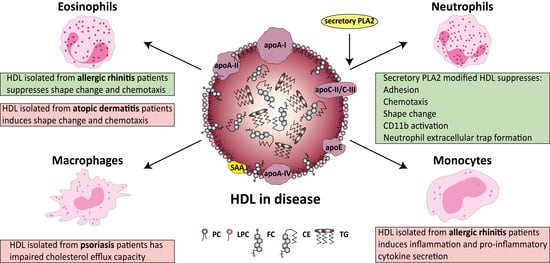
High-Density Lipoprotein (HDL) in Allergy and Skin Diseases: Focus on Immunomodulating Functions
Abstract
:1. Introduction
2. HDL Metabolism, Composition and Function
3. Potential Role of HDL in Atopic Allergic Diseases
3.1. Relation of HDL with Asthma
3.2. Allergic Rhinitis is Associated with Complex Alterations in HDL Composition and Function
4. HDL in Inflammatory Skin Diseases
4.1. Atopic Dermatitis is Associated with Complex Alterations in HDL Composition and Function
4.2. HDL in Psoriasis
4.3. HDL in Urticaria
4.4. HDL in Angioedema
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ABC | ATP-binding cassette |
| ACE | angiotensin-converting enzyme |
| AMPK | adenosine monophosphate-activated protein kinase |
| apo | apolipoprotein |
| CD | cluster of differentiation |
| CE | cholesteryl ester |
| CETP | cholesteryl ester transfer protein |
| EL | endothelial lipase |
| FC | free cholesterol |
| FEV1 | forced expiratory volume in one second |
| FVC | forced vital capacity |
| HDL | high-density lipoprotein |
| HL | hepatic lipase |
| IgE | immunoglobulin E |
| IL | interleukin |
| JAK | janus kinase |
| LCAT | lecithin-cholesterol acyltransferase |
| LDL | low-density lipoprotein |
| LDLR | low-density lipoprotein receptor |
| LPC | lyso-phosphatidylcholine |
| LPL | lipoprotein lipase |
| Lp-PLA2 | lipoprotein-associated phospholipase A2 |
| LPS | lipopolysaccharide |
| NET | neutrophil extracellular trap |
| NF-κB | nuclear factor-κB |
| PAF | platelet-activating factor |
| PAF-AH | platelet-activating factor-acetylhydrolase |
| PC | phosphatidylcholine |
| PLA2 | phospholipase A2 |
| PLTP | phospholipid transfer protein |
| PON | paraoxonase |
| PUVA | psoralen and ultraviolet A |
| RCT | reverse cholesterol transport |
| SAA | serum amyloid A |
| SR-BI | scavenger receptor class B type I |
| TG | triglyceride |
| TLRs | toll-like receptors |
| TNF | tumor necrosis factor |
| UVB | ultraviolet B |
| VLDL | very low-density lipoprotein |
References
- Karimkhani, C.; Dellavalle, R.P.; Coffeng, L.E.; Flohr, C.; Hay, R.J.; Langan, S.M.; Nsoesie, E.O.; Ferrari, A.J.; Erskine, H.E.; Silverberg, J.I.; et al. Global skin disease morbidity and mortality an update from the global burden of disease study 2013. JAMA Dermatol. 2017, 153, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Pawankar, R. Allergic diseases and asthma: A global public health concern and a call to action. World Allergy Organ. J. 2014, 7, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, B.; Wang, S.; Peng, D.; Zhao, S. HDL and immunomodulation: An emerging role of HDL against atherosclerosis. Immunol. Cell Biol. 2010, 88, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Catapano, A.L.; Pirillo, A.; Bonacina, F.; Norata, G.D. HDL in innate and adaptive immunity. Cardiovasc. Res. 2014, 103, 372–383. [Google Scholar] [CrossRef] [Green Version]
- Fessler, M.B. Next stop for HDL: The lung. Clin. Exp. Allergy 2012, 42, 340–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shenoy, C.; Shenoy, M.M.; Rao, G.K. Dyslipidemia in dermatological disorders. N. Am. J. Med. Sci. 2015, 7, 421–428. [Google Scholar] [CrossRef] [Green Version]
- Di Angelantonio, E.; Sarwar, N.; Perry, P.; Kaptoge, S.; Ray, K.K.; Thompson, A.; Wood, A.M.; Lewington, S.; Sattar, N.; Packard, C.J.; et al. Major lipids, apolipoproteins, and risk of vascular disease. JAMA J. Am. Med. Assoc. 2009, 302, 1993–2000. [Google Scholar] [CrossRef] [Green Version]
- Gordon, T.; Castelli, W.; Hjortland, M.C. High density lipoprotein as a protective factor against coronary heart disease: The Framingham Study. Am. J. Med. 1977, 62, 707–714. [Google Scholar] [CrossRef]
- Wilson, P.W.F.; Abbott, R.D.; Castelli, W.P. High density lipoprotein cholesterol and mortality. The Framingham heart study. Arteriosclerosis 1988, 8, 737–741. [Google Scholar] [CrossRef] [Green Version]
- Gordon, D.J.; Probstfield, J.L.; Garrison, R.J.; Neaton, J.D.; Castelli, W.P.; Knoke, J.D.; Jacobs, D.R.; Bangdiwala, S.; Tyroler, H.A. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation 1989, 79, 8–15. [Google Scholar] [CrossRef] [Green Version]
- Karalis, I.; Jukema, J.W. HDL Mimetics Infusion and Regression of Atherosclerosis: Is It Still Considered a Valid Therapeutic Option? Curr. Cardiol. Rep. 2018, 20, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicholls, S.J.; Andrews, J.; Kastelein, J.J.P.; Merkely, B.; Nissen, S.E.; Ray, K.K.; Schwartz, G.G.; Worthley, S.G.; Keyserling, C.; Dasseux, J.L.; et al. Effect of serial infusions of CER-001, a pre-β High-density lipoprotein mimetic, on coronary atherosclerosis in patients following acute coronary syndromes in the CER-001 atherosclerosis regression acute coronary syndrome trial: A randomized clinical trial. JAMA Cardiol. 2018, 3, 815–822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armitage, J.; Holmes, M.V.; Preiss, D. Cholesteryl Ester Transfer Protein Inhibition for Preventing Cardiovascular Events: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2019, 73, 477–487. [Google Scholar] [CrossRef]
- Holmes, M.V.; Asselbergs, F.W.; Palmer, T.M.; Drenos, F.; Lanktree, M.B.; Nelson, C.P.; Dale, C.E.; Padmanabhan, S.; Finan, C.; Swerdlow, D.I. Mendelian randomization of blood lipids for coronary heart disease. Eur. Heart J. 2015, 36, 539–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norata, G.D.; Pirillo, A.; Ammirati, E.; Catapano, A.L. Emerging role of high density lipoproteins as a player in the immune system. Atherosclerosis 2012, 220, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Madsen, C.M.; Varbo, A.; Nordestgaard, B.G. Low HDL Cholesterol and high risk of autoimmune disease: Two population-based cohort studies including 117341 individuals. Clin. Chem. 2019, 65, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Davidson, W.S.; Thompson, T.B. The structure of apolipoprotein A-I in high density lipoproteins. J. Biol. Chem. 2007, 282, 22249–22253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Javaheri, A.; Rader, D.J. Apolipoprotein A-I and cholesterol efflux: The good, the bad, and the modified. Circ. Res. 2014, 114, 1681–1683. [Google Scholar] [CrossRef] [Green Version]
- Sorci-Thomas, M.G.; Thomas, M.J. High density lipoprotein biogenesis, cholesterol efflux, and immune cell function. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2561–2565. [Google Scholar] [CrossRef] [Green Version]
- Fessler, M.B.; Parks, J.S. Intracellular Lipid Flux and Membrane Microdomains as Organizing Principles in Inflammatory Cell Signaling. J. Immunol. 2011, 187, 1529–1535. [Google Scholar] [CrossRef] [Green Version]
- Varshney, P.; Yadav, V.; Saini, N. Lipid rafts in immune signalling: Current progress and future perspective. Immunology 2016, 149, 13–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Yuan, S.; Peng, D.; Zhao, S. HDL and ApoA-I inhibit antigen presentation-mediated T cell activation by disrupting lipid rafts in antigen presenting cells. Atherosclerosis 2012, 225, 105–114. [Google Scholar] [CrossRef]
- Law, S.-H.; Chan, M.-L.; Marathe, G.K.; Parveen, F.; Chen, C.-H.; Ke, L.-Y. An Updated Review of Lysophosphatidylcholine Metabolism in Human Diseases. Int. J. Mol. Sci. 2019, 20, 1149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carneiro, A.B.; Iaciura, B.M.F.; Nohara, L.L.; Lopes, C.D.; Veas, E.M.C.; Mariano, V.S.; Bozza, P.T.; Lopes, U.G.; Atella, G.C.; Almeida, I.C.; et al. Lysophosphatidylcholine Triggers TLR2- and TLR4-Mediated Signaling Pathways but Counteracts LPS-Induced NO Synthesis in Peritoneal Macrophages by Inhibiting NF-κB Translocation and MAPK/ERK Phosphorylation. PLoS ONE 2013, 8, e76233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, L.S.; Modlin, R.L. Toll-like receptors in the skin. Semin. Immunopathol. 2007, 29, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Kawai, T. Toll-like receptor signaling pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.S.W.; Kauls, L.S.; Gaspari, A.A. Toll-like receptors: Applications to dermatologic disease. J. Am. Acad. Dermatol. 2006, 54, 951–983. [Google Scholar] [CrossRef]
- McInturff, J.E.; Modlin, R.L.; Kim, J. The role of toll-like receptors in the pathogenesis and treatment of dermatological disease. J. Investig. Dermatol. 2005, 125, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Trakaki, A.; Sturm, G.J.; Pregartner, G.; Scharnagl, H.; Eichmann, T.O.; Trieb, M.; Knuplez, E.; Holzer, M.; Stadler, J.T.; Heinemann, A.; et al. Allergic rhinitis is associated with complex alterations in high-density lipoprotein composition and function. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 1280–1292. [Google Scholar] [CrossRef]
- Holzer, M.; Wolf, P.; Curcic, S.; Birner-Gruenberger, R.; Weger, W.; Inzinger, M.; El-Gamal, D.; Wadsack, C.; Heinemann, A.; Marsche, G. Psoriasis alters HDL composition and cholesterol efflux capacity. J. Lipid Res. 2012, 53, 1618–1624. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Sheth, N.; Krishnamoorthy, P.; Saboury, B.; Raper, A.; Baer, A.; Ochotony, R.; Doveikis, J.; Derohannessian, S.; Van Voorhees, A.S.; et al. Aortic vascular inflammation in psoriasis is associated with HDL particle size and concentration: A pilot study. Am. J. Cardiovasc. Dis. 2012, 2, 285–292. [Google Scholar] [PubMed]
- Tom, W.L.; Playford, M.P.; Admani, S.; Natarajan, B.; Joshi, A.A.; Eichenfield, L.F.; Mehta, N.N. Characterization of lipoprotein composition and function in pediatric psoriasis reveals a more atherogenic profile. J. Investig. Dermatol. 2016, 136, 67–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holzer, M.; Wolf, P.; Inzinger, M.; Trieb, M.; Curcic, S.; Pasterk, L.; Weger, W.; Heinemann, A.; Marsche, G. Anti-psoriatic therapy recovers high-density lipoprotein composition and function. J. Investig. Dermatol. 2014, 134, 635–642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehta, N.N.; Li, R.; Krishnamoorthy, P.; Yu, Y.D.; Farver, W.; Rodrigues, A.; Raper, A.; Wilcox, M.; Baer, A.; DerOhannesian, S.; et al. Abnormal lipoprotein particles and cholesterol efflux capacity in patients with psoriasis. Atherosclerosis 2012, 224, 218–221. [Google Scholar] [CrossRef] [Green Version]
- Trieb, M.; Wolf, P.; Knuplez, E.; Weger, W.; Schuster, C.; Peinhaupt, M.; Holzer, M.; Trakaki, A.; Eichmann, T.; Lass, A.; et al. Abnormal composition and function of high-density lipoproteins in atopic dermatitis patients. Allergy Eur. J. Allergy Clin. Immunol. 2019, 74, 398. [Google Scholar] [CrossRef] [Green Version]
- Birjmohun, R.S.; Kees Hovingh, G.; Stroes, E.S.G.; Hofstra, J.J.; Dallinga-Thie, G.M.; Meijers, J.C.M.; Kastelein, J.J.P.; Levi, M. Effects of short-term and long-term danazol treatment on lipoproteins, coagulation, and progression of atherosclerosis: Two clinical trials in healthy volunteers and patients with hereditary angioedema. Clin. Ther. 2008, 30, 2314–2323. [Google Scholar] [CrossRef]
- Zannis, V.I.; Chroni, A.; Kypreos, K.E.; Kan, H.Y.; Cesar, T.B.; Zanni, E.E.; Kardassis, D. Probing the pathways of chylomicron and HDL metabolism using adenovirus-mediated gene transfer. Curr. Opin. Lipidol. 2004, 15, 151–166. [Google Scholar] [CrossRef]
- Zannis, V.I.; Cole, F.S.; Jackson, C.L.; Kurnit, D.M.; Karathanasis, S.K. Distribution of Apolipoprotein A-I, C-II, C-III, and E mRNA in Fetal Human Tissues. Time-Dependent Induction of Apolipoprotein E mRNA by Cultures of Human Monocyte-Macrophages. Biochemistry 1985, 24, 4450–4455. [Google Scholar] [CrossRef]
- Zannis, V.I.; Fotakis, P.; Koukos, G.; Kardassis, D.; Ehnholm, C.; Jauhiainen, M.; Chroni, A. Hdl biogenesis, remodeling, and catabolism. In High Density Lipoproteins; Von Eckardstein, A., Kardassis, D., Eds.; Handbook of Experimental Pharmacology; Springer New York LLC: New York, NY, USA, 2015; Volume 224, pp. 53–111. [Google Scholar]
- Mackness, M.I.; Durrington, P.N. HDL, its enzymes and its potential to influence lipid peroxidation. Atherosclerosis 1995, 115, 243–253. [Google Scholar] [CrossRef]
- Meilhac, O.; Tanaka, S.; Couret, D. High-density lipoproteins are bug scavengers. Biomolecules 2020, 10, 598. [Google Scholar] [CrossRef]
- Barter, P.J.; Brewer, H.B.; Chapman, M.J.; Hennekens, C.H.; Rader, D.J.; Tall, A.R. Cholesteryl Ester Transfer Protein. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhai, X.; Li, J.; Albers, J.J.; Vuletic, S.; Ren, G. Structural basis of the lipid transfer mechanism of phospholipid transfer protein (PLTP). Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2018, 1863, 1082–1094. [Google Scholar] [CrossRef] [PubMed]
- Albers, J.J.; Cheung, M.C. Emerging roles for phospholipid transfer protein in lipid and lipoprotein metabolism. Curr. Opin. Lipidol. 2004, 15, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Von Eckardstein, A.; Kardassis, D. High Density Lipoproteins: From Biological Understanding to Clinical Exploitation; Handbook of Experimental Pharmacology; Springer New York LLC: New York, NY, USA, 2015; Volume 224. [Google Scholar] [CrossRef]
- Lewis, G.F.; Rader, D.J. New insights into the regulation of HDL metabolism and reverse cholesterol transport. Circ. Res. 2005, 96, 1221–1232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yvan-Charvet, L.; Wang, N.; Tall, A.R. The role of HDL, ABCA1, and ABCG1 transporters in cholesterol efflux and immune responses. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 139–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maugeais, C.; Tietge, U.J.F.; Broedl, U.C.; Marchadier, D.; Cain, W.; McCoy, M.G.; Lund-Katz, S.; Glick, J.M.; Rader, D.J. Dose-Dependent Acceleration of High-Density Lipoprotein Catabolism by Endothelial Lipase. Circulation 2003, 108, 2121–2126. [Google Scholar] [CrossRef] [Green Version]
- Santamarina-Fojo, S.; González-Navarro, H.; Freeman, L.; Wagner, E.; Nong, Z. Hepatic lipase, lipoprotein metabolism, and atherogenesis. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1750–1754. [Google Scholar] [CrossRef]
- Acton, S.; Rigotti, A.; Landschulz, K.T.; Xu, S.; Hobbs, H.H.; Kriegert, M. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science (80-) 1996, 271, 518–520. [Google Scholar] [CrossRef]
- Krieger, M. Charting the Fate of the “Good Cholesterol”: Identification and Characterization of the High-Density Lipoprotein Receptor SR-BI. Annu. Rev. Biochem. 1999, 68, 523–558. [Google Scholar] [CrossRef]
- Pagler, T.A.; Rhode, S.; Neuhofer, A.; Laggner, H.; Strobl, W.; Hinterndorfer, C.; Volf, I.; Pavelka, M.; Eckhardt, E.R.M.; Van Der Westhuyzen, D.R.; et al. SR-BI-mediated high density lipoprotein (HDL) endocytosis leads to HDL resecretion facilitating cholesterol efflux. J. Biol. Chem. 2006, 281, 11193–11204. [Google Scholar] [CrossRef] [Green Version]
- Kontush, A.; Lindahl, M.; Lhomme, M.; Calabresi, L.; Chapman, M.J.; Davidson, W.S. Structure of HDL: Particle subclasses and molecular components. In Handbook of Experimental Pharmacology; Springer New York LLC: New York, NY, USA, 2015; Volume 224, pp. 3–51. [Google Scholar]
- Camont, L.; Chapman, M.J.; Kontush, A. Biological activities of HDL subpopulations and their relevance to cardiovascular disease. Trends Mol. Med. 2011, 17, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Vaisar, T. Proteomics investigations of HDL: Challenges and promise. Curr. Vasc. Pharmacol. 2012, 10, 410–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilkins, J.T.; Seckler, H.S. HDL modification: Recent developments and their relevance to atherosclerotic cardiovascular disease. Curr. Opin. Lipidol. 2019, 30, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Davidson, W.S.; Silva, R.A.G.D.; Chantepie, S.; Lagor, W.R.; Chapman, M.J.; Kontush, A. Proteomic analysis of defined hdl subpopulations reveals particle-specific protein clusters: Relevance to antioxidative function. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 870–876. [Google Scholar] [CrossRef]
- Holzer, M.; Kern, S.; Birner-Grünberger, R.; Curcic, S.; Heinemann, A.; Marsche, G. Refined purification strategy for reliable proteomic profiling of HDL 2/3: Impact on proteomic complexity. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef]
- Shao, B.; Heinecke, J.W. Quantifying HDL proteins by mass spectrometry: How many proteins are there and what are their functions? Expert Rev. Proteom. 2018, 15, 31–40. [Google Scholar] [CrossRef] [Green Version]
- Davidson, W.S. HDL Proteome Watch. The Davidson & Shah Lab Website. Available online: https://homepages.uc.edu/~davidswm/HDLproteome.html (accessed on 25 November 2020).
- Wiesner, P.; Leidl, K.; Boettcher, A.; Schmitz, G.; Liebisch, G. Lipid profiling of FPLC-separated lipoprotein fractions by electrospray ionization tandem mass spectrometry. J. Lipid Res. 2009, 50, 574–585. [Google Scholar] [CrossRef] [Green Version]
- Scherer, M.; Böttcher, A.; Liebisch, G. Lipid profiling of lipoproteins by electrospray ionization tandem mass spectrometry. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2011, 1811, 918–924. [Google Scholar] [CrossRef]
- Vuorela, T.; Catte, A.; Niemelä, P.S.; Hall, A.; Hyvönen, M.T.; Marrink, S.J.; Karttunen, M.; Vattulainen, I. Role of Lipids in Spheroidal High Density Lipoproteins. PLoS Comput. Biol. 2010, 6, e1000964. [Google Scholar] [CrossRef] [Green Version]
- Frame, N.M.; Gursky, O. Structure of serum amyloid A suggests a mechanism for selective lipoprotein binding and functions: SAA as a hub in macromolecular interaction networks. FEBS Lett. 2016, 590, 866–879. [Google Scholar] [CrossRef] [Green Version]
- Ferretti, G.; Bacchetti, T.; Moroni, C.; Savino, S.; Liuzzi, A.; Balzola, F.; Bicchiega, V. Paraoxonase Activity in High-Density Lipoproteins: A Comparison between Healthy and Obese Females. J. Clin. Endocrinol. Metab. 2005, 90, 1728–1733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stafforini, D.M.; McIntyre, T.M.; Carter, M.E.; Prescott, S.M. Human plasma platelet-activating factor acetylhydrolase. Association with lipoprotein particles and role in the degradation of platelet-activating factor. J. Biol. Chem. 1987, 262, 4215–4222. [Google Scholar] [PubMed]
- Snyder, F. Platelet-activating factor and its analogs: Metabolic pathways and related intracellular processes. Biochim. Biophys. Acta (BBA)/Lipids Lipid Metab. 1995, 1254, 231–249. [Google Scholar] [CrossRef]
- Stafforini, D.M.; McIntyre, T.M.; Zimmerman, G.A.; Prescott, S.M. Platelet-activating factor acetylhydrolases. J. Biol. Chem. 1997, 272, 17895–17898. [Google Scholar] [CrossRef] [Green Version]
- Chowaniec, Z.; Skoczyńska, A. Plasma lipid transfer proteins: The role of PLTP and CETP in atherogenesis. Adv. Clin. Exp. Med. 2018, 27, 429–436. [Google Scholar] [CrossRef]
- Von Eckardstein, A. Tachometer for Reverse Cholesterol Transport? J. Am. Heart Assoc. 2012, 1, e003723. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, A.B.; Zhang, Z. Allergic asthma: Influence of genetic and environmental factors. J. Biol. Chem. 2011, 286, 32883–32889. [Google Scholar] [CrossRef] [Green Version]
- Idani, E.; Raji, H.; Madadizadeh, F.; Cheraghian, B.; Haddadzadeh Shoshtari, M.; Dastoorpoor, M. Prevalence of asthma and other allergic conditions in adults in Khuzestan, southwest Iran, 2018. BMC Public Health 2019, 19, 303. [Google Scholar] [CrossRef]
- Locksley, R.M. Asthma and Allergic Inflammation. Cell 2010, 140, 777–783. [Google Scholar] [CrossRef] [Green Version]
- Quirt, J.; Hildebrand, K.J.; Mazza, J.; Noya, F.; Kim, H. Asthma. Allergy Asthma Clin. Immunol. 2018, 14, 50. [Google Scholar] [CrossRef]
- Fenger, R.V.; Gonzalez-Quintela, A.; Linneberg, A.; Husemoen, L.L.N.; Thuesen, B.H.; Aadahl, M.; Vidal, C.; Skaaby, T.; Sainz, J.C.; Calvo, E. The relationship of serum triglycerides, serum HDL, and obesity to the risk of wheezing in 85,555 adults. Respir. Med. 2013, 107, 816–824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schäfer, T.; Ruhdorfer, S.; Weigl, L.; Wessner, D.; Heinrich, J.; Döring, A.; Wichmann, H.E.; Ring, J. Intake of unsaturated fatty acids and HDL cholesterol levels are associated with manifestations of atopy in adults. Clin. Exp. Allergy 2003, 33, 1360–1367. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, D.; Jung, M.; Strizich, G.; Shaw, P.A.; Davis, S.M.; Klein, O.L.; Penedo, F.J.; Ries, A.L.; Daviglus, M.L.; Moreiras, J.J.; et al. Association of systemic inflammation, adiposity, and metabolic dysregulation with asthma burden among Hispanic adults. Respir. Med. 2017, 125, 72–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sobko, E.A.; Solovyeva, I.A.; Demko, I.V.; Kraposhina, A.Y.; Ishchenko, O.P.; Razzakova, N.M.; Egorov, S.A.; Vtyurina, S.S.; Prugova, V.L. Functional and laboratory characteristics in the concomitance of asthma and obesity at a young age. Ter. Arkh. 2016, 88, 40–46. [Google Scholar] [CrossRef]
- Shenoi, A.; Kumar, L.; Sarihyan, S.; Gangully, N. High density lipoprotein cholesterol and total cholesterol in children with asthma and allergic rhinitis. Acta Paediatr. 1992, 81, 150–152. [Google Scholar] [CrossRef]
- Fessler, M.B.; Massing, M.W.; Spruell, B.; Jaramillo, R.; Draper, D.W.; Madenspacher, J.H.; Arbes, S.J.; Calatroni, A.; Zeldin, D.C. Novel relationship of serum cholesterol with asthma and wheeze in the United States. J. Allergy Clin. Immunol. 2009, 124, 967. [Google Scholar] [CrossRef] [Green Version]
- Enright, P.L.; Ward, B.J.; Tracy, R.P.; Lasser, E.C. Asthma and its association with cardiovascular disease in the elderly. J. Asthma 1996, 33, 45–53. [Google Scholar] [CrossRef]
- Park, S.; Choi, N.K.; Kim, S.; Lee, C.H. The relationship between metabolic syndrome and asthma in the elderly. Sci. Rep. 2018, 8, 9378. [Google Scholar] [CrossRef]
- Picado, C.; Deulofeu, R.; Lleonart, R.; Agustí, M.; Casals, E.; Quintó, L.; Mullol, J. Lipid and protein metabolism in asthma. Effects of diet and corticosteroid therapy. Allergy Eur. J. Allergy Clin. Immunol. 1999, 54, 569–575. [Google Scholar] [CrossRef]
- Erel, F.; Gulec, M.; Kartal, O.; Caliskaner, Z.; Ozturk, S.; Yaman, H.; Kurt, Y.; Gocgeldi, E.; Ors, F.; Karaayvaz, M. Serum Leptin Levels and Lipid Profiles in Patients with Allergic Rhinitis and Mild Asthma. Allergol. Immunopathol. (Madr.) 2007, 35, 232–238. [Google Scholar] [CrossRef]
- Lu, M.; Wu, B.; Qiao, R.; Gu, H.; Din, Y.; Dong, X. No associations between serum lipid levels or HOMA-IR and asthma in children and adolescents: A NHANES analysis. JCRPE J. Clin. Res. Pediatr. Endocrinol. 2019, 11, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.J.; Huang, C.S.; Liu, Y.C.; Su, Y.M.; Wan, K.S. The lipid profile in obese asthmatic children compared to non-obese asthmatic children. Allergol. Immunopathol. (Madr.) 2016, 44, 346–350. [Google Scholar] [CrossRef] [PubMed]
- Cottrell, L.; Neal, W.A.; Ice, C.; Perez, M.K.; Piedimonte, G. Metabolic abnormalities in children with asthma. Am. J. Respir. Crit. Care Med. 2011, 183, 441–448. [Google Scholar] [CrossRef] [Green Version]
- Yiallouros, P.K.; Savva, S.C.; Kolokotroni, O.; Dima, K.; Zerva, A.; Kouis, P.; Bousquet, J.; Middleton, N. Asthma: The Role of Low High-Density-Lipoprotein Cholesterol in Childhood and Adolescence. Int. Arch. Allergy Immunol. 2014, 165, 91–99. [Google Scholar] [CrossRef]
- Yiallouros, P.K.; Savva, S.C.; Kolokotroni, O.; Behbod, B.; Zeniou, M.; Economou, M.; Chadjigeorgiou, C.; Kourides, Y.A.; Tornaritis, M.J.; Lamnisos, D.; et al. Low serum high-density lipoprotein cholesterol in childhood is associated with adolescent asthma. Clin. Exp. Allergy 2012, 42, 423–432. [Google Scholar] [CrossRef]
- Cakmak, A.; Zeyrek, D.; Atas, A.; Selek, S.; Erel, O. Oxidative status and paraoxonase activity in children with asthma. Clin. Investig. Med. 2009, 32, E327–E334. [Google Scholar] [CrossRef] [Green Version]
- Gulcan, E.; Bulut, I.; Toker, A.; Gulcan, A. Evaluation of glucose tolerance status in patients with asthma bronchiale. J. Asthma 2009, 46, 207–209. [Google Scholar] [CrossRef]
- Scichilone, N.; Rizzo, M.; Benfante, A.; Catania, R.; Giglio, R.V.; Nikolic, D.; Montalto, G.; Bellia, V. Serum low density lipoprotein subclasses in asthma. Respir. Med. 2013, 107, 1866–1872. [Google Scholar] [CrossRef] [Green Version]
- Ekmekci, O.B.; Donma, O.; Ekmekci, H.; Yildirim, N.; Uysal, O.; Sardogan, E.; Demirel, H.; Demir, T. Plasma paraoxonase activities, lipoprotein oxidation, and trace element interaction in asthmatic patients. Biol. Trace Elem. Res. 2006, 111, 41–52. [Google Scholar] [CrossRef]
- Dominic, J.C.; Yuri Agrawal, P.A.C. Lipids and Pulmonary Function in the Third National Health and Nutrition Examination Survey. Am. J. Epidemiol. 2002, 155, 842–848. [Google Scholar] [CrossRef] [Green Version]
- Peng, J.; Huang, Y. Meta-analysis of the association between asthma and serum levels of high-density lipoprotein cholesterol and low-density lipoprotein cholesterol. Ann. Allergy Asthma Immunol. 2017, 118, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Barochia, A.V.; Kaler, M.; Cuento, R.A.; Gordon, E.M.; Weir, N.A.; Sampson, M.; Fontana, J.R.; MacDonald, S.; Moss, J.; Manganiello, V.; et al. Serum Apolipoprotein A-I and large high-density lipoprotein particles are positively correlated with fev1 in atopic asthma. Am. J. Respir. Crit. Care Med. 2015, 191, 990–1000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barochia, A.V.; Kaler, M.; Cuento, R.; Gordon, E.M.; Theard, P.; Figueroa, D.; Weir, N.; Sampson, M.; Remaley, A.T. Serum High Density Lipoprotein (hdl) Cholesterol And Large Hdl Particles Are Negatively Correlated With Blood Eosinophils In Atopic Asthma. Am. J. Respir. Crit. Care Med. 2016, 193, A1451. [Google Scholar] [CrossRef]
- Barochia, A.V.; Gordon, E.M.; Kaler, M.; Cuento, R.A.; Theard, P.; Figueroa, D.M.; Yao, X.; Weir, N.A.; Sampson, M.L.; Stylianou, M.; et al. High density lipoproteins and type 2 inflammatory biomarkers are negatively correlated in atopic asthmatics. J. Lipid Res. 2017, 58, 1713–1721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rastogi, D.; Fraser, S.; Oh, J.; Huber, A.M.; Schulman, Y.; Bhagtani, R.H.; Khan, Z.S.; Tesfa, L.; Hall, C.B.; Macian, F. Inflammation, metabolic dysregulation, and pulmonary function among obese urban adolescents with asthma. Am. J. Respir. Crit. Care Med. 2015, 191, 149–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouyang, F.; Kumar, R.; Pongracic, J.; Story, R.E.; Liu, X.; Wang, B.; Xing, H.; Liu, X.; Li, Z.; Zhang, W.; et al. Adiposity, serum lipid levels, and allergic sensitization in Chinese men and women. J. Allergy Clin. Immunol. 2009, 123, 940–948.e10. [Google Scholar] [CrossRef] [Green Version]
- Su, X.; Ren, Y.; Li, M.; Zhao, X.; Kong, L.; Kang, J. Association between lipid profile and the prevalence of asthma: A meta-analysis and systemic review. Curr. Med. Res. Opin. 2018, 34, 423–433. [Google Scholar] [CrossRef]
- Rasmussen, F.; Hancox, R.J.; Nair, P.; Hansen, H.S.; Siersted, H.C.; Nybo, M. Associations between airway hyperresponsiveness, obesity and lipoproteins in a longitudinal cohort. Clin. Respir. J. 2013, 7, 268–275. [Google Scholar] [CrossRef]
- Park, J.H.; Mun, S.; Choi, D.P.; Lee, J.Y.; Kim, H.C. Association between high-density lipoprotein cholesterol level and pulmonary function in healthy Korean adolescents: The JS high school study. BMC Pulm. Med. 2017, 17, 190. [Google Scholar] [CrossRef] [Green Version]
- Park, S.W.; Lee, E.H.; Lee, E.J.; Kim, H.J.; Bae, D.J.; Han, S.; Kim, D.; Jang, A.S.; Uh, S.T.; Kim, Y.H.; et al. Apolipoprotein A1 potentiates lipoxin A4 synthesis and recovery of allergen-induced disrupted tight junctions in the airway epithelium. Clin. Exp. Allergy 2013, 43, 914–927. [Google Scholar] [CrossRef]
- Yao, X.; Gordon, E.M.; Barochia, A.V.; Remaley, A.T.; Levine, S.J. The A’s Have It: Developing Apolipoprotein A-I Mimetic Peptides Into a Novel Treatment for Asthma. Chest 2016, 150, 283–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thorne, P.S.; Mendy, A.; Metwali, N.; Salo, P.; Co, C.; Jaramillo, R.; Rose, K.M.; Zeldin, D.C. Endotoxin exposure: Predictors and prevalence of associated asthma outcomes in the United States. Am. J. Respir. Crit. Care Med. 2015, 192, 1287–1297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thorne, P.S.; Kulhánková, K.; Yin, M.; Cohn, R.; Arbes, S.J.; Zeldin, D.C. Endotoxin exposure is a risk factor for asthma: The national survey of endotoxin in United States housing. Am. J. Respir. Crit. Care Med. 2005, 172, 1371–1377. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.D.; Lim, H.Y.; Lee, H.G.; Yoon, D.Y.; Choe, Y.K.; Choi, I.; Paik, S.G.; Kim, Y.S.; Yang, Y.; Lim, J.S. Apolipoprotein A-I induces IL-10 and PGE2 production in human monocytes and inhibits dendritic cell differentiation and maturation. Biochem. Biophys. Res. Commun. 2005, 338, 1126–1136. [Google Scholar] [CrossRef] [PubMed]
- Tiniakou, I.; Drakos, E.; Sinatkas, V.; Van Eck, M.; Zannis, V.I.; Boumpas, D.; Verginis, P.; Kardassis, D. High-Density Lipoprotein Attenuates Th1 and Th17 Autoimmune Responses by Modulating Dendritic Cell Maturation and Function. J. Immunol. 2015, 194, 4676–4687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicholas, B.L.; Skipp, P.; Barton, S.; Singh, D.; Bagmane, D.; Mould, R.; Angco, G.; Ward, J.; Guha-Niyogi, B.; Wilson, S.; et al. Identification of lipocalin and apolipoprotein A1 as biomarkers of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2010, 181, 1049–1060. [Google Scholar] [CrossRef] [Green Version]
- Kuczia, P.; Mastalerz, L.; Potaczek, D.P.; Cybulska, A.; Zareba, L.; Bazan-Socha, S.; Undas, A. Increased activity of lipoprotein-associated phospholipase A2 in non-severe asthma. Allergol. Int. 2019, 68, 450–455. [Google Scholar] [CrossRef]
- Grandel, K.E.; Wardlow, M.L.; Farr, R.S. Platelet activating factor in sputum of patients with asthma and COPD. J. Allergy Clin. Immunol. 1985, 75, 184. [Google Scholar] [CrossRef]
- Stenton, S.C.; Court, E.N.; Kingston, W.P.; Goadby, P.; Kelly, C.A.; Duddridge, M.; Ward, C.; Hendrick, D.J.; Walters, E.H. Platelet-activating factor in bronchoalveolar lavage fluid from asthmatic subjects. Eur. Respir. J. 1990, 3, 408–413. [Google Scholar]
- Kurosawa, M.; Yamashita, T.; Kurimoto, F. Increased levels of blood platelet-activating factor in bronchial asthmatic patients with active symptoms. Allergy 1994, 49, 60–63. [Google Scholar] [CrossRef]
- Tsukioka, K.; Matsuzaki, M.; Nakamata, M.; Kayahara, H. Increased Plasma Level of Platelet-Activating Factor (PAF) and Decreased Serum PAF Acetylhydrolase (PAFAH) Activity in Adults With Bronchial Asthma. J. Investig. Allergol. Clin. Immunol. 1996, 6, 22–29. [Google Scholar]
- Hsieh, K.H.; Ng, C.K. Increased plasma platelet-activating factor in children with acute asthmatic attacks and decreased in vivo and in vitro production of platelet-activating factor after immunotherapy. J. Allergy Clin. Immunol. 1993, 91, 650–657. [Google Scholar] [CrossRef]
- Chan-Yeung, M.; Lam, S.; Chan, H.; Tse, K.S.; Salari, H. The release of platelet-activating factor into plasma during allergen-induced bronchoconstriction. J. Allergy Clin. Immunol. 1991, 87, 667–673. [Google Scholar] [CrossRef]
- Shirasaki, H.; Nishikawa, M.; Adcock, I.M.; Mak, J.C.; Sakamoto, T.; Shimizu, T.; Barnes, P.J. Expression of platelet-activating factor receptor mRNA in human and guinea pig lung. Am. J. Respir. Cell Mol. Biol. 1994, 10, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xu, H.; Shi, Y.; Nandedkar, S.; Zhang, H.; Gao, H.; Feroah, T.; Weihrauch, D.; Schulte, M.L.; Jones, D.W.; et al. Genetic deletion of apolipoprotein A-I increases airway hyperresponsiveness, inflammation, and collagen deposition in the lung. J. Lipid Res. 2010, 51, 2560–2570. [Google Scholar] [CrossRef] [Green Version]
- Yao, X.; Gordon, E.M.; Figueroa, D.M.; Barochia, A.V.; Levine, S.J. Emerging roles of apolipoprotein e and apolipoprotein A-I in the pathogenesis and treatment of lung disease. Am. J. Respir. Cell Mol. Biol. 2016, 55, 159–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, C.; Yao, X.; Keeran, K.J.; Zywicke, G.J.; Qu, X.; Yu, Z.X.; Dagur, P.K.; McCoy, J.P.; Remaley, A.T.; Levine, S.J. Apolipoprotein A-I attenuates ovalbumin-induced neutrophilic airway inflammation via a granulocyte colony-stimulating factor-dependent mechanism. Am. J. Respir. Cell Mol. Biol. 2012, 47, 186–195. [Google Scholar] [CrossRef] [Green Version]
- Yao, X.; Vitek, M.P.; Remaley, A.T.; Levine, S.J. Apolipoprotein mimetic peptides: A new approach for the treatment of asthma. Front. Pharmacol. 2012, 3, 37. [Google Scholar] [CrossRef] [Green Version]
- Nandedkar, S.D.; Weihrauch, D.; Xu, H.; Shi, Y.; Feroah, T.; Hutchins, W.; Rickaby, D.A.; Duzgunes, N.; Hillery, C.A.; Konduri, K.S.; et al. D-4F, an apoA-1 mimetic, decreases airway hyperresponsiveness, inflammation, and oxidative stress in a murine model of asthma. J. Lipid Res. 2011, 52, 499–508. [Google Scholar] [CrossRef] [Green Version]
- Eifan, A.O.; Durham, S.R. Pathogenesis of rhinitis. Clin. Exp. Allergy 2016, 46, 1139–1151. [Google Scholar] [CrossRef]
- Small, P.; Keith, P.K.; Kim, H. Allergic rhinitis. Allergy Asthma Clin. Immunol. 2018, 14, 51. [Google Scholar] [CrossRef] [Green Version]
- Vinding, R.K.; Stokholm, J.; Chawes, B.L.K.; Bisgaard, H. Blood lipid levels associate with childhood asthma, airway obstruction, bronchial hyperresponsiveness, and aeroallergen sensitization. J. Allergy Clin. Immunol. 2016, 137, 68–74.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomazic, P.V.; Birner-Gruenberger, R.; Leitner, A.; Obrist, B.; Spoerk, S.; Lang-Loidolt, D. Nasal mucus proteomic changes reflect altered immune responses and epithelial permeability in patients with allergic rhinitis. J. Allergy Clin. Immunol. 2014, 133, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Tomazic, P.V.; Birner-Gruenberger, R.; Leitner, A.; Darnhofer, B.; Spoerk, S.; Lang-Loidolt, D. Apolipoproteins have a potential role in nasal mucus of allergic rhinitis patients: A proteomic study. Laryngoscope 2015, 125, E91–E96. [Google Scholar] [CrossRef] [PubMed]
- Tomazic, P.; Birner-Grünberger, R.; Britta, O.; Spörk, S.; Leitner, A.; Lang-Loidolt, D. The (potential) role of apolipoproteins in nasal mucus of allergic rhinitis patients. Clin. Transl. Allergy 2014, 4, P19. [Google Scholar] [CrossRef] [Green Version]
- Kåredal, M.H.; Mortstedt, H.; Jeppsson, M.C.; Kronholm Diab, K.; Nielsen, J.; Jonsson, B.A.G.; Lindh, C.H. Time-dependent proteomic iTRAQ analysis of nasal lavage of hairdressers challenged by persulfate. J. Proteome Res. 2010, 9, 5620–5628. [Google Scholar] [CrossRef]
- Jaramillo, R.; Cohn, R.D.; Crockett, P.W.; Gowdy, K.M.; Zeldin, D.C.; Fessler, M.B. Relation between objective measures of atopy and myocardial infarction in the United States. J. Allergy Clin. Immunol. 2013, 131, 405–411.e11. [Google Scholar] [CrossRef] [Green Version]
- Trasande, L.; Fiorino, E.K.; Attina, T.; Berger, K.; Goldring, R.; Chemtob, C.; Levy-Carrick, N.; Shao, Y.; Liu, M.; Urbina, E.; et al. Associations of World Trade Center exposures with pulmonary and cardiometabolic outcomes among children seeking care for health concerns. Sci. Total Environ. 2013, 444, 320–326. [Google Scholar] [CrossRef] [Green Version]
- Lee, E.J.; Hwang, H.J.; Jung, C.M.; Kim, M.K.; Kang, M.S.; Kim, K.S. The relationship between chronic rhinosinusitis and metabolic syndrome. Am. J. Rhinol. Allergy 2017, 31, 222–227. [Google Scholar] [CrossRef]
- Roula, D.; Theiler, A.; Luschnig, P.; Sturm, G.J.; Tomazic, P.V.; Marsche, G.; Heinemann, A.; Sturm, E.M. Apolipoprotein A-IV acts as an endogenous anti-inflammatory protein and is reduced in treatment-naïve allergic patients and allergen-challenged mice. Allergy 2020, 75, 392–402. [Google Scholar] [CrossRef] [Green Version]
- Makino, Y.; Noguchi, E.; Takahashi, N.; Matsumoto, Y.; Kubo, S.; Yamada, T.; Imoto, Y.; Ito, Y.; Osawa, Y.; Shibasaki, M.; et al. Apolipoprotein A-IV is a candidate target molecule for the treatment of seasonal allergic rhinitis. J. Allergy Clin. Immunol. 2010, 126, 1163–1169.e5. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.W.; Cha, J.; Han, S.; Chen, Y.; Gucek, M.; Cho, H.J.; Nakahira, K.; Choi, A.M.K.; Ryu, J.H.; Yoon, J.H. Apolipoprotein e and periostin are potential biomarkers of nasal mucosal inflammation a parallel approach of in vitro and in vivo secretomes. Am. J. Respir. Cell Mol. Biol. 2020, 62, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Dykewicz, M.S.; Hamilos, D.L. Rhinitis and sinusitis. J. Allergy Clin. Immunol. 2010, 125, S103–S115. [Google Scholar] [CrossRef] [PubMed]
- Upton, D.C.; Welham, N.V.; Kuo, J.S.; Walker, J.W.; Pasic, T.R. Chronic rhinosinusitis with nasal polyps: A proteomic analysis. Ann. Otol. Rhinol. Laryngol. 2011, 120, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Ozkaya, E.; Akduman, H.; Erenberk, U.; Demir, A.; Dundaroz, M.R. Plasma paraoxonase activity and oxidative stress and their relationship to disease severity in children with allergic rhinitis. Am. J. Rhinol. Allergy 2013, 27, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Fonacier, L.S.; Dreskin, S.C.; Leung, D.Y.M.; Mineola, C.; Denver, A. Allergic skin diseases. J Allergy Clin Immunol. 2010, 125, S138–S149. [Google Scholar] [CrossRef]
- Williams, H.; Robertson, C.; Stewart, A.; Aït-Khaled, N.; Anabwani, G.; Anderson, R.; Asher, I.; Beasley, R.; Björkstén, B.; Burr, M.; et al. Worldwide variations in the prevalence of symptoms of atopic eczema in the international study of asthma and allergies in childhood. J. Allergy Clin. Immunol. 1999, 103, 125–138. [Google Scholar] [CrossRef]
- Bieber, T. Atopic dermatitis 2.0: From the clinical phenotype to the molecular taxonomy and stratified medicine. Allergy Eur. J. Allergy Clin. Immunol. 2012, 67, 1475–1482. [Google Scholar] [CrossRef] [Green Version]
- Garmhausen, D.; Hagemann, T.; Bieber, T.; Dimitriou, I.; Fimmers, R.; Diepgen, T.; Novak, N. Characterization of different courses of atopic dermatitis in adolescent and adult patients. Allergy Eur. J. Allergy Clin. Immunol. 2013, 68, 498–506. [Google Scholar] [CrossRef]
- Wu, L.C.; Hwang, C.Y.; Chung, P.I.; Hua, T.C.; Da Chen, Y.; Chu, S.Y.; Lee, D.D.; Chang, Y.T.; Wang, W.J.; Liu, H.N.; et al. Autoimmune disease comorbidities in patients with atopic dermatitis: A nationwide case-control study in Taiwan. Pediatr. Allergy Immunol. 2014, 25, 586–592. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Greenland, P. Eczema and cardiovascular risk factors in 2 US adult population studies. J. Allergy Clin. Immunol. 2015, 135, 721–728.e6. [Google Scholar] [CrossRef] [PubMed]
- Andersen, Y.M.F.; Egeberg, A.; Gislason, G.H.; Hansen, P.R.; Skov, L.; Thyssen, J.P. Risk of myocardial infarction, ischemic stroke, and cardiovascular death in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2016, 138, 310–312.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, V.Y.F.; Chen, T.J.; Yeh, C.M.; Chou, K.T.; Hung, M.H.; Chu, S.Y.; Su, K.C.; Chang, Y.S.; Lin, Y.H.; Liu, C.J. Atopic dermatitis and risk of ischemic stroke: A nationwide population-based study. Ann. Med. 2014, 46, 84–89. [Google Scholar] [CrossRef]
- Silverberg, J.I. Association between adult atopic dermatitis, cardiovascular disease, and increased heart attacks in three population-based studies. Allergy Eur. J. Allergy Clin. Immunol. 2015, 70, 1300–1308. [Google Scholar] [CrossRef] [PubMed]
- Standl, M.; Tesch, F.; Baurecht, H.; Rodríguez, E.; Müller-Nurasyid, M.; Gieger, C.; Peters, A.; Wang-Sattler, R.; Prehn, C.; Adamski, J.; et al. Association of Atopic Dermatitis with Cardiovascular Risk Factors and Diseases. J. Investig. Dermatol. 2017, 137, 1074–1081. [Google Scholar] [CrossRef]
- Agón-Banzo, P.J.; Sanmartin, R.; García-Malinis, A.J.; Hernández-Martín, Á.; Puzo, J.; Doste, D.; Pardos, C.; Gilaberte, Y. Body mass index and serum lipid profile: Association with atopic dermatitis in a paediatric population. Australas. J. Dermatol. 2020, 61, e60–e64. [Google Scholar] [CrossRef]
- Yamane, Y.; Moriyama, K.; Yasuda, C.; Miyata, S.; Aihara, M.; Ikezawa, Z.; Miyazaki, K. New horny layer marker proteins for evaluating skin condition in atopic dermatitis. Int. Arch. Allergy Immunol. 2009, 150, 89–101. [Google Scholar] [CrossRef]
- Liu, F.T.; Goodarzi, H.; Chen, H.Y. IgE, mast cells, and eosinophils in atopic dermatitis. Clin. Rev. Allergy Immunol. 2011, 41, 298–310. [Google Scholar] [CrossRef]
- Azfar, R.S.; Gelfand, J.M. Psoriasis and metabolic disease: Epidemiology and pathophysiology. Curr. Opin. Rheumatol. 2008, 20, 416–422. [Google Scholar] [CrossRef]
- Boehncke, W.H. Systemic inflammation and cardiovascular comorbidity in psoriasis patients: Causes and consequences. Front. Immunol. 2018, 9, 579. [Google Scholar] [CrossRef]
- Boehncke, W.H.; Schön, M.P. Psoriasis. Lancet 2015, 386, 983–994. [Google Scholar] [CrossRef]
- Lande, R.; Gregorio, J.; Facchinetti, V.; Chatterjee, B.; Wang, Y.H.; Homey, B.; Cao, W.; Wang, Y.H.; Su, B.; Nestle, F.O.; et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature 2007, 449, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Zaba, L.C.; Fuentes-Duculan, J.; Eungdamrong, N.J.; Abello, M.V.; Novitskaya, I.; Pierson, K.C.; Gonzalez, J.; Krueger, J.G.; Lowes, M.A. Psoriasis is characterized by accumulation of immunostimulatory and Th1/Th17 cell-polarizing myeloid dendritic cells. J. Investig. Dermatol. 2009, 129, 79–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lowes, M.A.; Russell, C.B.; Martin, D.A.; Towne, J.E.; Krueger, J.G. The IL-23/T17 pathogenic axis in psoriasis is amplified by keratinocyte responses. Trends Immunol. 2013, 34, 174–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferretti, G.; Alleva, R.; Taus, M.; Simonetti, O.; Cinti, B.; Offidani, A.M.; Bossi, G.; Curatola, G. Abnormalities of plasma lipoprotein composition and fluidity in psoriasis. Acta Derm. Venereol. 1994, 74, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, G.; Simonetti, O.; Offidani, A.M.; Messini, L.; Cinti, B.; Marshiseppe, I.; Bossi, G.; Curatola, G. Changes of plasma lipids and erythrocyte membrane fluidity in psoriatic children. Pediatr. Res. 1993, 33, 506–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tam, L.-S.; Tomlinson, B.; Chu, T.T.-W.; Li, M.; Leung, Y.-Y.; Kwok, L.-W.; Li, T.K.; Yu, T.; Zhu, Y.-E.; Wong, K.-C.; et al. Cardiovascular risk profile of patients with psoriatic arthritis compared to controls—the role of inflammation. Rheumatology 2008, 47, 718–723. [Google Scholar] [CrossRef] [Green Version]
- Mallbris, L.; Granath, F.; Hamsten, A.; Ståhle, M. Psoriasis is associated with lipid abnormalities at the onset of skin disease. J. Am. Acad. Dermatol. 2006, 54, 614–621. [Google Scholar] [CrossRef]
- Borska, L.; Kremlacek, J.; Andrys, C.; Krejsek, J.; Hamakova, K.; Borsky, P.; Palicka, V.; Rehacek, V.; Malkova, A.; Fiala, Z. Systemic inflammation, oxidative damage to nucleic acids, and metabolic syndrome in the pathogenesis of psoriasis. Int. J. Mol. Sci. 2017, 18, 2238. [Google Scholar] [CrossRef] [Green Version]
- Nakhwa, Y.C.; Rashmi, R.; Basavaraj, K.H. Dyslipidemia in Psoriasis: A Case Controlled Study. Int. Sch. Res. Not. 2014, 2014. [Google Scholar] [CrossRef] [Green Version]
- Miao, C.; Li, J.; Li, Y.; Zhang, X. Obesity and dyslipidemia in patients with psoriasis: A case-control study. Medicine (Baltimore) 2019, 98, e16323. [Google Scholar] [CrossRef] [PubMed]
- Ferdinando, L.B.; Fukumoto, P.K.; Sanches, S.; Fabricio, L.H.Z.; Skare, T.L. Metabolic syndrome and psoriasis: A study in 97 patients. Rev. Assoc. Med. Bras. 2018, 64, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Pietrzak, A.; Chabros, P.; Grywalska, E.; Kiciński, P.; Franciszkiewicz-Pietrzak, K.; Krasowska, D.; Kandzierski, G. Serum lipid metabolism in psoriasis and psoriatic arthritis—An update. Arch. Med. Sci. 2019, 15, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Pietrzak, A.; Kadzielewski, J.; Janowski, K.; Roliński, J.; Krasowska, D.; Chodorowska, G.; Paszkowski, T.; Kapeć, E.; Jastrzbska, I.; Tabarkiewicz, J.; et al. Lipoprotein (a) in patients with psoriasis: Associations with lipid profiles and disease severity. Int. J. Dermatol. 2009, 48, 379–387. [Google Scholar] [CrossRef]
- Pietrzak, A.; Grywalska, E.; Walankiewicz, M.; Lotti, T.; Roliński, J.; Myśliński, W.; Chabros, P.; Piekarska-Myślińska, D.; Reich, K. Psoriasis and metabolic syndrome in children: Current data. Clin. Exp. Dermatol. 2017, 42, 131–136. [Google Scholar] [CrossRef]
- Sarvtin, M.T.; Hedayati, M.T.; Tahereh Shokohi, Z.H. Serum Lipids and Lipoproteins in Patients With Psoriasis. Arch. Iran Med. 2014, 17, 343–346. [Google Scholar]
- Khan, S.; Agrawal, S.; Baral, N.; Lamsal, M. Evaluation of ADA activity as a potential marker of disease severity in psoriasis patients. Psoriasis Targets Ther. 2018, 8, 59–63. [Google Scholar] [CrossRef] [Green Version]
- Sabry, H.H.; Sabry, J.H.; Daifalla, A.E.H.; Akl, E.M.; Hamed, A.M.; Torky, A.A.A. Serum markers for asymptomatic atherosclerosis in Egyptian psoriatic patients: Study controlled by doppler estimation of carotid intima-media thickness. Vasc. Health Risk Manag. 2018, 14, 145–152. [Google Scholar] [CrossRef] [Green Version]
- Cao, L.Y.; Soler, D.C.; Debanne, S.M.; Grozdev, I.; Rodriguez, M.E.; Feig, R.L.; Carman, T.L.; Gilkeson, R.C.; Orringer, C.E.; Kern, E.F.; et al. Psoriasis and Cardiovascular Risk Factors: Increased Serum Myeloperoxidase and Corresponding Immunocellular Overexpression by Cd11b(+) CD68(+) Macrophages in Skin Lesions. Am. J. Transl. Res. 2014, 6, 16–27. [Google Scholar]
- Komorowska, O.; Bohdan, M.; Szczerkowska-Dobosz, A.; Rawicz-Zegrzda, D.; Dudziak, M.; Zdrojewski, T.; Gruchala, M.; Dorota Purzycka-Bohdan, R.N. Assessment of Cardiovascular Risk Factors in Patients With Psoriasis. Acta Dermatovenerol. Croat. 2016, 24, 261–267. [Google Scholar]
- El Asmi, M.A.; Zidi, W.; Mebazaa, A.; Zayani, Y.; Ayadi, I.; Feki, M.; Osman, A.B.; Kaabachi, N. Serum lipid level in tunisian patients with psoriasis. Clin. Lab. 2014, 60, 1043–1047. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Lin, K.; Liu, W.; Zhang, S.Z. Characterization of the Abnormal Lipid Profile in Chinese Patients With Psoriasis. Int. J. Clin. Exp. Pathol. 2015, 8, 15280–15284. [Google Scholar] [PubMed]
- Akkara Veetil, B.M.; Matteson, E.L.; Maradit-Kremers, H.; McEvoy, M.T.; Crowson, C.S. Trends in lipid profiles in patients with psoriasis: A population-based analysis. BMC Dermatol. 2012, 12, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coimbra, S.; Oliveira, H.; Reis, F.; Belo, L.; Rocha, S.; Quintanilha, A.; Figueiredo, A.; Teixeira, F.; Castro, E.; Rocha-Pereira, P.; et al. Psoriasis therapy and cardiovascular risk factors: A 12-week follow-up study. Am. J. Clin. Dermatol. 2010, 11, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Yuksel, E.P.; Yuksel, S.; Yenercag, M.; Soylu, K.; Aydin, F.; Senturk, N.; Yucel, H.; Canturk, T.; Turanli, A.Y. Impaired heart rate recovery indices in psoriasis patients. Med. Sci. Monit. 2014, 20, 350–354. [Google Scholar] [CrossRef] [Green Version]
- Mebazaa, A.; El Asmi, M.; Zidi, W.; Zayani, Y.; Cheikh Rouhou, R.; El Ounifi, S.; Kanoun, F.; Mokni, M.; Osman, A.B.; Feki, M.; et al. Metabolic syndrome in Tunisian psoriatic patients: Prevalence and determinants. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 705–709. [Google Scholar] [CrossRef]
- Sirin, M.C.; Korkmaz, S.; Erturan, I.; Filiz, B.; Aridogan, B.C.; Cetin, E.S.; Yildirim, M. Evaluation of monocyte to HDL cholesterol ratio and other inflammatory markers in patients with psoriasis. An. Bras. Dermatol. 2020, 95, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, S.; Mandal, S.; Singh, K.G.; Prajapati, R. Metabolic Diseases and Associated Complications in Patients with Psoriasis. J. Assoc. Physicians India 2020, 68, 44–46. [Google Scholar]
- Rocha-Pereira, P.; Santos-Silva, A.; Rebelo, I.; Figueiredo, A.; Quintanilha, A.; Teixeira, F. Dislipidemia and oxidative stress in mild and in severe psoriasis as a risk for cardiovascular disease. Clin. Chim. Acta 2001, 303, 33–39. [Google Scholar] [CrossRef] [Green Version]
- Tekin, N.S.; Tekin, I.O.; Barut, F.; Sipahi, E.Y. Accumulation of oxidized low-density lipoprotein in psoriatic skin and changes of plasma lipid levels in psoriatic patients. Mediat. Inflamm. 2007, 2007, 78454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, L.; Qin, S.; Dang, L.; Song, G.; Yao, S.; Yang, N.; Li, Y. Psoriasis decreases the anti-oxidation and anti-inflammation properties of high-density lipoprotein. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2014, 1841, 1709–1715. [Google Scholar] [CrossRef] [PubMed]
- Usta, M.; Turan, E.; Aral, H.; Inal, B.B.; Gurel, M.S.; Guvenen, G. Serum paraoxonase-1 activities and oxidative status in patients with plaque-type psoriasis with/without metabolic syndrome. J. Clin. Lab. Anal. 2011, 25, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Reynoso-von Drateln, C.; Martínez-Abundis, E.; Balcázar-Muñoz, B.R.; Bustos-Saldaña, R.; González-Ortiz, M. Lipid profile, insulin secretion, and insulin sensitivity in psoriasis. J. Am. Acad. Dermatol. 2003, 48, 882–885. [Google Scholar] [CrossRef]
- Thungaturthi, S.; Vadakedath, S.; Pavuluri, P.; Rani, J.; Gundu, R.; Bheem, J.; Kandi, V. Atherogenesis in Psoriasis: Evaluation of the Serum Activities of Non-high-density Lipoprotein Cholesterol and Other Lipids Among Newly Diagnosed Psoriasis Patients. Cureus 2019, 11, e4203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uyanik, B.S.; Ari, Z.; Onur, E.; Gündüz, K.; Tanülkü, S.; Durkan, K. Serum lipids and apolipoproteins in patients with psoriasis. Clin. Chem. Lab. Med. 2002, 40, 65–68. [Google Scholar] [CrossRef]
- Shreberk-Hassidim, R.; Galili, E.; Hassidim, A.; Ramot, Y.; Merdler, I.; Baum, S.; Zlotogorski, A.; Barzilai, A.; Astman, N. Epidemiology and Comorbidities of Psoriasis among Israeli Adolescents: A Large Cross-Sectional Study. Dermatology 2019, 235, 488–494. [Google Scholar] [CrossRef]
- Asha, K.; Singal, A.; Sharma, S.B.; Arora, V.K.; Aggarwal, A. Dyslipidaemia & oxidative stress in patients of psoriasis: Emerging cardiovascular risk factors. Indian J. Med. Res. 2017, 146, 708–713. [Google Scholar] [CrossRef]
- Madanagobalane, S.; Anandan, S. Prevalence of metabolic syndrome in south Indian patients with psoriasis vulgaris and the relation between disease severity and metabolic syndrome: A hospital-based case-control study. Indian J. Dermatol. 2012, 57, 353–357. [Google Scholar] [CrossRef]
- Uczniak, S.; Gerlicz, Z.A.; Kozłowska, M.; Kaszuba, A. Presence of selected metabolic syndrome components in patients with psoriasis vulgaris. Postep. Dermatol. Alergol. 2016, 33, 114–119. [Google Scholar] [CrossRef]
- Akcali, C.; Buyukcelik, B.; Kirtak, N.; Inaloz, S. Clinical and laboratory parameters associated with metabolic syndrome in Turkish patients with psoriasis. J. Int. Med. Res. 2014, 42, 386–394. [Google Scholar] [CrossRef]
- Zindancı, I.; Albayrak, O.; Kavala, M.; Kocaturk, E.; Can, B.; Sudogan, S.; Koç, M. Prevalence of Metabolic Syndrome in Patients with Psoriasis. Sci. World J. 2012, 2012, 312463. [Google Scholar] [CrossRef] [Green Version]
- Love, T.J.; Qureshi, A.A.; Karlson, E.W.; Gelfand, J.M.; Choi, H.K. Prevalence of the metabolic syndrome in psoriasis: Results from the national health and nutrition examination survey, 2003–2006. Arch. Dermatol. 2011, 147, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Miller, I.M.; Skaaby, T.; Ellervik, C.; Jemec, G.B.E. Quantifying cardiovascular disease risk factors in patients with psoriasis: A meta-analysis. Br. J. Dermatol. 2013, 169, 1180–1187. [Google Scholar] [CrossRef]
- Piskin, S.; Gurkok, F.; Ekuklu, G.; Senol, M. Serum lipid levels in psoriasis. Yonsei Med. J. 2003, 44, 24–26. [Google Scholar] [CrossRef] [PubMed]
- Seçkin, D.; Tokgözoğlu, L.; Akkaya, S. Are lipoprotein profile and lipoprotein (a) levels altered in men with psoriasis? J. Am. Acad. Dermatol. 1994, 31, 445–449. [Google Scholar] [CrossRef]
- Akhyani, M.; Ehsani, A.H.; Robati, R.M.; Robati, A.M. The lipid profile in psoriasis: A controlled study. J. Eur. Acad. Dermatol. Venereol. 2007, 21, 1330–1332. [Google Scholar] [CrossRef]
- Seishima, M.; Seishima, M.; Mori, S.; Noma, A. Serum lipid and apolipoprotein levels in patients with psoriasis. Br. J. Dermatol. 1994, 130, 738–742. [Google Scholar] [CrossRef]
- Farshchian, M.; Zamanian, A.; Farshchian, M.; Monsef, A.-R.; Mahjub, H. Serum lipid level in Iranian patients with psoriasis. J. Eur. Acad. Dermatol. Venereol. 2007, 21, 802–805. [Google Scholar] [CrossRef]
- Ferretti, G.; Bacchetti, T.; Campanati, A.; Simonetti, O.; Liberati, G.; Offidani, A. Correlation between lipoprotein(a) and lipid peroxidation in psoriasis: Role of the enzyme paraoxonase-1. Br. J. Dermatol. 2012, 166, 204–207. [Google Scholar] [CrossRef]
- Toker, A.; Kadi, M.; Yildirim, A.K.; Aksoy, H.; Akçay, F. Serum lipid profile paraoxonase and arylesterase activities in psoriasis. Cell Biochem. Funct. 2009, 27, 176–180. [Google Scholar] [CrossRef]
- Sorokin, A.V.; Kotani, K.; Elnabawi, Y.A.; Dey, A.K.; Sajja, A.P.; Yamada, S.; Ueda, M.; Harrington, C.L.; Baumer, Y.; Rodante, J.A.; et al. Association between oxidation-modified lipoproteins and coronary plaque in psoriasis an observational cohort study. Circ. Res. 2018, 123, 1244–1254. [Google Scholar] [CrossRef] [PubMed]
- Golden, J.B.; McCormick, T.S.; Ward, N.L. IL-17 in psoriasis: Implications for therapy and cardiovascular co-morbidities. Cytokine 2013, 62, 195–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolk, R.; Armstrong, E.J.; Hansen, P.R.; Thiers, B.; Lan, S.; Tallman, A.M.; Kaur, M.; Tatulych, S. Effect of tofacitinib on lipid levels and lipid-related parameters in patients with moderate to severe psoriasis. J. Clin. Lipidol. 2017, 11, 1243–1256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.J.; Strober, B.E.; Hansen, P.R.; Ahlehoff, O.; Egeberg, A.; Qureshi, A.A.; Robertson, D.; Valdez, H.; Tan, H.; Wolk, R. Effects of tofacitinib on cardiovascular risk factors and cardiovascular outcomes based on phase III and long-term extension data in patients with plaque psoriasis. J. Am. Acad. Dermatol. 2016, 75, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Papp, K.A.; Menter, A.; Strober, B.; Langley, R.G.; Buonanno, M.; Wolk, R.; Gupta, P.; Krishnaswami, S.; Tan, H.; Harness, J.A. Efficacy and safety of tofacitinib, an oral Janus kinase inhibitor, in the treatment of psoriasis: A Phase 2b randomized placebo-controlled dose-ranging study. Br. J. Dermatol. 2012, 167, 668–677. [Google Scholar] [CrossRef]
- Singh, S.; Bhansali, A. Randomized placebo control study of metformin in psoriasis patients with metabolic syndrome (systemic treatment cohort). Indian J. Endocrinol. Metab. 2017, 21, 581–587. [Google Scholar] [CrossRef]
- Zangrilli, A.; Bavetta, M.; Scaramella, M.; Bianchi, L. Long-term treatment of psoriatic patients with adalimumab reduces disease severity and maintains a favorable lipid pattern and a low Atherogenic Index. G. Ital. Dermatol. Venereol. 2018, 153, 146–154. [Google Scholar] [CrossRef]
- Puig, L.; Strohal, R.; Fuiman, J.; Pedersen, R.; Szumski, A.; Koenig, A.S.; Robertson, D.; Drexel, H. Cardiometabolic biomarkers in chronic plaque psoriasis before and after etanercept treatment. J. Dermatolog. Treat. 2014, 25, 470–481. [Google Scholar] [CrossRef]
- Dey, A.K.; Joshi, A.A.; Chaturvedi, A.; Lerman, J.B.; Aberra, T.M.; Rodante, J.A.; Teague, H.L.; Harrington, C.L.; Rivers, J.P.; Chung, J.H.; et al. Association between skin and aortic vascular inflammation in patients with psoriasis: A case-cohort study using positron emission tomography/computed tomography. JAMA Cardiol. 2017, 2, 1013–1018. [Google Scholar] [CrossRef] [Green Version]
- Kilic, S.; Emre, S.; Metin, A.; Isikoglu, S.; Erel, O. Effect of the systemic use of methotrexate on the oxidative stress and paraoxonase enzyme in psoriasis patients. Arch. Dermatol. Res. 2013, 305, 495–500. [Google Scholar] [CrossRef]
- Corbetta, S.; Angioni, R.; Cattaneo, A.; Becke-Peccoz, P.; Spada, A. Effects of retinoid therapy on insulin sensitivity, lipid profile and circulating adipocytokines. Eur. J. Endocrinol. 2006, 154, 83–86. [Google Scholar] [CrossRef] [Green Version]
- Bacchetti, T.; Campanati, A.; Ferretti, G.; Simonetti, O.; Liberati, G.; Offidani, A.M. Oxidative stress and psoriasis: The effect of antitumour necrosis factor-α inhibitor treatment. Br. J. Dermatol. 2013, 168, 984–989. [Google Scholar] [CrossRef]
- Egeberg, A.; Wu, J.J.; Korman, N.; Solomon, J.A.; Goldblum, O.; Zhao, F.; Mallbris, L. Ixekizumab treatment shows a neutral impact on cardiovascular parameters in patients with moderate-to-severe plaque psoriasis: Results from UNCOVER-1, UNCOVER-2, and UNCOVER-3. J. Am. Acad. Dermatol. 2018, 79, 104–109.e8. [Google Scholar] [CrossRef] [Green Version]
- Gelfand, J.M.; Shin, D.B.; Duffin, K.C.; Armstrong, A.W.; Blauvelt, A.; Tyring, S.K.; Menter, A.; Gottlieb, S.; Lockshin, B.N.; Simpson, E.L.; et al. A Randomized Placebo-Controlled Trial of Secukinumab on Aortic Vascular Inflammation in Moderate-to-Severe Plaque Psoriasis (VIP-S). J. Investig. Dermatol. 2020, 140, 1784–1793.e2. [Google Scholar] [CrossRef]
- Ahlehoff, O.; Hansen, P.R.; Gislason, G.H.; Frydland, M.; Bryld, L.E.; Elming, H.; Jemec, G.B.E. Myocardial function and effects of biologic therapy in patients with severe psoriasis: A prospective echocardiographic study. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 819–823. [Google Scholar] [CrossRef]
- Mehta, N.N.; Shin, D.B.; Joshi, A.A.; Dey, A.K.; Armstrong, A.W.; Duffin, K.C.; Fuxench, Z.C.; Harrington, C.L.; Hubbard, R.A.; Kalb, R.E.; et al. Effect of 2 psoriasis treatments on vascular inflammation and novel inflammatory cardiovascular biomarkers: A randomized placebo-controlled trial. Circ. Cardiovasc. Imaging 2018, 11, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Staniak, H.L.; Bittencourt, M.S.; de Souza Santos, I.; Sharovsky, R.; Sabbag, C.; Goulart, A.C.; Lotufo, P.A.; Benseñor, I.M. Association between psoriasis and coronary calcium score. Atherosclerosis 2014, 237, 847–852. [Google Scholar] [CrossRef] [Green Version]
- Asefi, M.; Vaisi-Raygani, A.; Bahrehmand, F.; Kiani, A.; Rahimi, Z.; Nomani, H.; Ebrahimi, A.; Tavilani, H.; Pourmotabbed, T. Paraoxonase 1 (PON1) 55 polymorphism, lipid profiles and psoriasis. Br. J. Dermatol. 2012, 167, 1279–1286. [Google Scholar] [CrossRef]
- Houshang, N.; Reza, K.; Sadeghi, M.; Ali, E.; Mansour, R.; Vaisi-Raygani, A. Antioxidant status in patients with psoriasis. Cell Biochem. Funct. 2014, 32, 268–273. [Google Scholar] [CrossRef]
- Bacchetti, T.; Simonetti, O.; Ricotti, F.; Offidani, A.; Ferretti, G. Plasma oxidation status and antioxidant capacity in psoriatic children. Arch. Dermatol. Res. 2020, 312, 33–39. [Google Scholar] [CrossRef]
- Husni, M.E.; Wilson Tang, W.H.; Lucke, M.; Chandrasekharan, U.M.; Brennan, D.M.; Hazen, S.L. Correlation of High-Density Lipoprotein–Associated Paraoxonase 1 Activity With Systemic Inflammation, Disease Activity, and Cardiovascular Risk Factors in Psoriatic Disease. Arthritis Rheumatol. 2018, 70, 1240–1250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuberbier, T.; Asero, R.; Bindslev-Jensen, C.; Walter Canonica, G.; Church, M.K.; Giménez-Arnau, A.M.; Grattan, C.E.H.; Kapp, A.; Maurer, M.; Merk, H.F.; et al. EAACI/GALEN/EDF/WAO guideline: Management of urticaria. In Allergy: European Journal of Allergy and Clinical Immunology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2009; Volume 64, pp. 1427–1443. [Google Scholar]
- Kanani, A.; Betschel, S.D.; Warrington, R. Urticaria and angioedema. Allergy Asthma Clin. Immunol. 2018, 14, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuberbier, T.; Balke, M.; Worm, M.; Edenharter, G.; Maurer, M. Epidemiology of urticaria: A representative cross-sectional population survey. Clin. Exp. Dermatol. 2010, 35, 869–873. [Google Scholar] [CrossRef] [PubMed]
- Maurer, M.; Ortonne, J.P.; Zuberbier, T. Chronic urticaria: An internet survey of health behaviours, symptom patterns and treatment needs in European adult patients. Br. J. Dermatol. 2009, 160, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Amar, S.M.; Dreskin, S.C. Urticaria. Prim. Care Clin. Off. Pract. 2008, 35, 141–157. [Google Scholar] [CrossRef] [PubMed]
- Antia, C.; Baquerizo, K.; Korman, A.; Bernstein, J.A.; Alikhan, A. Urticaria: A comprehensive review: Epidemiology, diagnosis, and work-up. J. Am. Acad. Dermatol. 2018, 79, 599–614. [Google Scholar] [CrossRef]
- Amin, M.M.; Rushdy, M. Hyperlipidemia in association with pro-inflammatory cytokines among chronic spontaneous urticaria: Case-control study. Eur. Ann. Allergy Clin. Immunol. 2018, 50, 245–261. [Google Scholar] [CrossRef] [Green Version]
- Yaldiz, M.; Asil, K. Evaluation of carotid intima media thickness and hematologic inflammatory markers in patients with chronic spontaneous urticaria. Adv. Dermatol. Allergol. 2020, 37, 214–220. [Google Scholar] [CrossRef]
- Kaplan, A.P.; Greaves, M.W. Angioedema. J. Am. Acad. Dermatol. 2005, 53, 373–388. [Google Scholar] [CrossRef]
- Sloane, D.E.; Lee, C.W.; Sheffer, A.L. Hereditary angioedema: Safety of long-term stanozolol therapy. J. Allergy Clin. Immunol. 2007, 120, 654–658. [Google Scholar] [CrossRef]
- Széplaki, G.; Varga, L.; Valentin, S.; Kleiber, M.; Karádi, I.; Romics, L.; Füst, G.; Farkas, H. Adverse effects of danazol prophylaxis on the lipid profiles of patients with hereditary angioedema. J. Allergy Clin. Immunol. 2005, 115, 864–869. [Google Scholar] [CrossRef] [PubMed]
- Szegedi, R.; Széplaki, G.; Varga, L.; Prohászka, Z.; Széplaki, Z.; Karádi, I.; Füst, G.; Farkas, H. Long-term danazol prophylaxis does not lead to increased carotid intima-media thickness in hereditary angioedema patients. Atherosclerosis 2008, 198, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Nebenführer, Z.; Szabó, E.; Kajdácsi, E.; Kőhalmi, K.V.; Karádi, I.; Zsáry, A.; Farkas, H.; Cervenak, L. Flow-mediated vasodilation assay indicates no endothelial dysfunction in hereditary angioedema patients with C1-inhibitor deficiency. Ann. Allergy Asthma Immunol. 2019, 122, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Wurfel, M.M.; Kunitake, S.T.; Lichenstein, H.; Kane, J.P.; Wright, S.D. Lipopolysaccharide (LPS)-binding protein is carried on lipoproteins and acts as a cofactor in the neutralization of LPS. J. Exp. Med. 1994, 180, 1025–1035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levels, J.H.M.; Abraham, P.R.; Van Barreveld, E.P.; Meijers, J.C.M.; Van Deventer, S.J.H. Distribution and kinetics of lipoprotein-bound lipoteichoic acid. Infect. Immun. 2003, 71, 3280–3284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levels, J.H.M.; Abraham, P.R.; Van den Ende, A.; Van Deventer, S.J.H. Distribution and kinetics of lipoprotein-bound endotoxin. Infect. Immun. 2001, 69, 2821–2828. [Google Scholar] [CrossRef] [Green Version]
- Curcic, S.; Holzer, M.; Frei, R.; Pasterk, L.; Schicho, R.; Heinemann, A.; Marsche, G. Neutrophil effector responses are suppressed by secretory phospholipase A2 modified HDL. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2015, 1851, 184–193. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trakaki, A.; Marsche, G. High-Density Lipoprotein (HDL) in Allergy and Skin Diseases: Focus on Immunomodulating Functions. Biomedicines 2020, 8, 558. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines8120558
Trakaki A, Marsche G. High-Density Lipoprotein (HDL) in Allergy and Skin Diseases: Focus on Immunomodulating Functions. Biomedicines. 2020; 8(12):558. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines8120558
Chicago/Turabian StyleTrakaki, Athina, and Gunther Marsche. 2020. "High-Density Lipoprotein (HDL) in Allergy and Skin Diseases: Focus on Immunomodulating Functions" Biomedicines 8, no. 12: 558. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines8120558