Arterial Pulse Wave Velocity Signal Reconstruction Using Low Sampling Rates
Abstract
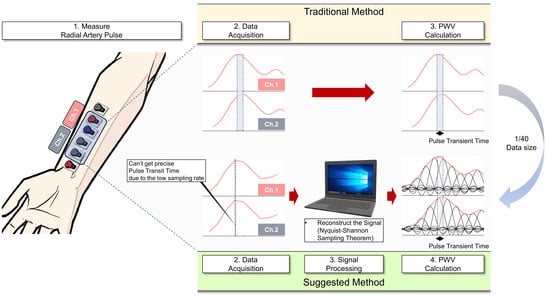
:1. Introduction
2. Materials and Methods
2.1. Measurement Setup for Radial Artery Pulse Using Bioimpedance
2.1.1. Hardware Setup Configuration
2.1.2. Software Setup Configuration
2.1.3. Data Acquisition
2.2. Biosignal Reconstruction and Processing
2.3. Data Validation
3. Results
3.1. Signal Reconstruction Results
3.2. Resolution Comparison with the Traditional High-Frequency Sampling Method
3.3. PWV Measurement Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Willum Hansen, T.; Staessen, J.A.; Torp-Pedersen, C.; Rasmussen, S.; Thijs, L.; Ibsen, H.; Jeppesen, J. Prognostic Value of Aortic Pulse Wave Velocity as Index of Arterial Stiffness in the General Population. Circulation 2006, 113, 664–670. [Google Scholar] [CrossRef]
- Sutton-Tyrrell, K.; Najjar, S.S.; Boudreau, R.M.; Venkitachalam, L.; Kupelian, V.; Simonsick, E.M.; Havlik, R.; Lakatta, E.G.; Spurgeon, H.; Kritchevsky, S. Elevated Aortic Pulse Wave Velocity, a Marker of Arterial Stiffness, Predicts Cardiovascular Events in Well-Functioning Older Adults. Circulation 2005, 111, 3384–3390. [Google Scholar] [CrossRef]
- Sugawara, J.; Hayashi, K.; Yokoi, T.; Cortez-Cooper, M.Y.; DeVan, A.E.; Anton, M.A.; Tanaka, H. Brachial–Ankle Pulse Wave Velocity: An Index of Central Arterial Stiffness? J. Hum. Hypertens. 2005, 19, 401–406. [Google Scholar] [CrossRef]
- Cecelja, M.; Chowienczyk, P. Dissociation of Aortic Pulse Wave Velocity with Risk Factors for Cardiovascular Disease Other than Hypertension: A Systematic Review. Hypertension 2009, 54, 1328–1336. [Google Scholar] [CrossRef]
- Meaume, S.; Rudnichi, A.; Lynch, A.; Bussy, C.; Sebban, C.; Benetos, A.; Safar, M.E. Aortic Pulse Wave Velocity as a Marker of Cardiovascular Disease in Subjects over 70 Years Old. J. Hypertens. 2001, 19, 871–877. [Google Scholar] [CrossRef]
- Ohkuma, T.; Ninomiya, T.; Tomiyama, H.; Kario, K.; Hoshide, S.; Kita, Y.; Inoguchi, T.; Maeda, Y.; Kohara, K.; Tabara, Y. Brachial-Ankle Pulse Wave Velocity and the Risk Prediction of Cardiovascular Disease: An Individual Participant Data Meta-Analysis. Hypertension 2017, 69, 1045–1052. [Google Scholar] [CrossRef]
- Nichols, W.W.; Denardo, S.J.; Wilkinson, I.B.; McEniery, C.M.; Cockcroft, J.; O’Rourke, M.F. Effects of Arterial Stiffness, Pulse Wave Velocity, and Wave Reflections on the Central Aortic Pressure Waveform. J. Clin. Hypertens. 2008, 10, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Fok, H.; Jiang, B.; Clapp, B.; Chowienczyk, P. Regulation of Vascular Tone and Pulse Wave Velocity in Human Muscular Conduit Arteries: Selective Effects of Nitric Oxide Donors to Dilate Muscular Arteries Relative to Resistance Vessels. Hypertension 2012, 60, 1220–1225. [Google Scholar] [CrossRef] [PubMed]
- Woolam, G.L.; Schnur, P.L.; Vallbona, C.; Hoff, H.E. The Pulse Wave Velocity as an Early Indicator of Atherosclerosis in Diabetic Subjects. Circulation 1962, 25, 533–539. [Google Scholar] [CrossRef]
- Chan, P.-K.; Chen, C.-C.; Yang, C.-L. Systolic and Diastolic Blood Pressure Estimation from Pulse Transit Time Using Dual Split-Ring Resonators with Notch Structure. In Proceedings of the 2019 IEEE MTT-S International Microwave Symposium (IMS), Boston, MA, USA, 2–7 June 2019; pp. 361–364. [Google Scholar]
- Meyer, M.D.; Agrawal, D.P. A High Sampling Rate Delayed LMS Filter Architecture. IEEE Trans. Circuits Syst. II Analog Digit. Signal Process. 1993, 40, 727–729. [Google Scholar] [CrossRef]
- Marino, D.; Musuvathi, M.; Narayanasamy, S. LiteRace: Effective Sampling for Lightweight Data-Race Detection. In Proceedings of the 30th ACM SIGPLAN Conference on Programming Language Design and Implementation, Dublin, Ireland, 15–21 June 2009; pp. 134–143. [Google Scholar]
- Dieter, W.R.; Datta, S.; Kai, W.K. Power Reduction by Varying Sampling Rate. In Proceedings of the 2005 International Symposium on Low Power Electronics and Design, San Diego, CA, USA, 8–10 August 2005; pp. 227–232. [Google Scholar]
- Tobola, A.; Streit, F.J.; Espig, C.; Korpok, O.; Sauter, C.; Lang, N.; Schmitz, B.; Hofmann, C.; Struck, M.; Weigand, C. Sampling Rate Impact on Energy Consumption of Biomedical Signal Processing Systems. In Proceedings of the 2015 IEEE 12th International Conference on Wearable and Implantable Body Sensor Networks (BSN), Cambridge, MA, USA, 9–12 June 2015; IEEE: Piscataway, NJ, USA, 2015; pp. 1–6. [Google Scholar]
- Zurbuchen, N.; Wilde, A.; Bruegger, P. A Machine Learning Multi-Class Approach for Fall Detection Systems Based on Wearable Sensors with a Study on Sampling Rates Selection. Sensors 2021, 21, 938. [Google Scholar] [CrossRef]
- Eisele, G.; Vachon, H.; Lafit, G.; Kuppens, P.; Houben, M.; Myin-Germeys, I.; Viechtbauer, W. The Effects of Sampling Frequency and Questionnaire Length on Perceived Burden, Compliance, and Careless Responding in Experience Sampling Data in a Student Population. Assessment 2022, 29, 136–151. [Google Scholar] [CrossRef]
- Landau, H.J. Sampling, Data Transmission, and the Nyquist Rate. Proc. IEEE 1967, 55, 1701–1706. [Google Scholar] [CrossRef]
- Haykin, S.; Van Veen, B. Signals and Systems; John Wiley & Sons: New York, NY, USA, 2007; ISBN 8126512652. [Google Scholar]
- Namkoong, M.; Baskar, B.; Singh, L.; Guo, H.; McMurray, J.; Branan, K.; Rahman, M.S.; Hsiao, C.-T.; Kuriakose, J.; Hernandez, J.; et al. Add-On Soft Electronic Interfaces for Continuous Cuffless Blood Pressure Monitoring. Adv. Mater. Technol. 2023, 8, 2300158. [Google Scholar] [CrossRef] [PubMed]
- Yazdanian, H.; Samani, M.M.; Mahanm, A. Characteristics of the Howland Current Source for Bioelectric Impedance Measurements Systems. In Proceedings of the 2013 20th Iranian Conference on Biomedical Engineering (ICBME), Tehran, Iran, 18–20 December 2013; pp. 189–193. [Google Scholar]
- Liu, J.; Qiao, X.; Wang, M.; Zhang, W.; Li, G.; Lin, L. The Differential Howland Current Source with High Signal to Noise Ratio for Bioimpedance Measurement System. Rev. Sci. Instrum. 2014, 85, 055111. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.-C.; Kim, J.-Y.; Cho, S.H. A Bio-Impedance Measurement System for Portable Monitoring of Heart Rate and Pulse Wave Velocity Using Small Body Area. In Proceedings of the 2009 IEEE International Symposium on Circuits and Systems (ISCAS), Taipei, Taiwan, 24–27 May 2009; IEEE: Piscataway, NJ, USA, 2009; pp. 3106–3109. [Google Scholar]
- Obeid, H.; Fortier, C.; Garneau, C.-A.; Pare, M.; Boutouyrie, P.; Bruno, R.M.; Khettab, H.; Goupil, R.; Agharazii, M. Radial-Digital Pulse Wave Velocity: A Noninvasive Method for Assessing Stiffness of Small Conduit Arteries. Am. J. Physiol. Circ. Physiol. 2021, 320, H1361–H1369. [Google Scholar] [CrossRef] [PubMed]
- Namkoong, M.; Guo, H.; Rahman, M.S.; Wang, D.; Pfeil, C.J.; Hager, S.; Tian, L. Moldable and Transferrable Conductive Nanocomposites for Epidermal Electronics. Npj Flex. Electron. 2022, 6, 41. [Google Scholar] [CrossRef] [PubMed]
- Cornish, B.H.; Ward, L.C.; Thomas, B.J.; Jebb, S.A.; Elia, M. Evaluation of Multiple Frequency Bioelectrical Impedance and Cole-Cole Analysis for the Assessment of Body Water Volumes in Healthy Humans. Eur. J. Clin. Nutr. 1996, 50, 159–164. [Google Scholar] [PubMed]
- Moqadam, S.M.; Grewal, P.K.; Haeri, Z.; Ingledew, P.A.; Kohli, K.; Golnaraghi, F. Cancer Detection Based on Electrical Impedance Spectroscopy: A Clinical Study. J. Electr. Bioimpedance 2018, 9, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Sherlock, B.G.; Monro, D.M. Moving Discrete Fourier Transform. IEE Proc. F (Radar Signal Process.) 1992, 139, 279–282. [Google Scholar] [CrossRef]
- Theußl, T.; Hauser, H.; Gröller, E. Mastering Windows: Improving Reconstruction. In Proceedings of the 2000 IEEE Symposium on Volume Visualization, Salt Lake City, UT, USA, 8–13 October 2000; pp. 101–108. [Google Scholar]
- Podder, P.; Khan, T.Z.; Khan, M.H.; Rahman, M.M. Comparative Performance Analysis of Hamming, Hanning and Blackman Window. Int. J. Comput. Appl. 2014, 96, 1–7. [Google Scholar] [CrossRef]
- Venkataramani, R.; Bresler, Y. Perfect Reconstruction Formulas and Bounds on Aliasing Error in Sub-Nyquist Nonuniform Sampling of Multiband Signals. IEEE Trans. Inf. Theory 2000, 46, 2173–2183. [Google Scholar] [CrossRef]
- Por, E.; van Kooten, M.; Sarkovic, V. Nyquist–Shannon Sampling Theorem; Leiden University: Leiden, The Netherlands, 2019; Volume 1. [Google Scholar]
- Jerri, A.J. The Shannon Sampling Theorem—Its Various Extensions and Applications: A Tutorial Review. Proc. IEEE 1977, 65, 1565–1596. [Google Scholar] [CrossRef]
- Grenander, U. Probability and Statistics: The Harald Cramér Volume; Almqvist & Wiksell: Stockholm, Sweden, 1959. [Google Scholar]
- Stiltz, H.L. Aerospace Telemetry; Prentice Hall: Hoboken, NJ, USA, 1961. [Google Scholar]
- Leis, J.W. Digital Signal Processing Using MATLAB for Students and Researchers; John Wiley & Sons: New York, NY, USA, 2011; ISBN 1118033809. [Google Scholar]
- Condon, J.J.; Ransom, S.M. Essential Radio Astronomy; Princeton University Press: Princeton, NJ, USA, 2016; Volume 2, ISBN 1400881161. [Google Scholar]
- Pauca, A.L.; O’Rourke, M.F.; Kon, N.D. Prospective Evaluation of a Method for Estimating Ascending Aortic Pressure from the Radial Artery Pressure Waveform. Hypertension 2001, 38, 932–937. [Google Scholar] [CrossRef] [PubMed]
- Duprez, D.A.; Kaiser, D.R.; Whitwam, W.; Finkelstein, S.; Belalcazar, A.; Patterson, R.; Glasser, S.; Cohn, J.N. Determinants of Radial Artery Pulse Wave Analysis in Asymptomatic Individuals. Am. J. Hypertens. 2004, 17, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Tyan, C.-C.; Liu, S.-H.; Chen, J.-Y.; Chen, J.-J.; Liang, W.-M. A Novel Noninvasive Measurement Technique for Analyzing the Pressure Pulse Waveform of the Radial Artery. IEEE Trans. Biomed. Eng. 2007, 55, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Tahmasebi, P.; Hezarkhani, A.; Sahimi, M. Multiple-Point Geostatistical Modeling Based on the Cross-Correlation Functions. Comput. Geosci. 2012, 16, 779–797. [Google Scholar] [CrossRef]
- Pan, B. Reliability-Guided Digital Image Correlation for Image Deformation Measurement. Appl. Opt. 2009, 48, 1535–1542. [Google Scholar] [CrossRef]
- Ott, H.W. Electromagnetic Compatibility Engineering; John Wiley & Sons: New York, NY, USA, 2011; ISBN 1118210654. [Google Scholar]
- Braun, S. Spectrum Analysis and EMI Measurements Based on Time-Domain Methods. In Proceedings of the 15th Conference on Microwave Techniques COMITE 2010, Brno, Czech Republic, 19–21 April 2010; IEEE: Piscataway, NJ, USA, 2010; pp. 13–18. [Google Scholar]
- Azpúrua, M.A.; Pous, M.; Çakir, S.; Çetinta, M.; Silva, F. Improving Time-Domain EMI Measurements through Digital Signal Processing. IEEE Electromagn. Compat. Mag. 2015, 4, 82–91. [Google Scholar] [CrossRef]
- Díaz, A.; Galli, C.; Tringler, M.; Ramírez, A.; Cabrera Fischer, E.I. Reference Values of Pulse Wave Velocity in Healthy People from an Urban and Rural Argentinean Population. Int. J. Hypertens. 2014, 2014, 653239. [Google Scholar] [CrossRef]
- Murakami, M.; Maeda, N.; Takemasa, S.; Tomoeda, M.; Kato, J. Effects of A Single Bout of Cycling Exercise on Pulse Wave Velocity (PWV)in Healthy Middle-Aged Individuals. J. Phys. Ther. Sci. 2012, 24, 427–429. [Google Scholar] [CrossRef]
- Papaioannou, T.G.; Oikonomou, E.; Lazaros, G.; Christoforatou, E.; Vogiatzi, G.; Tsalamandris, S.; Chasikidis, C.; Kalambogias, A.; Mystakidi, V.-X.; Galiatsatos, N. The Influence of Resting Heart Rate on Pulse Wave Velocity Measurement is Mediated by Blood Pressure and Depends on Aortic Stiffness Levels: Insights from the Corinthia Study. Physiol. Meas. 2019, 40, 55005. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Liu, Y.; Ni, S.; Cheng, G.; Hu, X. LossDetection: Real-Time Packet Loss Monitoring System for Sampled Traffic Data. IEEE Trans. Netw. Serv. Manag. 2023, 20, 30–45. [Google Scholar] [CrossRef]
- Liu, J.; Khattak, A.; Han, L.; Yuan, Q. How Much Information Is Lost When Sampling Driving Behavior Data? Indicators to Quantify the Extent of Information Loss. J. Intell. Connect. Veh. 2020, 3, 17–29. [Google Scholar] [CrossRef]
- Young, A.D.; Ling, M.J. Minimising Loss-Induced Errors in Real Time Wireless Sensing by Avoiding Data Dependency. In Proceedings of the 2009 Sixth International Workshop on Wearable and Implantable Body Sensor Networks, Berkeley, CA, USA, 3–5 June 2009; pp. 327–332. [Google Scholar]










| Sampling Rate | 250 Hz | 500 Hz | 1000 Hz | Traditional | ||||
|---|---|---|---|---|---|---|---|---|
| Conditions | Normal | Exercise | Normal | Exercise | Normal | Exercise | Normal | Exercise |
| PWV [m/s] | 6.04 | 6.41 | 6.27 | 6.89 | 6.35 | 6.06 | 6.87 | 7.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, S.; Coté, G. Arterial Pulse Wave Velocity Signal Reconstruction Using Low Sampling Rates. Biosensors 2024, 14, 92. https://0-doi-org.brum.beds.ac.uk/10.3390/bios14020092
Hong S, Coté G. Arterial Pulse Wave Velocity Signal Reconstruction Using Low Sampling Rates. Biosensors. 2024; 14(2):92. https://0-doi-org.brum.beds.ac.uk/10.3390/bios14020092
Chicago/Turabian StyleHong, Sungcheol, and Gerard Coté. 2024. "Arterial Pulse Wave Velocity Signal Reconstruction Using Low Sampling Rates" Biosensors 14, no. 2: 92. https://0-doi-org.brum.beds.ac.uk/10.3390/bios14020092