Serum Insufficiency Induces RANKL-Independent Osteoclast Formation during Developing Ischemic ONFH
Abstract
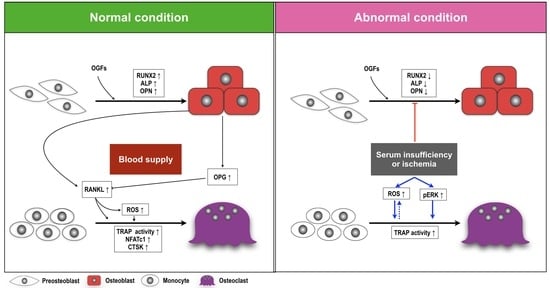
:1. Introduction
2. Results
2.1. Serum Insufficiency Suppressed Proliferation of Monocytes but Induced Osteoclastogenesis
2.2. Serum Reperfusion Inhibited Serum Insufficiency-Induced Osteoclastogenesis
2.3. Ischemia Suppressed Calcification, Alkaline Phosphatase Activity, and the Activation of Osteoblasts after Stimulation of Osteogenic Growth Factors
2.4. Ischemia-Induced Pattern Changes in Osteoblast and Osteoclast Proteins in Patients with and without Osteonecrosis
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Treatments
4.2. Protein Extraction and Immunoblotting Analysis
4.3. ALP Activity and Calcification
4.4. TRAP Staining and Activity
4.5. ROS Assay
4.6. Sample Collection from Patients
4.7. Image Analysis
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Larson, E.; Jones, L.C.; Goodman, S.B.; Koo, K.-H.; Cui, Q. Early-stage osteonecrosis of the femoral head: Where are we and where are we going in year 2018? Int. Orthop. 2018, 42, 1723–1728. [Google Scholar] [CrossRef] [PubMed]
- Lavernia, C.J.; Sierra, R.J.; Grieco, F.R. Osteonecrosis of the Femoral Head. J. Am. Acad. Orthop. Surg. 1999, 7, 250–261. [Google Scholar] [CrossRef]
- Weinstein, R.S.; Nicholas, R.W.; Manolagas, S.C. Apoptosis of Osteocytes in Glucocorticoid-Induced Osteonecrosis of the Hip 1. J. Clin. Endocrinol. Metab. 2000, 85, 2907–2912. [Google Scholar] [CrossRef]
- Pouya, F.; Kerachian, M.A. Avascular Necrosis of the Femoral Head: Are Any Genes Involved? Arch. Bone Jt. Surg. 2015, 3, 149–155. [Google Scholar]
- Li, D.; Liu, J.; Guo, B.; Liang, C.; Dang, L.; Lu, C.; He, X.; Cheung, H.Y.-S.; Xu, L.; Lu, C.; et al. Osteoclast-derived exosomal miR-214-3p inhibits osteoblastic bone formation. Nat. Commun. 2016, 7, 10872. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Meng, H.; Wang, Y.; Zhao, B.; Zhao, C.; Sun, W.; Zhu, Y.; Han, B.; Yuan, X.; Liu, R.; et al. Analysis of early stage osteonecrosis of the human femoral head and the mechanism of femoral head collapse. Int. J. Biol. Sci. 2018, 14, 156–164. [Google Scholar] [CrossRef]
- Wang, C.; Wang, X.; Xu, X.-L.; Yuan, X.-L.; Gou, W.-L.; Wang, A.-Y.; Guo, Q.-Y.; Peng, J.; Lu, S.-B. Bone Microstructure and Regional Distribution of Osteoblast and Osteoclast Activity in the Osteonecrotic Femoral Head. PLoS ONE 2014, 9, e96361. [Google Scholar] [CrossRef]
- Arnett, T.R.; Gibbons, D.C.; Utting, J.C.; Orriss, I.R.; Hoebertz, A.; Rosendaal, M.; Meghji, S. Hypoxia is a major stimulator of osteoclast formation and bone resorption. J. Cell. Physiol. 2003, 196, 2–8. [Google Scholar] [CrossRef]
- Yang, D.-C.; Yang, M.-H.; Tsai, C.-C.; Huang, T.-F.; Chen, Y.-H.; Hung, S.-C. Hypoxia Inhibits Osteogenesis in Human Mesenchymal Stem Cells through Direct Regulation of RUNX2 by TWIST. PLoS ONE 2011, 6, e23965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.H.; Lee, N.K.; Lee, A.S.Y. Current Understanding of RANK Signaling in Osteoclast Differentiation and Maturation. Mol. Cells 2017, 40, 706–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gianakos, A.L.; Moya-Angeler, J.; Duggal, S.; Zambrana, L.; Fields, K.G.; Mintz, D.N.; Cornell, C.N.; Lane, J.M. The Efficacy of Bisphosphonates with Core Decompression and Mesenchymal Stem Cells Compared with Bisphosphonates Alone in the Treatment of Osteonecrosis of the Hip: A Retrospective Study. HSS J. ® 2016, 12, 137–144. [Google Scholar] [CrossRef] [Green Version]
- Koo, K.H.; Kim, R.; Ko, G.H.; Song, H.R.; Jeong, S.T.; Cho, S.H. Preventing collapse in early osteonecrosis of the femoral head. A randomised clinical trial of core decompression. J. Bone Joint Surg. Br. 1995, 77, 870–874. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhang, Y.; Li, X.; Li, J.; Yang, S.; Xing, X.; Fan, G.; Yokota, H.; Zhang, P. eIF2α signaling regulates ischemic osteonecrosis through endoplasmic reticulum stress. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Vezzani, G.; Quartesan, S.; Cancellara, P.; Camporesi, E.; Mangar, D.; Bernasek, T.; Dalvi, P.; Yang, Z.; Paoli, A.; Rizzato, A.; et al. Hyperbaric oxygen therapy modulates serum OPG/RANKL in femoral head necrosis patients. J. Enzym. Inhib. Med. Chem. 2017, 32, 707–711. [Google Scholar] [CrossRef]
- Steinberg, E.M. Core decompression of the femoral head for avascular necrosis: indications and results. Can. J. Surg. 1995, 38, 18–24. [Google Scholar]
- Rackwitz, L.; Eden, L.; Reppenhagen, S.; Reichert, J.C.; Jakob, F.; Walles, H.; Pullig, O.; Tuan, R.S.; Rudert, M.; Nöth, U. Stem cell- and growth factor-based regenerative therapies for avascular necrosis of the femoral head. Stem Cell Res. Ther. 2012, 3, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helbig, L.; Simank, H.G.; Kroeber, M.; Schmidmaier, G.; Grützner, P.A.; Guehring, T. Core decompression combined with implantation of a demineralised bone matrix for non-traumatic osteonecrosis of the femoral head. Arch. Orthop. Trauma Surg. 2012, 132, 1095–1103. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, S.; Su, X. Core decompression and implantation of bone marrow mononuclear cells with porous hydroxylapatite composite filler for the treatment of osteonecrosis of the femoral head. Arch. Orthop. Trauma Surg. 2012, 133, 125–133. [Google Scholar] [CrossRef]
- Liu, B.-Y.; Zhao, D.-W.; Yu, X.-B.; Yang, L.; Guo, L.; Wang, B.-J. Effect of superior retinacular artery damage on osteonecrosis of the femoral head. Chin. Med. J. 2013, 126, 3845–3850. [Google Scholar] [PubMed]
- Miron, R.J.; Zhang, Y. Osteoinduction: A Review of Old Concepts with New Standards. J. Dent. Res. 2012, 91, 736–744. [Google Scholar] [CrossRef]
- Perez-Amodio, S.; Jansen, D.C.; Schoenmaker, T.; Vogels, I.M.C.; Reinheckel, T.; Hayman, A.R.; Cox, T.M.; Saftig, P.; Beertsen, W.; Everts, V. Calvarial Osteoclasts Express a Higher Level of Tartrate-Resistant Acid Phosphatase than Long Bone Osteoclasts and Activation Does not Depend on Cathepsin K or L Activity. Calcif. Tissue Int. 2006, 79, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Zenger, S.; Hollberg, K.; Ljusberg, J.; Norgård, M.; Ek-Rylander, B.; Kiviranta, R.; Andersson, G. Proteolytic processing and polarized secretion of tartrate-resistant acid phosphatase is altered in a subpopulation of metaphyseal osteoclasts in cathepsin K-deficient mice. Bone 2007, 41, 820–832. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, J.; Dou, C.; Li, N.; Kang, F.; Wang, Y.; Cao, Z.; Yang, X.; Dong, S. Alliin Attenuated RANKL-Induced Osteoclastogenesis by Scavenging Reactive Oxygen Species through Inhibiting Nox1. Int. J. Mol. Sci. 2016, 17, 1516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ichiseki, T.; Kaneuji, A.; Ueda, Y.; Nakagawa, S.; Mikami, T.; Fukui, K.; Matsumoto, T. Osteonecrosis development in a novel rat model characterized by a single application of oxidative stress. Arthritis Rheum. 2011, 63, 2138–2141. [Google Scholar] [CrossRef]
- Tsukamoto, I.; Iitsuka, N.; Hie, M.; Nakanishi, A. Ethanol increases osteoclastogenesis associated with the increased expression of RANK, PU.1 and MITF in vitro and in vivo. Int. J. Mol. Med. 2012, 30, 165–172. [Google Scholar] [CrossRef]
- Rao, L.G.; MacKinnon, E.S.; Josse, R.G.; Murray, T.M.; Strauss, A.; Rao, A.V. Lycopene consumption decreases oxidative stress and bone resorption markers in postmenopausal women. Osteoporos. Int. 2007, 18, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.K.; Choi, Y.G.; Baik, J.Y.; Han, S.Y.; Jeong, D.-W.; Bae, Y.S.; Kim, N.; Lee, S.Y. A crucial role for reactive oxygen species in RANKL-induced osteoclast differentiation. Blood 2005, 106, 852–859. [Google Scholar] [CrossRef] [Green Version]
- Han, B.; Geng, H.; Liu, L.; Wu, Z.; Wang, Y. GSH attenuates RANKL-induced osteoclast formation in vitro and LPS-induced bone loss in vivo. Biomed. Pharmacother. 2020, 128, 110305. [Google Scholar] [CrossRef] [PubMed]
- Pratap, J.; Galindo, M.; Zaidi, K.; Vradii, D.; Bhat, B.M.; A Robinson, J.; Choi, J.-Y.; Komori, T.; Stein, J.L.; Lian, J.B.; et al. Cell growth regulatory role of Runx2 during proliferative expansion of preosteoblasts. Cancer Res. 2003, 63, 5357–5362. [Google Scholar] [PubMed]
- Galindo, M.; Pratap, J.; Young, D.W.; Hovhannisyan, H.; Im, H.-J.; Choi, J.-Y.; Lian, J.B.; Stein, J.L.; Stein, G.S.; van Wijnen, A.J. The Bone-specific Expression of Runx2 Oscillates during the Cell Cycle to Support a G1-related Antiproliferative Function in Osteoblasts. J. Biol. Chem. 2005, 280, 20274–20285. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Shi, X.; Wu, J.; Wang, Y.; Lin, J.; Zhao, Y.; Li, H.; Ren, M.; Hu, R.; Liu, F.; et al. CoCl2 induced hypoxia enhances osteogenesis of rat bone marrow mesenchymal stem cells through cannabinoid receptor 2. Arch. Oral Biol. 2019, 108, 104525. [Google Scholar] [CrossRef] [PubMed]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsiao, Y.-m.; Hu, C.-C.; Chen, M.-F.; Chang, C.-H.; Chiu, Y.-T.; Chang, Y. Serum Insufficiency Induces RANKL-Independent Osteoclast Formation during Developing Ischemic ONFH. Biomedicines 2021, 9, 685. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines9060685
Hsiao Y-m, Hu C-C, Chen M-F, Chang C-H, Chiu Y-T, Chang Y. Serum Insufficiency Induces RANKL-Independent Osteoclast Formation during Developing Ischemic ONFH. Biomedicines. 2021; 9(6):685. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines9060685
Chicago/Turabian StyleHsiao, Yi-min, Chih-Chien Hu, Mei-Feng Chen, Chih-Hsiang Chang, Yu-Tien Chiu, and Yuhan Chang. 2021. "Serum Insufficiency Induces RANKL-Independent Osteoclast Formation during Developing Ischemic ONFH" Biomedicines 9, no. 6: 685. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines9060685