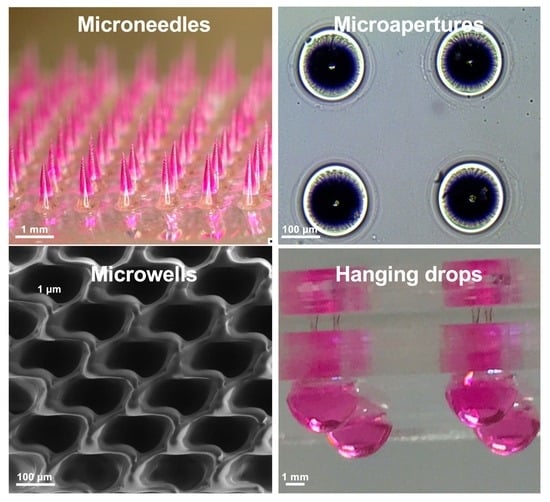
1. Introduction
Microfabrication techniques have revolutionized the way biologists and medical scientists conduct studies in the last few decades [
1]. The well-developed semiconductor workflow enables not only the creation of physical microstructures conducive to the dimension of cells and tissues but also integrates these structures for various downstream functional assays [
2]. However, although microfabrication techniques have been widely applied in various areas of biomedical research, the dependence on lithographic procedures that require either chemical etching with special equipment and cleanroom facilities or a silicon master for replica molding has been criticized as a barrier to entry. Even minor changes in the design of a microstructure require a cumbersome and laborious process accompanied by a significant increase in the fabrication and materials cost [
3]. Although most biomedical laboratories can utilize alternative commercial products with relative ease, the lack of flexibility in changing the design and protocol integration as well as the costly nature often hamper the utility of these alternatives. Identifying a rapid and economical method for the reliable generation of microstructures has thus become crucial, especially in terms of methods that allow for quick iterations in design modification during initial stages of validation, which is imperative for individual labs.
Laser machining has long been utilized as a salient alternative or supplementary tool to some lithography-based approaches for microfabrication since the late 1980s [
3,
4]. CO
2 lasers are one of the most conventionally used laser sources for rapid prototyping and cost-effective microfabrication. Microchannel engraving has been demonstrated as a rapid fabrication method for microfluidic devices [
5,
6]. Laser drilling, termed ablation, of a single spot is another conventional technique to generate an array of microstructures, i.e., concavities or through-holes applied in many industrial and research applications [
7,
8]. Combined with the use of different substrate materials, a wide range of downstream tissue or cellular-based utilities can be readily achieved [
3,
9,
10,
11]. For example, microscale needles, termed microneedles (MNs), enable the delivery of large molecules, such as proteins, DNA and vaccines, into the skin in a painless, rapid and efficient way [
12]. The female MN polydimethylsiloxane (PDMS) mold, used for fabrication of polymeric MNs, can be easily and conveniently made by laser drilling. Additionally, microwell technology fabricated by CO
2 lasers has been applied in generating cellular spheroids [
3,
13] and circulating tumor cell cultures [
14]. Microwells are conducive to the formation of uniform, size-controlled, multicellular tumor spheroids (MCTSs). MCTSs imitate three-dimensional (3D) growth in a way similar to avascular tumors in vivo in terms of their cell–cell interaction. Studies have also indicated that drug screening carried out on MCTSs is more relevant than that of traditional two-dimensional (2D) cultures, suggesting the potential use of microwells in future drug screening and exploration of different cancer treatment modalities [
15].
However, a major barrier to fabricating microstructure arrays using CO
2 laser drilling is that different studies have only been reported sporadically, and these studies have involved different systems, substrate materials, and applications [
3,
8,
9,
16,
17]. On the one hand, these outcomes have prohibited this technique from reaching the greater scientific community because of the concern that specific and unique protocols as well as meticulous pre/postmaterial processes are required for different applications. On the other hand, varying microstructure geometries and qualities from different studies [
13,
14,
16] suggest ample room for further improvement based on a comprehensive and systematic understanding of the choice of both the materials and the laser system. With these notions, we hope to generate a holistic viewpoint of the simplicity and consistency of the method as well as of the diverse microstructures and applications that can be achieved through the integration of various common laboratory-accessible consumables.
In this report, we present a facile laser ablation technique using a laboratory-built CO
2 laser drilling system that can be conveniently applied to fabricate different biocompatible materials for generating arrayed microstructures. The primary goal was to establish a set of protocols that are similar in laser parameters and also applicable to various downstream cellular-based and drug discovery applications (
Figure 1). Detailed laser parameters were inspected using different pulse numbers for single-dot laser drilling. PDMS was first assessed for its ability to form different MN mold geometries, and polyvinylpyrrolidone/polyvinyl alcohol (PVP/PVA) were employed to cast an MN array patch for transdermal drug delivery. Polystyrene (PS) microwells were optimized in an arrangement to better prevent cell loss during cell seeding steps and to form size-controlled MCTSs. Penetrated glass microapertures were assessed for their ability to capture MCTSs by applying negative suction. PS was also validated as a substrate to perforate a through-hole that was advantageous for media exchange in the hanging drop method for large MCTSs. The proposed methodology highlights a highly versatile and facile microfabrication technique synergizing the conventional CO
2 laser and several biocompatible materials for a wide range of downstream applications.
2. Experimental Section
2.1. Laser Setup and Substrate Materials
A Synrad 48-1 CO2 laser (10 W, Synrad, Inc., Mukilteo, WA, USA) with a 10.6 µm wavelength was horizontally mounted on an adjustable frame that allowed adjustment along the z-axis. The diameter of the laser beam (3.5 mm) was broadened by a 2× beam expander and then focused by a plano-convex lens (2”). Two step motor X–Y stages positioned the substrate materials at the location of interest for laser ablation. The parts mentioned above were assembled by Laser Solution Technology Inc. (Taipei, Taiwan) based on the author’s conceptual design. Laser pulse commends were operated under pulse width modulations, in which the duty cycle was set at 60% duty at 5 kHz and the power was determined based on the number of pulses ablated on the choice of the materials. PDMS was fabricated by mixing elastomer (Sylgard-184A, Dow Corning Corp., Midland, MI, USA) and curing agent (Sylgard-184B, Dow Corning Corp.) at a weight ratio of 10 to 1. PS microscope slides (1 mm thick) were purchased from EMS (Electron Microscopy Sciences Inc., Hatfield, PA, USA). Commercial No.1 borosilicate coverglass (22 × 22 mm, Paul Marienfeld GmbH and Co. KG., Lauda-Königshofen, BW, Germany) was used as the glass substrate. Poly(methyl methacrylate) (PMMA) slides (1 mm thick) were acquired from a local manufacturer (Yi-Shiou Co. Ltd., Tainan, Taiwan).
2.2. Cell Culture
Human hepatocellular carcinoma cell lines, HepG2 and Huh7, were cultured at 37 °C in a 5% CO2 humidified incubator and maintained in high-glucose Dulbecco’s Modified Eagle’s Medium (DMEM, Sigma-Aldrich Corp., St. Louis, MO, USA) supplemented with 1% penicillin/streptomycin (Sigma-Aldrich Corp.) and 10% fetal bovine serum (FBS, Gibco, Thermo Fisher Scientific Co. Ltd., Waltham, MA, USA). Cultured cells were monitored daily and the supernatants were replaced with fresh medium every two or three days or at approximately 80–90% confluency.
2.3. MN Fabrication
A 50 wt% poly(vinyl pyrrolidone) (PVP, molecular weight (MW) 10,000 kDa, Sigma-Aldrich Corp.)/poly(vinyl alcohol) (PVA, MW 6000 kDa, Polysciences, Inc., Warrington, PA, USA) aqueous solution was prepared at a weight ratio of 1:1. PDMS molds were made by direct laser ablation on PDMS. A 30 W plasma treatment (Harrick Plasma Inc., Ithaca, NY, USA) was performed on the surface of the PDMS mold for 30 s. Tetramethylrhodamine isothiocyanate–dextran (TRITC-dextran, MW 155 kDa, Sigma-Aldrich Corp.) was added to deionized water and stirred until completely dissolved. The prepared PVP/PVA solution was mixed well with the TRITC-dextran aqueous solution to obtain a homogeneous solution containing 40 wt% PVP/PVA. As a first layer, the TRITC-dextran-containing PVP/PVA solution was added to the PDMS mold surface under a vacuum to ensure the complete filling of the MN mold cavities by the solution. Residual solution that did not enter into the mold cavities was removed from the mold surface. Under the same vacuum operation, the PVP/PVA solution without TRITC-dextran was then added to the mold surface as a second layer to constitute the patch. The filled molds were placed at room temperature for 30 min and dried in an oven at 37 °C for at least one day. The PVP/PVA MN array was then gently peeled from the mold for skin insertion tests.
2.4. MN Skin Insertion
To evaluate the MN skin insertion ability, the fabricated MNs were inserted into fully thick porcine skin with a thickness of 1186 ± 136 μm (n = 5) by using a custom-made applicator for 10 min. The applicator device provided a consistent force for MN application and ensured consistent MN penetration. After the complete dissolution of MNs in the skin, the MN-treated site was excised for histological examination.
2.5. PDMS Chamber Fabrication
PDMS chambers were made for both microwell and hanging drop applications. The PS slide was cleaned with 75% ethanol and air-dried, then treated with 30 W air plasma cleaner for 30 s and placed in an aqueous solution of 1% volume(v)/v (3-aminopropyl)triethoxysilane (APTES, Sigma-Aldrich Corp.) for at least 20 min. The slides were then washed with deionized water and air-dried. PDMS was also treated with air plasma under the same conditions used for the PS slide. Activated slides and PDMS were kept in conformal contact at 70 °C in an oven for 1 h.
2.6. MCTS Formation in Microwells
Microwells were first soaked within 75% ethanol to remove debris from the laser ablation and for disinfection. The microwells were then placed under irradiation with UV light for 30 min. Before cell seeding, microwells were coated with 0.2% Pluronic F-127 (Sigma-Aldrich Corp.) in 1× PBS for 30 min to prevent cell attachment to the PS substrate and then washed twice with 1× PBS. Then, the desired cell suspension concentrations (50, 100, 150, 200 cells microwell−1) of both Huh7 and HepG2 cells were loaded into the chamber. Cells were maintained at 37 °C in a 5% CO2 humidified incubator for 4 days, and the aggregation of the cells was recorded daily. Formed MCTSs were harvested by pipetting the medium twice to flush them from the microwells and then transferred to the container of interest for different downstream applications.
2.7. MCTS Trapping
The Huh-7 MCTS suspension solution was diluted to 300 MCTSs suspended in serum-free medium (50 µL). Hoechst solution (1:2000, Invitrogen, Thermo Fisher Scientific Co. Ltd.) was added to the cell suspension, and MCTSs were then incubated for 30 min at 37 °C. A PDMS chamber was made and sealed by a coverglass attachment. The cell suspension was then added to the top of the coverglass, and suction was applied to the sealed PDMS chamber by syringe (1 mL). The MCTCs were then trapped within an array of funnel-like glass apertures, and excessive MCTSs were removed by washing with 1× PBS.
2.8. MCTS Formation by the Hanging Drop Method
After disinfection with 75% ethanol spray, hybrid devices for use with the hanging drop method were exposed to UV light for 30 min for sanitizing and drying. The device was then placed on a bracing frame of sterilized PDMS in a culture dish (150 mm), and 0.2% Pluronic F-127 in 1× PBS (50 µL) was loaded into both sides of the PDMS wells for 30 min. Then, the Pluronic F-127 was aspirated, and the device was kept dry for further usage. After dilution in culture medium to obtain desired cell densities, cell solutions (20 µL) with specific cell numbers (500, 1000, 5000 cells droplet−1) were dispensed into the bottom side of the PDMS wells of each device (after inverting the device). Next, medium (50 μL) was added to the top side of the through-hole well to promote fluid exchange to the bottom side by gravity. Before maintaining the cultures at 37 °C in a 5% CO2 humidified incubator, the device was placed into a 100 mm culture dish that also contained sterilized ultrapure water (2 mL) in a culture dish (30 mm), which significantly reduced the rate of evaporation within the incubator. Owing to gravity, cells within hanging drops settled at the nadir of the medium/air interface at the bottom of the droplets and developed into a 3D aggregates or microtissue structures spontaneously during the 4 days of formation. Note that fresh culture medium exchange was performed daily, and we also ensured that the droplet structures on the bottom side of the PDMS wells were intact every day.
2.9. MCTS Anticancer Drug Screening and Viability Analysis
Doxorubicin hydrochloride (DOX, Sigma-Aldrich Corp.) at 5 mg mL−1 in DMSO was diluted with culture medium to 3.16, 10, 31.6, and 100 μM, dispensed into the hanging drops after 3 days of MCTS development, and then treated for 24 h. The morphology and viability of the MCTSs were monitored using a LIVE/DEAD™ Viability/Cytotoxicity Kit (Invitrogen, Thermo Fisher Scientific Co. Ltd.), where the calcein-AM and ethidium homodimer-1 were added at dilutions of 1:2000 and 1:1000, respectively. After incubation at 37 °C in a 5% CO2 humidified incubator for 1 h, MCTSs were recorded under an inverted fluorescence microscope. The quantitative viability measurement of MCTSs in hanging drops was also assessed using a WST-1 Cell Proliferation Assay Kit (Takara Bio Inc., Kusatsu, Shiga, Japan), in which WST-1 reagent (10 μL) was added to culture medium (100 μL) containing a spheroid in each drop, followed by transfer to a 96-well plate. After incubation for 2 h in the incubator, the absorbance at 450 nm was measured using a spectrophotometer (Hitachi Ltd., Tokyo, Japan).
2.10. Statistical Analysis
Numerical values of dimensions in our study were expressed as the means ± standard deviations of more than three independent replicates. Statistical significance was determined via one-way analysis of variance (ANOVA) with Prism 7 (GraphPad Software Inc., San Diego, CA, USA). Statements of significance were based on p-values < 0.05.
4. Discussion
MNs can easily penetrate through the epidermis to deliver a broad range of molecules, especially macromolecules, into the skin without causing notable pain. Therefore, MNs have been considered as a convenient, safe, and effective system for transdermal drug delivery. Compared to MNs made of silicon and metals, polymeric MNs have attracted enormous attention because many polymers are biocompatible, biodegradable and nontoxic. Polymeric MNs can be fabricated on a large scale and can encapsulate large amounts of the drug [
19]. Additionally, polymers with different physical and chemical properties and degradation behaviors can be utilized to fabricate MNs with different drug release profiles for a variety of disease treatments.
Polymeric MNs are commonly made using a micromolding technique, which involves fabrication of a master MN structure, creation of a female PDMS mold from the master structure, and casting of polymers into the PDMS mold [
20]. A number of methods have been utilized to produce these master MN structures, such as the microelectromechanical systems (MEMS) technique for silicon-based structures and the electrodischarge machining process for metallic-based structures [
21]. However, these techniques are usually complicated and time-consuming, and require expensive equipment or cleanroom conditions. Nejad et al. previously investigated a high-aspect-ratio MN mold using CO
2 laser engraving on acrylic sheets [
22]. In this study, we demonstrated that PDMS molds with various shapes and geometries could be easily fabricated using the CO
2 laser ablation technique and adjusting the FPP and laser pulse number (
Figure 2). After tuning the laser parameters, PDMS molds with widths ranging from 250 to 700 μm and depths ranging from 150 to 1400 μm were generated (
Figure 2a,b). The obtained female PDMS molds can be directly used to fabricate polymeric MNs without the need for a master MN structure, thus eliminating many complicated steps and expensive cleanroom facilities.
A variety of polymeric MN shapes with different heights and tip sharpness were successfully prepared by casting the PVP/PVA solution into the laser-ablated mold (
Figure 2d,e). Both PVP and PVA are water-soluble polymers and have been widely used in biomedical applications due to their biocompatibility, biodegradability, and nontoxic nature [
23,
24,
25]. Additionally, these polymers have been approved by the FDA for clinical uses in humans [
24,
26]. Thus, PVP/PVA was used as a representative example to fabricate biodegradable MN arrays for proof of concept. We showed that the prepared drug-loaded PVP/PVA MNs had sufficient mechanical strength to be inserted into a porcine skin and that they were able to deliver the loaded macromolecular drugs into the skin (
Figure 2g). These results demonstrate the feasibility of using the proposed technique to fabricate biodegradable polymer-based MNs for transdermal drug delivery. This laser ablation approach enables the facile, on-demand and cleanroom-free fabrication of MN molds with the desired geometry, thus allowing mass production of MNs in a cost-effective way.
Rapid CO
2 laser prototyping has been previously demonstrated as an effective alternative for the fabrication of microwells for the formation of size-controlled 3D spheroids [
3,
11,
13,
16]. However, one major obstacle that has remained non-demonstrated in PS microwells is the inevitable loss of cells after cell seeding because of the required washing step to ensure that cells are not lodged into the microwells and can be efficiently removed without perturbing the size-uniformity of the MCTSs [
27]. To overcome this issue, the design of the microwell array arrangement should be as compact as possible to allow for complete surface utilization. Although PS microwells inherently possess a pair of wing recast structures due to the heat–melt process, the space between each microwell could be maintained with a marginal thickness and the formation of a barrier between microwells for the compartmentalization of the MCTSs (
Figure 3). Our results demonstrated that an improved microwell array arrangement could be utilized to yield uniform Huh7 and HepG2 MCTSs without an additional washing step after cell seeding. Additionally, choosing PS as the microwell substrate is favorable from a biologist’s perspective because PS is the most commonly utilized plastic for in vitro cell culture research. Compared to PDMS [
28] or polyester [
13] microwells, PS is hassle-free for the researchers in terms of the material accessibility, and more amenable in terms of compatibility with different forms of conventional culture plasticwares, such as Petri dishes [
3] or microtiter plates [
16]. In addition, the PS microwell displayed a gentle concave bottom, and curved microwell structures have been reported to promote the aggregation of spheroids [
29]. The exact time required for the formation of MCTS as well as the specific MCTS morphology is highly dependent on the cell number, microwell dimension and cell line [
30]. From our observation, initial fibroblast aggregation (data not shown) occurred rapidly after one day (on day 2) of cell seeding, which was also observed in a similar study [
31]. However, a similar aggregative morphology was observed for only HepG2 cells on day 3 and for Huh7 cells on day 4 (
Figure 3f).
In addition to generating a concave structure on the substrate, as demonstrated in the previous sections, penetrated microapertures can also result in several expanded utilizations. We demonstrated that a microaperture was conveniently generated on a conventional coverglass (
Figure 4). Given that CO
2 laser drilling provided an intense local heat source, the glass substrate reached the glass transition temperature and started to melt and flow to form an hourglass-shaped aperture with a size of ≈15 µm [
10]. By introducing two-stage laser drilling, the perforated aperture reached 1–3 µm, which is suitable for electrophysiological ion channel recordings [
10]. We previously demonstrated that the ablated hourglass-shaped glass aperture could be integrated with microfluidic channels for solution exchange, patch-clamp recordings and drug discovery [
17]. A few months ago, Ayan et al. showed that spheroid aspiration could assist in bioprinting control [
32]. Herein, the pattern of a microaperture array demonstrated that parallel spheroid trapping is possible for precise positioning.
The hanging drop method, which employs spheroid aggregation in a culture medium drop based on the drop shape and gravity, is one of the most commonly used methods for generating 3D models with minimal requirements for additional equipment [
33,
34,
35]. Although the typical hanging drop technique can be performed on the underside of culture plate lids, one of the major limitations of this technique is the difficultly in media exchange [
36]. Several commercial products and lab-developed platforms have included a microchannel or incorporated microfluidics for ease of liquid handling [
37,
38,
39]. Such modifications allow the media that spheroids are formed to be replaced by fresh media for better metabolic and intertissue communication [
39]. However, the implementation of clean-room microfabrication has again become inevitable. Additionally, high-throughput screening may not be necessary for preliminary lab investigations in which only a few data points a day can be sufficient. In this study, we fabricated uniform through-hole structures with a CO
2 laser, and we could easily generate uniformly shaped drops within the same drop volume (
Figure 5). In addition, direct media exchange by micropipetting was also accomplished without affecting the spheroids for studying the drug efficacy of DOX in HepG2 and Huh7 liver MCTSs (
Figure 6).
Finally, CO
2 laser-assisted microfabrication has been a preferred method for rapid in-house prototyping and manufacturing in the laboratory because of its cost-effectiveness and pattern flexibility as well as the ease of system accessibility [
3,
10,
17]. Several notable studies have demonstrated its feasibility for potential uses in biomedicine, such as in biochips and microfluidic devices for cellular-based assays [
40,
41]. Among various applications enabled by the CO
2 lasers, this work explicitly focused on providing an integrated viewpoint of single point-drilling in the generation a microstructure array. The results not only serve as a reference for researchers with different downstream applications but also provide a step-by-step workflow to identify potentially new structures in different materials. For instance, for researchers interested in the microstructure of laser-ablated PMMA (
Figure S2), the laser pulse parameters, FPP distance and methods for microstructure examination can all be determined based on the current work. This outcome, again, highlights that the seemingly disparate downstream applications of MN molds, microwells and microapertures were all derived from an identical protocol and unified parameters that involve minimal changes to the materials used. This work hopefully serves as a holistic guide for CO
2 laser microfabrication in regards to laser protocols, material choices and potential utilities to study biomedicine form a wide range of perspectives.