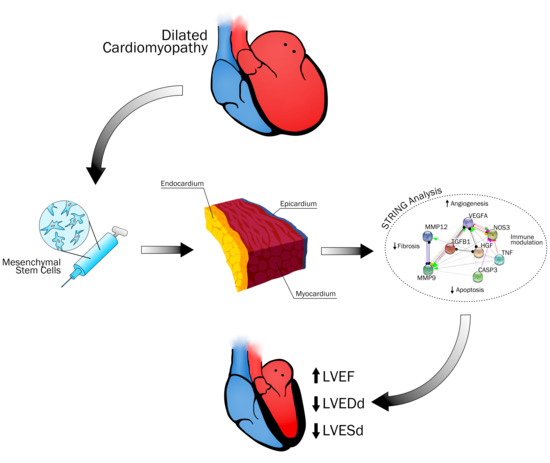
Efficacy and Mode of Action of Mesenchymal Stem Cells in Non-Ischemic Dilated Cardiomyopathy: A Systematic Review
Abstract
:1. Introduction
2. Experimental Section
2.1. Study Protocol, Search Strategy, and Data Sources
2.2. Study Criteria
2.3. Data Extraction
2.4. Protein-Protein Interaction Network
2.5. Assessment of Study Quality and Publication Bias
3. Results
3.1. Study Characteristics and Quality Assessment
3.2. Risk of Bias
3.3. Cell and Transplant Type
3.4. Administration Route
3.5. Cell Labelling
3.6. Effect on MSC Therapy on Cardiac Function
3.6.1. Clinical Evidence of Functional Effect
3.6.2. Preclinical Evidence of Functional Effect
3.7. Effect on MSC Therapy on Cardiac Regeneration
3.7.1. Fibrosis
3.7.2. Immunomodulation
3.7.3. Angiogenesis
3.7.4. Apoptosis
3.7.5. MSC Mode of Action in NIDCM
4. Challenges, Limitations and Future Perspectives
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. PICO Criteria
- Develop a question following the PICO criteria:
- Purpose: To describe efficacy and mode of action (MoA) of mesenchymal stem cell (MSC) therapy in patients with non-ischemic dilated cardiomyopathy (NIDCM)
- Condition or domain being studied: MSC efficacy and MoA in NIDCM
- Participants/populations being studied:
- Clinical trials, which have evaluated the efficacy of MSCs in adults ≥ 18 years diagnosed with NIDCM
- Clinical trials that evaluate the efficacy of MSCs in both NIDCM and ischemic heart disease, if the data from NIDCM patients can be extracted separately
- Studies of MSC efficacy and/or molecular/cellular effects performed in animals of NIDCM
- Intervention(s), exposure(s): We will include clinical trials and animal models of NIDCM, in which the intervention is administration of MSCs, regardless of administration route (intracoronary infusion, intramyocardial injection, intravenous injection).
- The MSC therapy may consist of MSCs of varying origin (bone-marrow, adipose tissue, wharton’s jelly, umbilical cord), transplant type (autologous, allogeneic, syngeneic, xenogeneic), status (cryopreserved or fresh), number of cells administered and number of cell administrations (single or repeated injections—if repeated, it must be stated)
- Comparator(s)/control: The study will include trials that have compared:
- MSC therapy, as defined above, versus placebo, sham intervention, or no intervention
- Different cell types or administration routes against each other
- Modified/preconditioned MSCs to normal MSCs, only if the data can be extracted separately for the normal MSC group
- Outcome: As we aim to describe and summarize the currently known MSC MoA in NIDCM, all outcomes (physiological mechanisms) will be eligible for inclusion. This is done to map current knowledge and inform future research. Key mechanisms of interest:
- Angiogenesis
- Fibrosis
- Immune response
- Chemotaxis
- Apoptosis
- Study design
- Clinical trials
- Animal models of NIDCM
- Inclusion and exclusion criteria are determined prior to the literature search
- Inclusion:
- Non-ischemic injury
- NIDCM induced by autoimmune myocarditis
- Anthracycline-induced NIDCM
- Isoproterenol-induced non ischemic heart failure
- Genetic NIDCM
- MSCs including BM-MSCs, hUCB-MSCs, AT-MSCs
- Treatment consisting of exosomes, conditioned medium etc. from MSCs
- Exclusion
- Ischemic injury
- Any other kind of stem cell therapy than MSCs, also iPS-MSCs
- Modified MSCs (preconditioning, gene modified, differentiated etc.), unless data from a control group with “normal” MSCs can be extracted separately
- Any kind of co-intervention, regardless of character
- The following animal models; arrythmogenic and hypertrophic cardiomyopathies, TAC-model (HFpEF), pressure overload
- Patients with arrythmogenic and hypertrophic cardiomyopathies
- Reviews
- Editorial comments
Appendix B. PRISMA Checklist
| Section/Topic | # | Checklist Item | Reported on Page # |
| TITLE | |||
| Title | 1 | Identify the report as a systematic review, meta-analysis, or both. | 1 |
| ABSTRACT | |||
| Structured summary | 2 | Provide a structured summary including, as applicable: background; objectives; data sources; study eligibility criteria, participants, and interventions; study appraisal and synthesis methods; results; limitations; conclusions and implications of key findings; systematic review registration number. | 1 |
| INTRODUCTION | |||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known. | 1–2 |
| Objectives | 4 | Provide an explicit statement of questions being addressed with reference to participants, interventions, comparisons, outcomes, and study design (PICOS). | Appendix A |
| METHODS | |||
| Protocol and registration | 5 | Indicate if a review protocol exists, if and where it can be accessed (e.g., Web address), and, if available, provide registration information including registration number. | N/A |
| Eligibility criteria | 6 | Specify study characteristics (e.g., PICOS, length of follow-up) and report characteristics (e.g., years considered, language, publication status) used as criteria for eligibility, giving rationale. | 2–3 |
| Information sources | 7 | Describe all information sources (e.g., databases with dates of coverage, contact with study authors to identify additional studies) in the search and date last searched. | 2–3 |
| Search | 8 | Present full electronic search strategy for at least one database, including any limits used, such that it could be repeated. | 2 + Appendix C |
| Study selection | 9 | State the process for selecting studies (i.e., screening, eligibility, included in systematic review, and, if applicable, included in the meta-analysis). | 2–3 |
| Data collection process | 10 | Describe method of data extraction from reports (e.g., piloted forms, independently, in duplicate) and any processes for obtaining and confirming data from investigators. | 2–3 |
| Data items | 11 | List and define all variables for which data were sought (e.g., PICOS, funding sources) and any assumptions and simplifications made. | 3 |
| Risk of bias in individual studies | 12 | Describe methods used for assessing risk of bias of individual studies (including specification of whether this was done at the study or outcome level), and how this information is to be used in any data synthesis. | 3 + Appendix E |
| Summary measures | 13 | State the principal summary measures (e.g., risk ratio, difference in means). | N/A |
| Synthesis of results | 14 | Describe the methods of handling data and combining results of studies, if done, including measures of consistency (e.g., I2) for each meta-analysis. | N/A |
| Risk of bias across studies | 15 | Specify any assessment of risk of bias that may affect the cumulative evidence (e.g., publication bias, selective reporting within studies). | 5 |
| Additional analyses | 16 | Describe methods of additional analyses (e.g., sensitivity or subgroup analyses, meta-regression), if done, indicating which were pre-specified. | N/A |
| RESULTS | |||
| Study selection | 17 | Give numbers of studies screened, assessed for eligibility, and included in the review, with reasons for exclusions at each stage, ideally with a flow diagram. | 4 |
| Study characteristics | 18 | For each study, present characteristics for which data were extracted (e.g., study size, PICOS, follow-up period) and provide the citations. | Appendix D |
| Risk of bias within studies | 19 | Present data on risk of bias of each study and, if available, any outcome level assessment (see item 12). | Appendix E |
| Results of individual studies | 20 | For all outcomes considered (benefits or harms), present, for each study: (a) simple summary data for each intervention group (b) effect estimates and confidence intervals, ideally with a forest plot. | N/A |
| Synthesis of results | 21 | Present results of each meta-analysis done, including confidence intervals and measures of consistency. | N/A |
| Risk of bias across studies | 22 | Present results of any assessment of risk of bias across studies (see Item 15). | Appendix E |
| Additional analysis | 23 | Give results of additional analyses, if done (e.g., sensitivity or subgroup analyses, meta-regression (see Item 16)). | N/A |
| DISCUSSION | |||
| Summary of evidence | 24 | Summarize the main findings including the strength of evidence for each main outcome; consider their relevance to key groups (e.g., healthcare providers, users, and policy makers). | 4–12 |
| Limitations | 25 | Discuss limitations at study and outcome level (e.g., risk of bias), and at review-level (e.g., incomplete retrieval of identified research, reporting bias). | 13 |
| Conclusions | 26 | Provide a general interpretation of the results in the context of other evidence, and implications for future research. | 13 |
| FUNDING | |||
| Funding | 27 | Describe sources of funding for the systematic review and other support (e.g., supply of data); role of funders for the systematic review. | 13 |
Appendix C. Literature Search
| MeSH | #1 | AND | #4 |
| OR | Mesenchymal Stem Cells [MeSH] | Cardiomyopathy, Dilated [MeSH] | |
| TX/ALL | #2 | #5 | |
| OR | Mesenchymal Stem Cell * Bone Marrow Mesenchymal Stem Cells Bone Marrow Stromal Cell * Multipotent Bone Marrow Stromal Cells Adipose-Derived Mesenchymal * Adipose Derived Mesenchymal * Adipose Tissue-Derived Mesenchymal * Adipose Tissue Derived Mesenchymal * Mesenchymal Stromal Cell * Multipotent Mesenchymal Stromal Cells Mesenchymal Progenitor Cell * Wharton Jelly Cell * Wharton’s Jelly Cell * Bone Marrow Stromal Stem Cells | Dilated Cardiomyopath * Familial Idiopathic Cardiomyopath * Congestive Cardiomyopath * Idiopathic Dilated Cardiomyopath * Nonischemic dilated cardiomyopathy Doxorubicin-Induced Cardi * Doxorubicin-Induced Heart * Anthracycline-induced * Anthracycline cardio * Doxorubicin cardio * Doxorubicin heart * | |
| OR | Heart Failure [MeSH] | ||
| Cardiac Failure Heart Decompensation Right-Sided Heart Failure Right Sided Heart Failure Myocardial Failure Congestive Heart Failure Left-Sided Heart Failure Left Sided Heart Failure Non-ischemic heart failure Non Ischemic Heart Failure |
| Subject Headings | #1 | AND | #4 |
| OR | Mesenchymal Stem Cell | Congestive Cardiomyopathy | |
| TX/ALL | #2 | #5 | |
| OR | Mesenchymal Progenitor Cell Mesenchymal Stem Cells Stem Cell, Mesenchymal | Cardiomyopathy, congestive Cardiomyopathy, dilated Congestive heart disease Congestive myocardiopathy Congestive myocardiopathy, idiopathic Dilated cardiomyopathy Idiopathic congestive cardiomyopathy Idiopathic congestive myocardiopathy Idiopathic dilated cardiomyopathy Myocardiopathy, congestive Myocardiopathy, idiopathic congestive Doxorubicin-Induced Cardiomyopathy Doxorubicin-Induced Heart Failure Anthracycline-induced Cardiomyopathy Anthracycline cardiomyopathy Doxorubicin cardiotoxicity Doxorubicin heart | |
| OR | Heart Failure | ||
| Backward failure, heart Cardiac backward failure Cardiac decompensation Cardiac failure Cardiac incompetence Cardiac insufficiency Cardiac stand still Cardial decompensation Cardial insufficiency Chronic heart failure Chronic heart insufficiency Decompensatio cordis Decompensation, heart Heart backward failure Heart decompensation Heart incompetence Heart insufficiency Insufficientia cardis Myocardial failure Myocardial insufficiency |
Appendix D. Included Studies
| Study | Trial Type | NIDCM Phenotype | MSC Type and Concen-Tration | Admini-Stration Route | Functional Outcome | Molecular Outcome |
| Carmona, 2017 | Preclinical (Wistar rats, 8 weeks) | NIDCM induced by autoimmune myocarditis | Syngeneic BM-MNCs, BM-MSCs or AT-MSCs, 5 × 106 | Intramyocardial | BM-MSCs and AT-MSCs increased LVEF to 79.8% and 75.1%, respectively | BM-MSCs and AT-MSCs increased serum VEGF at 24 h (peak) and at four weeks post treatment. BM-MSC decreased BNP after four weeks and improved cardiac fiber organization, number of vessels and reduced fibrosis (IHC) |
| Deng, 2017 | Preclinical (Sprague Dawley rats, 8 weeks) | Adriamycin induced NIDCM | Syngeneic BM-MSCs, 1 × 107 | Intravenous | BM-MSCs increased LVEF | BM-MSCs upregulated Cx43, MEF2 and GATA4 and downregulated, TGF-β and Col-I (qPCR). BM-MSCs reduced CVF (IHC) |
| Fatkhutdinov, 2009 | Clinical (27 NIDCM patients) | Idiopathic NIDCM with LVEF ≤35% | Allogeneic BM-MSCs, dose N/A | Intracoronary | BM-MSCs increased walk distance and reduced NYHA class at one and three months follow-up | BM-MSCs reduced serum BNP one week after transplantation |
| Florea, 2020 (POSIEDON-DCM) | Clinical (34 NIDCM patients) | NIDCM with LVEF <40% | Allogeneic and autologous BM-MSCs, 1 × 108 | Trans-endocardial | LVEF was significantly increased in both males and females | BM-MSCs decreased serum TNF-α after six months in both males and females. EPC-CFU increased and FMD improved 3 months post treatment |
| Guo, 2013 | Preclinical (C57/BL6 mice, 10 weeks) | Doxorubicin induced NIDCM | Syngeneic BM-MSCs, 5 × 107 | Intravenous | BM-MSCs increased FS% and reduced LVDd and LVEDP | BM-MSCs reduced cardiac fibrosis and CVF (IHC) |
| Hare, 2017 (Poseidon-DCM) | Clinical (37 NIDCM patients) | NIDCM with LVEF <40% | Allogeneic and autologous BM-MSCs, 1 × 108 | Trans-endocardial | Allogeneic BM-MSCs increased LVEF to a greater degree than autologous BM-MSCs | Serum TNF-α decreased to a greater extent in the allogeneic group compared to the autologous group at six months follow-up. EPC-CFU increased in the allogeneic group compared to the autologous at three months follow-up. Both groups had a reduced percentage of T and B cell subtypes, normally associated with chronic inflammation, at six months follow-up |
| Kong, 2010 | Preclinical (Wistar rats, 3 months, 250 g) | Adriamycin induced NIDCM | Transplant type N/A, BM-MSCs, 2 × 106 | Intravenous for three days | BM-MSCs increased LVEF | BM-MSCs significantly reduced cardiac norepinephrine content and increased GAP-43, ChaT, and SYN density (IHC and WB) |
| Li, 2009 | Preclinical (Wistar rats, 180–200 g) | Isoproterenol induced HF | Syngeneic BM-MSCs, 3 × 106 | Intramyocardial | BM-MSCs increased LVEF and FS | BM-MSC attenuated myocardial fibrosis (IHC) and upregulated adrenomodullin (qPCR) |
| Mao, 2017 | Preclinical (Sprague Dawley rats, 180 g) | Doxorubicin induced NIDCM | Xenogeneic hUCB-MSCs or CM, 2.5 × 105 (low dose) or 1 × 106 (high dose) or 2.0 mL (CM) | Intramyocardial | Both low-dose and high-dose of hUCB-MSCs increased FS% and LVEF. | hUCB-MSCs attenuated mitochondrial swelling and maintained sarcolemma integrity (IHC). BM-MSCs increased serum LIF at both doses, HGF, GM-CSF, and VEGF at low dose and reduced BNP, cTNI (ELISA). Treatment increased HGF, VEGF, IGF-1 (qPCR) |
| Mörschbächer, 2016 | Preclinical (New Zealand rabbits, 3–4 months, 2–3.5 kg) | Doxorubicin induced NIDCM | Syngeneic AT-MSCs, 1 × 106 | Intramyocardial | No significant change in LVEF | AT-MSCs reduced histological lesions (IHC) |
| Premer, 2019 (POSEIDON-DCM) | Clinical (21 NIDCM patients) | NIDCM with LVEF <40% | Allogeneic and autologous BM-MSCs, 1 × 108 | Trans-endocardial | N/A | Allogeneic BM-MSCs increased EPC-CFUs and decreased plasma SDF-1α in both treatment groups. Plasma TNF-α negatively correlated with EPC-CFUs |
| Premer, 2015 (POSEIDON-DCM + TRIDENT) | Clinical (12 NIDCM patients) | Idiopathic NIDCM (inclusion criteria from POSEIDON-DCM) | Allogeneic and autologous BM-MSCs, 1 × 108 | Trans-endocardial | N/A | Allogeneic BM-MSCs led to increased EPC-CFUs and improved FMD compared to autologous BM-MSCs |
| Rieger, 2019 (POSEIDON-DCM) | Clinical (34 NIDCM patients) | NIDCM with LVEF <40% | Allogeneic and autologous BM-MSCs, 1 × 108 | Trans-endocardial | BM-MSCs increased LVEF in patients negative for any pathological variants (V-) and variants of uncertain significance at one year follow up, and improved MLHFQ score and NYHA class in V- patients only | N/A |
| Shabbir, 2009 | Preclinical (TO2 (cardiomyopathic) male hamsters, 4 months) | TO2 (cardiomyopathic) male hamsters | Xenogeneic BM-MSCs, 0.25 × 106, 1 × 106 or 4 × 106 or 0.8 mL CM | Two intramuscular injections (hamstring muscle) with 2 weeks interval or CM three times per week for 4 weeks | BM-MSCs increased FS% at all concentrations. 4 × 106 BM-MSCs increased FS% to the greatest degree | BM-MSCs decreased myocyte diameter, apoptotic myocytes, fibrosis (IHC), and circulating cTnI (ELISA). BM-MSCs downregulated Col3a1, MMP-9, MMP-13, TIMP-2, TIMP-3 (qPCR) |
| Xiao, 2017 | Clinical (53 NIDCM patients) | NIDCM with LVEF <40% | Autologous BM-MSCs or BM-MNCs, number of adherent cells in passage 3 | Intramyocardial | LVEF, LVEDd, NYHA class were improved after three and 12 months in both groups. Myocardial perfusion had increased in the BM-MSC group | N/A |
| Yu, 2014 | Preclinical (Sprague Dawley rats, 8 weeks) | Doxorubicin induced NIDCM | Syngeneic BM-MSCs, 5 × 106 | Intravenous every other day for 10 days | Repeated infusions of BM-MSCs increased LVEF to 79.6% and decreased LVEDd | BM-MSCs reduced CVF and Col-I/III ratio (IHC) MSCs downregulated Col-I, AT1, CYP11B2, TGF-β1 and upregulated Col-III (qPCR) |
| Yu, 2015 | Preclinical (Sprague Dawley rats, 37 g) | Furazolidone induced NIDCM | Syngeneic BM-MSCs, 1 × 105 | Intramyocardial | BM-MSCs increased LVEF to 74% | BM-MSCs reduced CVF (IHC), Col-I/III ratio and downregulated myocardial TGF-β1 (qPCR) |
| Zhang, 2019 | Preclinical (Lewis rats) | NIDCM induced by autoimmune myocarditis | Xenogeneic hUCB-MSCs, 1 × 106 | Intravenous | N/A | hUCB-MSCs decreased myocardial fibrosis (IHC) and activity of TGF-β1/ERK1/2 signaling |
| Labelled cells | ||||||
| Abd Allah, 2017 | Preclinical (Male albino rats, 150–200 g) | Doxorubicin induced NIDCM | Xenogeneic, PKH26-labelled c hUCB-MSCs, 1 × 106 | Intravenous | EDP, dP/dt max, and dP/dt min increased after six weeks | hUCB-MSCs decreased serum cTnI (ELISA) and collagen area (IHC) |
| Abdelmonem, 2019 | Preclinical (Wistar rats, 12 weeks old) | Isoprenaline induced HF | Syngeneic, PKH26-labelled BM-MSCs, 1 × 107 | Intravenous | BM-MSC increased LVEF to 74.47% and decreased LVESd | BM-MSCs decreased fibrosis and increased GATA4, desmin and cTNI (IHC) and increased eNOS (WB) and MEF2c (qPCR) |
| Ammar, 2015 | Preclinical (Wistar rats, 200–220 g) | Diabetic mellitus + doxorubicin induced NIDCM | Xenogeneic GFP labelled hBM-MSCs, 2 × 106, or GFP labelled hAT-MSCs, 1 × 106 | Intravenous | Both MSC types increased FS% and decreased arterial blood pressure (systolic and diastolic) | Both MSC groups led to an increased number of capillaries and decreased immune cell infiltration, collagen deposition and αSMA (IHC) |
| Chen, 2010 | Preclinical (Inbred Japanese rabbits, 1800–2000 g) | Doxorubicin induced NIDCM | Autologous BrdU labelled BM-MSCs, 5 × 105 | Intramyocardial | BM-MSCs increased LVEF to 68.38% and decreased LVESd | BM-MSCs were present in the myocardium four weeks after treatment, indicated by BrdU (IHC) |
| Gong, 2016 | Preclinical (cTnTR141W transgenic mice, 4 months) | Genetic NIDCM | Xenogeneic eGFP labelled hUCB-MSCs, 1.5 × 106 | Intramyocardial | hUCB-MSCs increased LVEF to 56.96% and decreased heart weight/body weight, LVEDd, LVESd | hUCB-MSCs reduced CVF, cytoplasmic vacuolisation and apoptotic nuclei and increased CD31+ vessels and αSMA+ aterioles (IHC). hUCB-MSCs increased Bcl-2/Bax ratio (WB), IGF-1 and VEGF and reduced serum CRP (ELISA) |
| Li, 2018 | Preclinical (Male Wistar rats, 180–200 g) | Isoproterenol induced HF | Syngeneic eGFP + DAPI labelled BM-MSCs, 5 × 106 | Intramyocardial | BM-MSCs increased LVEF to 69.24% and reduced LVESd | BM-MSCs decreased CVF (IHC), upregulated HGF and downregulated Col-I and III, MMP-2, MMP-9, TNF-β (qPCR), MMP-2 and MMP-9 (WB). BM-MSCs were present in the myocardium after four weeks |
| Li, 2008 | Preclinical (Male Wistar rats, 180–200 g) | Isoproterenol induced HF | Syngeneic DAPI labelled BM-MSCs, 3 × 106 | Intramyocardial | BM-MSCs increased LVEF to 78.51% and decreased LVESd | BM-MSCs increased HGF (qPCR and WB) and decreased CVF (IHC), Col-I and III, MMP-2 and MMP-9 (qPCR), Pro MMP-2, Active MMP-2 and MMP-9 (WB) |
| Mohamed, 2015 | Preclinical (Male Wistar rats, 170–190 g) | Isoproterenol induced HF | Syngeneic PKH26 labelled BM-MSCs, 1 × 106 | Intravenous | BM-MSCs increased LVEF to 58.33% | BM-MSCs reduced cardiac fibrosis (IHC) and increased eNOS, Cx43 (WB). BM-MSCs reduced caspase 3 (WB) and TGF- β (ELISA) |
| Nagaya, 2006 | Preclinical (Male Lewis rats, 220–250 g) | NIDCM induced by autoimmune myocarditis | Syngeneic BM-MSCs, 5 × 56 | Intramyocardial | BM-MSCs increased FS% and decreased LVEDP, LVDd | BM-MSCs increased myocardial capillary density and decreased CVF (IHC). BM-MSCs reduced MMP-2 (WB) |
| Psaltis, 2010 | Preclinical (Merino wether sheeps, 50 kg) | Doxorubicin induced NIDCM | Allogeneic GFP labelled Mesenchymal progenitor cells (MPCs), 1 × 109 ± 5 × 106 | Trans-endocardial | MPCs increased LVEF to 39.2% | MPC treatment reduced CVF and increased the density of karyokinetic cardiomyocytes and myocardial arterioles (IHC) |
| Yang, 2013 | Preclinical (Female Wistar rats, 210–240 g) | Adriamycin induced NIDCM | Syngeneic BrdU labelled BM-MSCs, 5 × 106 | One or two intravenous injections (1-day interval | Only two doses significantly increased LVEF. Two doses of BM-MSCs increased LVEF to 75.4%. BM-MSCs reduced mortality, LVESd and LVEDd, which were significantly improved by double infusion | BM-MSCs led to upregulation of VEGF (qPCR). Two injections decreased CVF (IHC) and serum BNP (ELISA). BM-MSCs improved fiber alignment (IHC) All parameters significantly improved by double infusion BrdU labelled BM-MSCs were present in the myocardium after Four weeks |
| Zhang, 2013 | Preclinical (Wistar rats, 6–7 weeks, 200–220 g) | Adriamycin induced NIDCM | Syngeneic BrdU labelled BM-MSCs, 1 × 107 | Intravenous | BM-MSCs increased LVEF ti 55.56% and decreased LVESd | BM-MSCs increased GATA-4, cTnI, Cx43 (IHC) and decreased serum BNP (ELISA) |
| Zhou, 2007 | Preclinical (New Zealand rabbits, 1.9 kg) | Adriamycin induced NIDCM | Autologous DAPI labelled BM-MSCs, 4 × 106 | Intramyocardial | No significant improvement in heart function. | BM-MSCs were present in the myocardium after two weeks and increased Bcl-2 (IHC) |
Appendix E. Risk of Bias Assessment

References
- Savvatis, K.; Schultheiss, H.P.; Tschöpe, C. Endomyocardial biopsy and ultrastructural changes in dilated cardiomyopathy: Taking a “deeper” look into patients’ prognosis. Eur. Heart J. 2015, 36, 708–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinto, Y.M.; Elliott, P.M.; Arbustini, E.; Adler, Y.; Anastasakis, A.; Böhm, M.; Duboc, D.; Gimeno, J.; De Groote, P.; Imazio, M.; et al. Proposal for a revised definition of dilated cardiomyopathy, hypokinetic non-dilated cardiomyopathy, and its implications for clinical practice: A position statement of the ESC working group on myocardial and pericardial diseases. Eur. Heart J. 2016, 37, 1850–1858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKenna, W.J.; Maron, B.J.; Thiene, G. Classification, epidemiology, and global burden of cardiomyopathies. Circ. Res. 2017, 121, 722–730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mestroni, L.; Brun, F.; Spezzacatene, A.; Sinagra, G.; Taylor, M.R.G. Genetic causes of Dilated Cardiomyopathy. Prog. Pediatr. Cardiol. 2014, 37, 13–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marstrand, P.; Picard, K.; Lakdawala, N.K. Second Hits in Dilated Cardiomyopathy. Curr. Cardiol. Rep. 2020, 22, 20–25. [Google Scholar] [CrossRef]
- Volkova, M.; Russell, R. Anthracycline Cardiotoxicity: Prevalence, Pathogenesis and Treatment. Curr. Cardiol. Rev. 2012, 7, 214–220. [Google Scholar] [CrossRef] [Green Version]
- Han, D.; Wang, Y.; Wang, Y.; Dai, X.; Zhou, T.; Chen, J.; Tao, B.; Zhang, J.; Cao, F. The Tumor-Suppressive Human Circular RNA CircITCH Sponges miR-330-5p to Ameliorate Doxorubicin-Induced Cardiotoxicity through Upregulating SIRT6, Survivin, and SERCA2a. Circ. Res. 2020, E108–E125. [Google Scholar] [CrossRef]
- Lotrionte, M.; Biondi-Zoccai, G.; Abbate, A.; Lanzetta, G.; D’Ascenzo, F.; Malavasi, V.; Peruzzi, M.; Frati, G.; Palazzoni, G. Review and meta-analysis of incidence and clinical predictors of anthracycline cardiotoxicity. Am. J. Cardiol. 2013, 112, 1980–1984. [Google Scholar] [CrossRef]
- Xie, Y.; Liao, J.; Yu, Y.; Guo, Q.; Yang, Y.; Ge, J.; Chen, H.; Chen, R. Endothelial-to-mesenchymal transition in human idiopathic dilated cardiomyopathy. Mol. Med. Rep. 2018, 17, 961–969. [Google Scholar] [CrossRef]
- Schultheiss, H.P.; Fairweather, D.L.; Caforio, A.L.P.; Escher, F.; Hershberger, R.E.; Lipshultz, S.E.; Liu, P.P.; Matsumori, A.; Mazzanti, A.; McMurray, J.; et al. Dilated cardiomyopathy. Nat. Rev. Dis. Prim. 2019, 5, 32. [Google Scholar] [CrossRef]
- Vrtovec, B. The Epidemiology and Pathophysiology of Heart Failure. Med. Clin. N. Am. 2018, 96, 881–890. [Google Scholar]
- Hare, J.M.; DiFede, D.L.; Rieger, A.C.; Florea, V.; Landin, A.M.; El-Khorazaty, J.; Khan, A.; Mushtaq, M.; Lowery, M.H.; Byrnes, J.J.; et al. Randomized Comparison of Allogeneic Versus Autologous Mesenchymal Stem Cells for Nonischemic Dilated Cardiomyopathy: POSEIDON-DCM Trial. J. Am. Coll. Cardiol. 2017, 69, 526–537. [Google Scholar] [CrossRef] [PubMed]
- Janssens, S.P. Mesenchymal Cell Therapy for Dilated Cardiomyopathy: Time to Test the Water. J. Am. Coll. Cardiol. 2017, 69, 538–540. [Google Scholar] [CrossRef] [PubMed]
- Dec, W.G.; Fuster, V. Idiopathic Dilated Cardiomyopathy. N. Engl. J. Med. 1994, 331, 1564–1575. [Google Scholar] [CrossRef]
- Mathiasen, A.B.; Qayyum, A.A.; Jørgensen, E.; Helqvist, S.; Fischer-Nielsen, A.; Kofoed, K.F.; Haack-Sørensen, M.; Ekblond, A.; Kastrup, J. Bone marrow-derived mesenchymal stromal cell treatment in patients with severe ischaemic heart failure: A randomized placebo-controlled trial (MSC-HF trial). Eur. Heart J. 2015, 36, 1744–1753. [Google Scholar] [CrossRef] [Green Version]
- Mias, C.; Lairez, O.; Trouche, E.; Roncalli, J.; Calise, D.; Seguelas, M.H.; Ordener, C.; Piercecchi-Marti, M.D.; Auge, N.; Salvayre, A.N.; et al. Mesenchymal stem cells promote matrix metalloproteinase secretion by cardiac fibroblasts and reduce cardiac ventricular fibrosis after myocardial infarction. Stem Cells 2009, 27, 2734–2743. [Google Scholar] [CrossRef]
- Premaratne, G.U.; Ma, L.-P.; Fujita, M.; Lin, X.; Bollano, E.; Fu, M. Stromal Vascular Fraction Transplantation as an Alternative Therapy for Ischemic Heart Failure: Anti-inflammatory Role. Period. Polytech. Civ. Eng. 2011, 50, 171–180. [Google Scholar] [CrossRef] [Green Version]
- Sato, T.; Iso, Y.; Uyama, T.; Kawachi, K.; Wakabayashi, K.; Omori, Y.; Soda, T.; Shoji, M.; Koba, S.; Yokoyama, S.I.; et al. Coronary vein infusion of multipotent stromal cells from bone marrow preserves cardiac function in swine ischemic cardiomyopathy via enhanced neovascularization. Lab. Investig. 2011, 91, 553–564. [Google Scholar] [CrossRef] [Green Version]
- Bao, L.; Meng, Q.; Li, Y.; Deng, S.; Yu, Z.; Liu, Z.; Zhang, L.; Fan, H. C-Kit Positive Cardiac Stem Cells and Bone Marrow–Derived Mesenchymal Stem Cells Synergistically Enhance Angiogenesis and Improve Cardiac Function After Myocardial Infarction in a Paracrine Manner. J. Card. Fail. 2017, 23, 403–415. [Google Scholar] [CrossRef]
- Golpanian, S.; Wolf, A.; Hatzistergos, K.E.; Hare, J.M. Rebuilding the Damaged Heart: Mesenchymal Stem Cells, Cell-Based Therapy, and Engineered Heart Tissue. Physiol. Rev. 2016, 96, 1127–1168. [Google Scholar] [CrossRef]
- Vrtovec, B. Cell Therapy for Nonischemic Cardiomyopathy. Circ. Res. 2018, 122, 28–30. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Li, Q.; Na, R.; Li, X.; Liu, B.; Meng, L.; Liutong, H.; Fang, W.; Zhu, N.; Zheng, X. Impact of repeated intravenous bone marrow mesenchymal stem cells infusion on myocardial collagen network remodeling in a rat model of doxorubicin-induced dilated cardiomyopathy. Mol. Cell. Biochem. 2014, 387, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Guo, S.; Gao, C.; Dai, G.; Gao, Y.; Li, M.; Wang, X.; Hu, D. A randomized comparative study on the efficacy of intracoronary infusion of autologous bone marrow mononuclear cells and mesenchymal stem cells in patients with dilated cardiomyopathy. Int. Heart J. 2017, 58, 238–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hooijmans, C.R.; Rovers, M.M.; De Vries, R.B.M.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rieger, A.C.; Myerburg, R.J.; Florea, V.; Tompkins, B.A.; Natsumeda, M.; Premer, C.; Khan, A.; Schulman, I.H.; Vidro-Casiano, M.; DiFede, D.L.; et al. Genetic determinants of responsiveness to mesenchymal stem cell injections in non-ischemic dilated cardiomyopathy. EBioMedicine 2019, 48, 377–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Premer, C.; Blum, A.; Bellio, M.A.; Schulman, I.H.; Hurwitz, B.E.; Parker, M.; Dermarkarian, C.R.; DiFede, D.L.; Balkan, W.; Khan, A.; et al. Allogeneic Mesenchymal Stem Cells Restore Endothelial Function in Heart Failure by Stimulating Endothelial Progenitor Cells. EBioMedicine 2015, 2, 467–475. [Google Scholar] [CrossRef] [Green Version]
- Premer, C.; Wanschel, A.; Porras, V.; Balkan, W.; Legendre-Hyldig, T.; Saltzman, R.G.; Dong, C.; Schulman, I.H.; Hare, J.M. Mesenchymal stem cell secretion of SDF-1α modulates endothelial function in dilated cardiomyopathy. Front. Physiol. 2019, 10, 1–11. [Google Scholar] [CrossRef]
- Florea, V.; Rieger, A.C.; Natsumeda, M.; Tompkins, B.A.; Banerjee, M.N.; Schulman, I.H.; Premer, C.; Khan, A.; Valasaki, K.; Mantero, A.; et al. The Impact of Patient Sex on the Response to Intramyocardial Mesenchymal Stem Cell Administration in Patients with Non-Ischemic Dilated Cardiomyopathy. Cardiovasc. Res. 2020, 116, 2131–2141. [Google Scholar] [CrossRef]
- Fatkhutdinov, T.K.; D’Yachkov, A.V.; Koroteyev, A.V.; Goldstein, D.V.; Bochkov, N.P. Safety and efficiency of transplantation of allogenic multipotent stromal cells in surgical treatment of dilatated cardiomyopathy. Bull. Exp. Biol. Med. 2010, 149, 119–124. [Google Scholar] [CrossRef]
- Nagaya, N.; Kangawa, K.; Itoh, T.; Iwase, T.; Murakami, S.; Miyahara, Y.; Fujii, T.; Uematsu, M.; Ohgushi, H.; Yamagishi, M.; et al. Transplantation of mesenchymal stem cells improves cardiac function in a rat model of dilated cardiomyopathy. Circulation 2005, 112, 1128–1135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carmona, M.D.; Cañadillas, S.; Romero, M.; Blanco, A.; Nogueras, S.; Herrera, C. Intramyocardial bone marrow mononuclear cells versus bone marrow–derived and adipose mesenchymal cells in a rat model of dilated cardiomyopathy. Cytotherapy 2017, 19, 947–961. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhou, Y.; Lai, X.; Zhou, G.; Wang, H.; Feng, X.; Chen, Y.; Wu, Y.; Wang, T.; Ma, L. Human Umbilical Cord Mesenchymal Stem Cells Alleviate Myocardial Endothelial-Mesenchymal Transition in a Rat Dilated Cardiomyopathy Model. Transplant. Proc. 2019, 51, 936–941. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, A.; Zisa, D.; Suzuki, G.; Lee, T. Heart failure therapy mediated by the trophic activities of bone marrow mesenchymal stem cells: A noninvasive therapeutic regimen. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, 1888–1897. [Google Scholar] [CrossRef]
- Gong, X.; Wang, P.; Wu, Q.; Wang, S.; Yu, L.; Wang, G. Human umbilical cord blood derived mesenchymal stem cells improve cardiac function in cTnTR141W transgenic mouse of dilated cardiomyopathy. Eur. J. Cell Biol. 2016, 95, 57–67. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, H.; Xiao, J.; Wu, J.; Ye, Y.; Li, Z.; Zou, Y.; Li, X. Monocyte chemotactic protein-1 promotes the myocardial homing of mesenchymal stem cells in dilated cardiomyopathy. Int. J. Mol. Sci. 2013, 14, 8164–8178. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Zhang, S.; Zhang, Y.; Yu, B.; Xu, Y.; Guan, Z. Paracrine action mediate the antifibrotic effect of transplanted mesenchymal stem cells in a rat model of global heart failure. Mol. Biol. Rep. 2009, 36, 725–731. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Li, Y.; Yu, B.; Xu, Y.; Zhao, S.D.; Guan, Z. Mesenchymal stem cell transplantation attenuates cardiac fibrosis associated with isoproterenol-induced global heart failure. Transpl. Int. 2008, 21, 1181–1189. [Google Scholar] [CrossRef]
- Li, L.L.; Peng, C.; Zhang, M.; Liu, Y.; Li, H.; Chen, H.; Sun, Y.; Zhu, C.; Zhang, Y. Mesenchymal stem cells overexpressing adrenomedullin improve heart function through antifibrotic action in rats experiencing heart failure. Mol. Med. Rep. 2018, 17, 1437–1444. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zhang, L.; Wu, Q.; Liu, H.; Huang, L. Recombinant human brain natriuretic peptide therapy combined with bone mesenchymal stem cell transplantation for treating heart failure in rats. Mol. Med. Rep. 2013, 7, 628–632. [Google Scholar] [CrossRef] [Green Version]
- Ammar, H.I.; Sequiera, G.L.; Nashed, M.B.; Ammar, R.I.; Gabr, H.M.; Elsayed, H.E.; Sareen, N.; El Rub, E.A.; Zickri, M.B.; Dhingra, S. Comparison of adipose tissue- and bone marrow- derived mesenchymal stem cells for alleviating doxorubicin-induced cardiac dysfunction in diabetic rats. Stem Cell Res. Ther. 2015, 6, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vrtovec, B.; Poglajen, G.; Sever, M.; Lezaic, L.; Socan, A.; Haddad, F.; Wu, J.C. CD34+ stem cell therapy in nonischemic dilated cardiomyopathy patients. Clin. Pharmacol. Ther. 2013, 94, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Psaltis, P.J.; Carbone, A.; Nelson, A.J.; Lau, D.H.; Jantzen, T.; Manavis, J.; Williams, K.; Itescu, S.; Sanders, P.; Gronthos, S.; et al. Reparative effects of allogeneic mesenchymal precursor cells delivered transendocardially in experimental nonischemic cardiomyopathy. JACC Cardiovasc. Interv. 2010, 3, 974–983. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Piao, J.; Jin, L.; Zhou, Y. Does pretreatment of bone marrow mesenchymal stem cells with 5-azacytidine or double intravenous infusion improve their therapeutic potential for dilated cardiomyopathy? Med. Sci. Monit. Basic Res. 2013, 19, 20–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, C.; Yang, C.; Xiao, S.; Fei, H. Dynamic propagation problems on mode III asymmetrical interface crack. J. Harbin Inst. Technol. 2007, 39, 1710–1714. [Google Scholar]
- Abd Allah, S.H.; Hussein, S.; Hasan, M.M.; Deraz, R.H.A.; Hussein, W.F.; Sabik, L.M.E. Functional and Structural Assessment of the Effect of Human Umbilical Cord Blood Mesenchymal Stem Cells in Doxorubicin-Induced Cardiotoxicity. J. Cell. Biochem. 2017, 118, 3119–3129. [Google Scholar] [CrossRef]
- Abdelmonem, M.; Shahin, N.N.; Rashed, L.A.; Amin, H.A.A.; Shamaa, A.A.; Shaheen, A.A. Hydrogen sulfide enhances the effectiveness of mesenchymal stem cell therapy in rats with heart failure: In vitro preconditioning versus in vivo co-delivery. Biomed. Pharmacother. 2019, 112, 108584. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, W.; Li, W.; Gao, C. Autologous bone marrow mesenchymal cell transplantation improves left ventricular function in a rabbit model of dilated cardiomyopathy. Exp. Mol. Pathol. 2010, 88, 311–315. [Google Scholar] [CrossRef]
- Mohamed, S.S.; Ahmed, L.A.; Attia, W.A.; Khattab, M.M. Nicorandil enhances the efficacy of mesenchymal stem cell therapy in isoproterenol-induced heart failure in rats. Biochem. Pharmacol. 2015, 98, 403–411. [Google Scholar] [CrossRef]
- Mörschbächer, P.D.; Alves Garcez, T.N.; Paz, A.H.; Magrisso, A.B.; Mello, H.F.; Rolim, V.M.; Neuwald, E.B.; Driemeier, D.; Contesini, E.A.; Cirne-Lima, E. Treatment of dilated cardiomyopathy in rabbits with mesenchymal stem cell transplantation and platelet-rich plasma. Vet. J. 2016, 209, 180–185. [Google Scholar] [CrossRef]
- Lopes, G.M.; Grudzinski, P.B.; Beyer Nardi, N.; Leguisamo, N.M. Cell therapy improves cardiac function in anthracycline-induced cardiomyopathy preclinical models—A systematic review and meta-analysis. Stem Cells Dev. 2020, 00. [Google Scholar] [CrossRef] [PubMed]
- Deng, B.; Wang, J.X.; Hu, X.X.; Duan, P.; Wang, L.; Li, Y.; Zhu, Q. Nkx2.5 enhances the efficacy of mesenchymal stem cells transplantation in treatment heart failure in rats. Life Sci. 2017, 182, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Fang, W.; Zhu, N.; Zheng, X.; Na, R.; Liu, B.; Meng, L.; Li, Z.; Li, Q.; Li, X. Beneficial effects of intramyocardial mesenchymal stem cells and VEGF165 plasmid injection in rats with furazolidone induced dilated cardiomyopathy. J. Cell. Mol. Med. 2015, 19, 1868–1876. [Google Scholar] [CrossRef] [PubMed]
- Pohlers, D.; Brenmoehl, J.; Löffler, I.; Müller, C.K.; Leipner, C.; Schultze-Mosgau, S.; Stallmach, A.; Kinne, R.W.; Wolf, G. TGF-β and fibrosis in different organs—Molecular pathway imprints. Biochim. Biophys. Acta Mol. Basis Dis. 2009, 1792, 746–756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, C.; Hou, X.; Wang, B.; Chi, J.; Jiang, Y.; Zhang, C.; Li, Z. Intramuscular injection of human umbilical cord-derived mesenchymal stem cells improves cardiac function in dilated cardiomyopathy rats. Stem Cell Res. Ther. 2017, 8, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Octavia, Y.; Tocchetti, C.G.; Gabrielson, K.L.; Janssens, S.; Crijns, H.J.; Moens, A.L. Doxorubicin-induced cardiomyopathy: From molecular mechanisms to therapeutic strategies. J. Mol. Cell. Cardiol. 2012, 52, 1213–1225. [Google Scholar] [CrossRef] [Green Version]
- Steffens, S.; Van Linthout, S.; Sluijter, J.P.G.; Tocchetti, C.G.; Thum, T.; Madonna, R. Stimulating pro-reparative immune responses to prevent adverse cardiac remodelling: Consensus document from the joint 2019 meeting of the ESC Working Groups of cellular biology of the heart and myocardial function. Cardiovasc. Res. 2020, 116, 1850–1862. [Google Scholar] [CrossRef]
- Progatzky, F.; Dallman, M.J.; Lo Celso, C. From seeing to believing: Labelling strategies for in vivo cell-tracking experiments. Interface Focus 2013, 3, 20130001. [Google Scholar] [CrossRef] [Green Version]
- Ansari, A.M.; Ahmed, A.K.; Matsangos, A.E.; Lay, F.; Born, L.J.; Marti, G.; Harmon, J.W.; Sun, Z. Cellular GFP Toxicity and Immunogenicity: Potential Confounders in in Vivo Cell Tracking Experiments. Stem Cell Rev. Rep. 2016, 12, 553–559. [Google Scholar] [CrossRef] [Green Version]
- Cho, D.I.; Kim, M.R.; Jeong, H.Y.; Jeong, H.C.; Jeong, M.H.; Yoon, S.H.; Kim, Y.S.; Ahn, Y. Mesenchymal stem cells reciprocally regulate the M1/M2 balance in mouse bone marrow-derived macrophages. Exp. Mol. Med. 2014, 46, e70. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoeeg, C.; Frljak, S.; Qayyum, A.A.; Vrtovec, B.; Kastrup, J.; Ekblond, A.; Follin, B. Efficacy and Mode of Action of Mesenchymal Stem Cells in Non-Ischemic Dilated Cardiomyopathy: A Systematic Review. Biomedicines 2020, 8, 570. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines8120570
Hoeeg C, Frljak S, Qayyum AA, Vrtovec B, Kastrup J, Ekblond A, Follin B. Efficacy and Mode of Action of Mesenchymal Stem Cells in Non-Ischemic Dilated Cardiomyopathy: A Systematic Review. Biomedicines. 2020; 8(12):570. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines8120570
Chicago/Turabian StyleHoeeg, Cecilie, Sabina Frljak, Abbas Ali Qayyum, Bojan Vrtovec, Jens Kastrup, Annette Ekblond, and Bjarke Follin. 2020. "Efficacy and Mode of Action of Mesenchymal Stem Cells in Non-Ischemic Dilated Cardiomyopathy: A Systematic Review" Biomedicines 8, no. 12: 570. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines8120570