the wound bed is a difficult approach -pH variations have multiple effects on several factors -Effects of pH depend on wound-specific conditions; no universal strategy possible | -Largely in vitro evidence with varied results | [115,116,117,118,119,120,121,122,123] |
| Negative Pressure Wound Therapy (NPWT) | -Standard of care in wound management -Almost no likelihood of resistance development -Well-suited for use in combination with antiseptic instillation | -Likely to be effective only in combination with conventional antiseptics but not as stand-alone therapy | -Already in use for wound care, can be leveraged to manage wound infections with more clinical studies and evidence-based practice | [124,125,126,127,128,129] |
| Hyperbaric Oxygen Therapy (HBOT) | -Standard of care in wound management -Almost no likelihood of resistance development -Can have minimal adverse effects if delivered locally | -Cumbersome delivery mechanism; local delivery devices need to be evaluated -Likely to be effective only in combination with other therapies but not as stand-alone therapy | -Already in use for wound care, can be leveraged to manage wound infections with more clinical studies and evidence-based practice | [130,131,132,133,134,135,136,137,138] |
| Surfactants | -Can be used to coat dressings, sutures, bandages -Less likelihood of resistance development | -Likely to be effective only in conjunction with antibiotics | -FDA approved surfactant polymer dressing available and in use | [139,140,141] |
| Electrical and Electrochemical approaches | -Almost no likelihood of resistance development -Can be combined with other agents in wound dressings | -Likely to be effective only in combination with other therapeutics but not as stand-alone therapy -Mode of delivery may not convenient | -Few commercial products available | [142,143,144,145,146,147] |
| Antimicrobial therapies that target bacteria and the chronic wound biofilm microenvironment, both directly and indirectly impacting microbial growth and survival | ||||
| Probiotics | -An established mode of therapy for other medical conditions -Less toxicity and adverse effects likely -Less likelihood of resistance development | -Could be counterintuitive to administer bacteria to treat an infection, this notion has to be overcome -Mode of delivery needs to be developed | -Reasonable body of in vitro and in vivo evidence; no specific wound infection product available | [148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166] |
| Mesenchymal stem cells | -Harness the ability of the innate immune system -Less likelihood of resistance development -Can be administered via bioengineered skin grafts or dressings | -Likely to be effective when combined with antibiotics -Ethical considerations (particularly for parenteral administration) | -In vivo preclinical evidence promising -Needs robust clinical evaluation to take it forward, approvals for which are likely to be rigorous | [167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kadam, S.; Shai, S.; Shahane, A.; Kaushik, K.S. Recent Advances in Non-Conventional Antimicrobial Approaches for Chronic Wound Biofilms: Have We Found the ‘Chink in the Armor’? Biomedicines 2019, 7, 35. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines7020035
Kadam S, Shai S, Shahane A, Kaushik KS. Recent Advances in Non-Conventional Antimicrobial Approaches for Chronic Wound Biofilms: Have We Found the ‘Chink in the Armor’? Biomedicines. 2019; 7(2):35. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines7020035
Chicago/Turabian StyleKadam, Snehal, Saptarsi Shai, Aditi Shahane, and Karishma S Kaushik. 2019. "Recent Advances in Non-Conventional Antimicrobial Approaches for Chronic Wound Biofilms: Have We Found the ‘Chink in the Armor’?" Biomedicines 7, no. 2: 35. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines7020035