High Energy Intake Induced Overexpression of Transcription Factors and Its Regulatory Genes Involved in Acceleration of Hepatic Lipogenesis: A Rat Model for Type 2 Diabetes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Design
2.3. Intraperitoneal Glucose Tolerance Test (IPGTT)
2.4. Calculation of Homeostasis Model Assessment of Insulin Resistance (HOMA-IR)
2.5. Estimation of Blood Biochemical Measurements
2.6. Fatty Acid Metabolic Genes and Regulatory Element Selection for Quantitative RT-PCR
2.7. Hepatic Gene Expression Study by Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.8. Histopathological Examinations of Various Tissues and Estimation of Tissue Injury by Densitometry
2.9. Statistical Analysis
3. Results
3.1. Standardization of High Fat Diet (HFD)
3.2. Animal Body Weight
3.3. All Groups Showed Non-Significant Differences in Serum Profile Initially
3.4. Assessment of Insulin Resistance After Eight Weeks (Before STZ Induction)
3.4.1. HFD Fed Animals Exhibited Glucose Intolerance
3.4.2. Homeostasis Model Assessment of Insulin Resistance (HOMA-IR)
3.5. Estimation of Serum Biochemical Parameters after Twelve Weeks (After STZ induction)
3.5.1. Detection of Elevated Serum Glucose Levels in DC Group
3.5.2. Diabetic Rats Showed Elevated Serum Lipid Profile
3.5.3. Elevation of Hepatic Enzymes in the Diabetic Rat Model
3.5.4. Assessment of Kidney Function
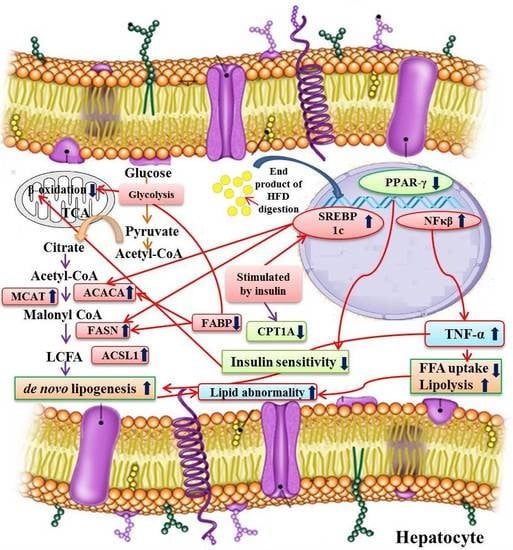
3.6. Expression Profiles of Fatty Acid Metabolism Genes, Inflammatory Marker, and Related Transcription Factors from Liver
3.6.1. Alteration in Transcription Factors Expression Results in Lipid Abnormalities
3.6.2. Modulation of Expression in Fatty Acid Metabolism Genes
3.6.3. Expression of the Inflammatory Marker in the Experimental Groups
3.7. Histological Examination of Liver, Kidney and Pancreas
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Guariguata, L.; Whiting, D.R.; Hambleton, I.; Beagley, J.; Linnenkamp, U.; Shaw, J.E. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res. Clin. Pract. 2014, 103, 137–149. [Google Scholar] [CrossRef] [PubMed]
- International Diabetes Federation. Diabetes Atlas, 7th ed.; International Diabetes Federation: Brussels, Belgium, 2015. [Google Scholar]
- Brendan, K.P.; David, F.A.; Michael, A.R.; James, E.D.; Bruce, P.; Randall, J.B. A model of type 2 diabetes in the guinea pig using sequential dietinduced glucose intolerance and streptozotocin treatment. Dis. Model. Mech. 2017, 10, 151–162. [Google Scholar]
- Beagley, J.; Guariguata, L.; Weil, C.; Motala, A. A Global estimates of undiagnosed diabetes in adults. Diabetes Res. Clin. Pract. 2014, 103, 150–160. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Status Report on Noncommunicable Diseases, 2014; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Saltiel, A.R.; Olefsky, J.M. Thiazolidinediones in the treatment of insulin resistance and type II diabetes. Diabetes 1996, 45, 1661–1669. [Google Scholar] [CrossRef] [PubMed]
- Lebovitz, H.E.; Banerji, M.A. Treatment of insulin resistance in diabetes mellitus. Eur. J. Pharmacol. 2004, 490, 135–146. [Google Scholar] [CrossRef]
- Srinivasan, K.; Viswanad, B.; Lydia, A.; Kaul, C.L.; Ramarao, P. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: A model for type 2 diabetes and pharmacological screening. Pharmacol. Res. 2005, 52, 313–320. [Google Scholar] [CrossRef]
- Garvey, W.T.; Kwon, S.; Zheng, D.; Shaughnessy, S.; Wallace, P.; Hutto, A.; Pugh, K.; Jenkins, A.J.; Klein, R.L.; Liao, Y. Effects of insulin resistance and type 2 diabetes on lipoprotein subclass particle size and concentration determined by nuclear magnetic resonance. Diabetes 2003, 52, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Krishnaswami, V. Treatment of dyslipidemia in patients with type 2 Diabetes. Lipids Health Dis. 2010, 9, 1–12. [Google Scholar]
- Shoelson, S.E.; Lee, J.; Goldfine, A.B. Inflammation and insulin resistance. J. Clin. Investig. 2006, 116, 1793–1801. [Google Scholar] [CrossRef]
- Arshag, D.M. Dyslipidemia in type 2 diabetes mellitus. Nat. Clin. Pract. Endocrinol. Metab. 2009, 5, 3150–3159. [Google Scholar]
- Zheng, X.K.; Zhang, L.; Wang, W.W.; Wu, Y.Y.; Zhang, Q.B.; Feng, W.S. Anti-diabetic activity and potential mechanism of total flavonoids of Selaginella tamariscina (Beauv.) Spring in rats induced by high fat diet and low dose STZ. J. Ethnopharmacol. 2011, 137, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Skovsø, S. Modeling type 2 diabetes in rats using high fat diet and streptozotocin. J. Diabetes Investig. 2014, 5, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, K.S.; Ipseeta, R.M.; Manjusha, K.B.; Ujwala, M.; Deshmukh, Y.A. Development of an experimental model of diabetes co-Existing with metabolic syndrome in rats. Adv. Pharmacol. Sci. 2016, 2016, 1–11. [Google Scholar]
- Rerup, C.C. Drugs producing diabetes through damage of the insulinsecreting cells. Pharmacol. Rev. 1970, 22, 485–518. [Google Scholar] [PubMed]
- Shafrir, E. Diabetes in animals: Contribution to the understanding of diabetes by study of its etiopathology in animal models. In Diabetes Mellitus; Porte, D., Sherwin, R.S., Baron, A., Eds.; McGraw-Hill: New York, NY, USA, 2003; pp. 231–255. [Google Scholar]
- Islam, M.S.; Wilson, R.D. Experimentally induced rodent models of type 2 diabetes. Methods Mol. Biol. 2012, 933, 161–174. [Google Scholar]
- Reed, M.J.; Meszaros, K.; Entes, L.J.; Claypool, M.D.; Pinkett, J.G.; Gadbois, T.M.; Reaven, G.M. New rat model of type 2 diabetes: The fat-fed, streptozotocin-treated rat. Metabolism 2000, 49, 1390–1394. [Google Scholar] [CrossRef] [PubMed]
- Binh, D.V.; Dung, N.T.K.; Thao, L.T.B.; Nhi, N.B.; Chi, P.V. Macro- and microvascular complications of diabetes induced by high-fat diet and low-dose streptozotocin injection in rats model. Int. J. Diabetes Res. 2013, 2, 50–55. [Google Scholar]
- Zhang, M.; Lv, X.Y.; Li, J.; Xu, Z.G.; Chen, L. The characterization of high-fat diet and multiple low-dose streptozotocin induced type 2 diabetes rat model. Exp. Diabetes Res. 2008, 2008, 1–9. [Google Scholar] [CrossRef]
- Franconi, F.; Seghieri, G.; Canu, S.; Straface, E.; Campesi, I.; Malorni, W. Are the available experimental models of type 2 diabetes appropriate for a gender perspective? Pharmacol. Res. 2007, 57, 6–18. [Google Scholar] [CrossRef]
- Unger, R.H.; Clark, G.O.; Scherer, P.E.; Orci, L. Lipid homeostasis, lipotoxicity and the metabolic syndrome. Biochim. Biophys. Acta 2010, 3, 209–214. [Google Scholar] [CrossRef]
- Uma, B.; Hemantkumar, S.C.; Geetika, K.; Abul, K.N. Antidiabetic effects of Embelia ribes extract in high fat diet and low dose streptozotocin-induced type 2 diabetic rats. Front. Life Sci. 2013, 7, 186–196. [Google Scholar]
- Campbell, J.E.; Oh, T.; Hurtgen, B.J.; Niemeyer, D.M.; Sheppard, F.R.; Lucca, J.J.D. Blast overpressure induced pulmonary and intestinal damage is ameliorated by post-injury decay accelerating factor injection. J. Clin. Immunol. Immunother. 2015, 2, 1–6. [Google Scholar]
- Stratton, I.M.; Adler, A.I.; Neil, H.A.; Matthews, D.R.; Manley, S.E.; Cull, C.A.; David, H.; Robert, C.T.; Rury, R.H. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): Prospective observational study. BMJ 2000, 321, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Cleeman, J.I.; Merz, C.N.; Brewer, H.B., Jr.; Clark, L.T.; Hunninghake, D.B.; Pasternak, R.C.; Smith, S.C., Jr.; Stone, N.J. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation 2004, 110, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Goff, D.C., Jr.; Gerstein, H.C.; Ginsberg, H.N.; Cushman, W.C.; Margolis, K.L.; Byington, R.P.; Buse, J.B.; Genuth, S.; Probstfield, J.L.; Simons-Morton, D.G. Prevention of cardiovascular disease in persons with type 2 diabetes mellitus: Current knowledge and rationale for the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Am. J. Cardiol. 2007, 99, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Brunzell, J.D.; Davidson, M.; Furberg, C.D.; Goldberg, R.B.; Howard, B.V.; Stein, J.H.; Witztum, J.L. Lipoprotein management in patients with cardiometabolic risk: Consensus statement from the American Diabetes Association and the American College of Cardiology Foundation. Diabetes Care 2008, 31, 811–822. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association: Standards of medical care in diabetes-2009. Diabetes Care 2009, 32, 13–61. [CrossRef] [PubMed]
- Luo, J.; Quan, J.; Tsai, J.; Hobensack, C.K.; Sullivan, C.; Hector, R. Nongenetic mouse models of non-insulin-dependent diabetes mellitus. Metabolism 1998, 47, 663–668. [Google Scholar] [CrossRef]
- Zhang, F.; Ye, C.; Li, G.; Ding, W.; Zhou, W.; Zhu, H.; Chen, G.; Luo, T.; Guang, M.; Liu, Y.; et al. The rat model of type 2 diabetic mellitus and its glycometabolism characters. Exp. Anim. 2003, 52, 401–407. [Google Scholar] [CrossRef]
- Kim, E.H.; Lee, J.B.; Kim, S.H.; Jo, M.W.; Hwang, J.Y.; Bae, S.J.; Jung, C.H.; Lee, W.J.; Park, J.Y.; Park, G.M.; et al. Serum triglyceride levels and cardiovascular disease events in Koreans. Cardiology 2015, 131, 228–235. [Google Scholar] [CrossRef]
- Zeljko, R. Hypertriglyceridaemia and risk of coronary artery disease. Nat. Rev. Cardiol. 2017, 14, 401–411. [Google Scholar]
- Storlien, L.H.; James, D.E.; Burleigh, K.M.; Chisholm, D.J.; Kraegen, E.W. Fat feeding causes widespread in-vivo insulin resistance, decreased energy expenditure and obesity in the rat. Am. J. Physiol. 1986, 251, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, K.; Patole, P.S.; Kaul, C.L.; Ramarao, P. Reversal of glucose intolerance by pioglitazone in high-fat diet fed rats. Methods Find. Exp. Clin. Pharmacol. 2004, 26, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Soumya, D.; Srilatha, B. Late stage complications of diabetes and insulin resistance. J. Diabetes Metab. 2011, 2, 167. [Google Scholar]
- Wang, H.J.; Jin, Y.X.; Shen, W.; Neng, J.; Wu, T.; Li, Y.J.; Fu, Z.W. Low dose streptozotocin (STZ) combined with high energy intake can effectively induce type 2 diabetes through altering the related gene expression. Asia Pac. J. Clin. Nutr. 2007, 16, 412–417. [Google Scholar] [PubMed]
- Horton, J.D.; Goldstein, J.L.; Brown, M.S. SREBPs: Activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Investig. 2002, 109, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Arkan, M.C.; Hevener, A.L.; Greten, F.R.; Maeda, S.; Li, Z.W.; Long, J.M.; Wynshaw-Boris, A.; Poli, G.; Olefsky, J.; Karin, M. IKK-β links inflammation to obesity-induced insulin resistance. Nat. Med. 2005, 11, 191–198. [Google Scholar] [CrossRef]
- Shimano, H.; Horton, J.D.; Shimomura, I.; Hammer, R.E.; Brown, M.S.; Goldstein, J.L. Isoform 1c of sterol regulatory element binding protein is less active than isoform 1a in livers of transgenic mice and in cultured cells. J. Clin. Investig. 1997, 99, 846–854. [Google Scholar] [CrossRef]
- Higuchi, N.; Kato, M.; Shundo, Y.; Tajiri, H.; Tanaka, M.; Yamashita, N.; Kohjima, M.; Kotoh, K.; Nakamuta, M.; Takayanagi, R.; et al. Liver X receptor in cooperation with SREBP-1c is a major lipid synthesis regulator in nonalcoholic fatty liver disease. Hepatol. Res. 2008, 38, 1122–1129. [Google Scholar] [CrossRef]
- Shimomura, I.; Bashmakov, Y.; Horton, J.D. Increased levels of nuclear SREBP-1c associated with fatty livers in two mouse models of diabetes mellitus. J. Biol. Chem. 1999, 274, 30028–30032. [Google Scholar] [CrossRef]
- Spiegelman, B.M. PPAR-gamma: Adipogenic regulator and thiazolidinedione receptor. Diabetes 1998, 47, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Schoonjans, K.; Staels, B.; Auwerx, J. The peroxisome proliferator activated receptors (PPARS) and their effects on lipid metabolism and adipocyte differentiation. Biochim. Biophys. Acta 1996, 1302, 93–109. [Google Scholar] [CrossRef]
- Shah, A.; Rader, D.J.; Millar, J.S. The effect of PPAR-alpha agonism on apolipoprotein metabolism in humans. Atherosclerosis 2010, 210, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Yuan, M.; Frantz, D.F.; Melendez, P.A.; Hansen, L.; Lee, J.; Shoelson, S.E. Local and systemic insulin resistance resulting from hepatic activation of IKK-β and NF-κβ. Nat. Med. 2005, 11, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Pickup, J.C.; Mattock, M.B.; Chusney, G.D.; Burt, D. NIDDM as a disease of the innate immune system: Association of acute-phase reactants and interleukin-6 with metabolic syndrome X. Diabetologia 1997, 40, 1286–1292. [Google Scholar] [CrossRef] [PubMed]
- Popa, C.; Netea, M.G.; van Riel, P.L.; van der Meer, J.W.; Stalenhoef, A.F. The role of TNF-α in A in chronic inflammatory conditions, intermediary metabolism, and cardiovascular risk. J. Lipid Res. 2007, 48, 751–762. [Google Scholar] [CrossRef]
- Chen, L.; Frister, A.; Wang, S.; Ludwig, A.; Behr, H.; Pippig, S.; Li, B.; Simm, A.; Hofmann, B.; Pilowski, C.; et al. Interaction of vascular smooth muscle cells and monocytes by soluble factors synergistically enhances IL-6 and MCP-1 production. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, 987–996. [Google Scholar] [CrossRef]
- Boord, J.B.; Fazio, S.; Linton, M.F. Cytoplasmic fatty acid-binding proteins: Emerging roles in metabolism and atherosclerosis. Curr. Opin. Lipidol. 2002, 13, 141–147. [Google Scholar] [CrossRef]
- Newberry, E.P.; Xie, Y.; Kennedy, S.; Han, X.; Buhman, K.; KLuo, J.; Gross, R.W.; Davidson, N.O. Decreased hepatic triglyceride accumulation and altered fatty acid uptake in mice with deletion of the liver fatty acid-binding protein gene. J. Biol. Chem. 2003, 78, 51664–51672. [Google Scholar] [CrossRef]
- Veerkamp, J.H.; van Moerkerk, H.T. Fatty acid-binding protein and its relation to fatty acid oxidation. Mol. Cell. Biochem. 1993, 123, 101–106. [Google Scholar] [CrossRef]






| Experimental Groups | Glucose (mM) | Insulin (µU/mL) |
|---|---|---|
| Healthy Control (HC) | 3.91 ± 0.08 | 8.30 ± 0.07 |
| High Fat diet Control (HFDC) | 5.65 ± 0.17 *** | 9.52 ± 0.16 ** |
| Diabetic Control (DC) | 5.51 ± 0.04 *** | 10.12 ± 0.30 *** |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khadke, S.P.; Kuvalekar, A.A.; Harsulkar, A.M.; Mantri, N. High Energy Intake Induced Overexpression of Transcription Factors and Its Regulatory Genes Involved in Acceleration of Hepatic Lipogenesis: A Rat Model for Type 2 Diabetes. Biomedicines 2019, 7, 76. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines7040076
Khadke SP, Kuvalekar AA, Harsulkar AM, Mantri N. High Energy Intake Induced Overexpression of Transcription Factors and Its Regulatory Genes Involved in Acceleration of Hepatic Lipogenesis: A Rat Model for Type 2 Diabetes. Biomedicines. 2019; 7(4):76. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines7040076
Chicago/Turabian StyleKhadke, Suresh P., Aniket A. Kuvalekar, Abhay M. Harsulkar, and Nitin Mantri. 2019. "High Energy Intake Induced Overexpression of Transcription Factors and Its Regulatory Genes Involved in Acceleration of Hepatic Lipogenesis: A Rat Model for Type 2 Diabetes" Biomedicines 7, no. 4: 76. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines7040076