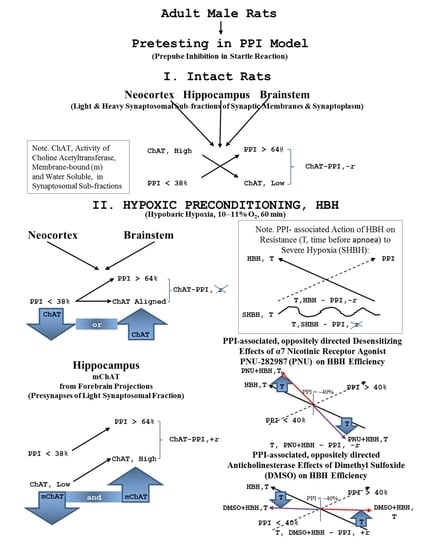
Opposite Pathways of Cholinergic Mechanisms of Hypoxic Preconditioning in the Hippocampus: Participation of Nicotinic α7 Receptors and Their Association with the Baseline Level of Startle Prepulse Inhibition
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Animals and Ethical Approval
2.2. Acoustic Startle Reaction Model
2.3. Hypoxic Models
2.4. Brain Tissue Preparation
2.5. Choline Acetyltransferase Assay
2.6. Drug Administration
2.7. Experimental Protocol
2.8. Reagents and Drugs
2.9. Data Analysis
3. Results
3.1. Biochemical Data Analysis
3.2. The Effects of PPI, HBH and PPI × HBH Interaction on the ChAT Activity in All Data Array (ANOVA Tests)
3.3. Comparison of the Synaptic ChAT Activity and PPI Values in Total Control and HBH Groups of Rats (Pearson’s r-Criterion)
3.4. PPI-Associated Level of ChAT Activity in Synaptic Sub-Fractions in the Control and HBH Sub-Groups
3.5. Pharmacological Data Analysis
3.6. PPI-Associated Effects of PNU and DMSO on HBH Efficiency
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, S.; Hafeez, A.; Noorulla, F.; Geng, X.; Shao, G.; Ren, C.; Lu, G.; Zhao, H.; Ding, Y.; Ji, X. Preconditioning in neuroprotection: From hypoxia to ischemia. Prog. Neurobiol. 2017, 157, 79–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Annachhatre, A.S.; Annachhatre, S.R. Preconditioning in cardiac anesthesia…… where are we? Ann. Card. Anaesth. 2019, 22, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Lee, K.S.; Kim, Y.S.; Kim, Y.H.; Kim, J.H. Effects of hypoxic preconditioning on memory evaluated using the T-maze behavior test. Anim. Cells Syst. (Seoul) 2019, 23, 10–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shojaei, F.; Rahmati, S.; Banitalebi Dehkordi, M. A review on different methods to increase the efficiency of mesenchymal stem cell-based wound therapy. Wound. Repair. Regen. 2019, 27, 661–671. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, M.; Li, H.; Yuan, J.; Li, J.; Wu, F.; Zhang, Y. Hypoxia preconditioning attenuates lung injury after thoracoscopic lobectomy in patients with lung cancer: A prospective randomized controlled trial. BMC Anesthesiol. 2019, 19, 209. [Google Scholar] [CrossRef]
- Späth, M.R.; Koehler, F.C.; Hoyer-Allo, K.J.R.; Grundmann, F.; Burst, V.; Müller, R.U. Preconditioning strategies to prevent acute kidney injury. F1000Res 2020, 9. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.-R.; Huang, Y.-Z.; Gao, H.-W.; Jiang, Y.-L.; Hu, J.-G.; Pi, J.-K.; Chen, A.-J.; Zhang, Y.; Zhou, L.; Xie, H.-Q. Hypoxic preconditioning of human urine-derived stem cell-laden small intestinal submucosa enhances wound healing potential. Stem Cell Res. Ther. 2020, 11, 150. [Google Scholar] [CrossRef] [Green Version]
- Saether, K.; Hilaire, G.; Monteau, R. Dorsal and ventral respiratory groups of neurons in the medulla of the rat. Brain Res. 1987, 419, 87–96. [Google Scholar] [CrossRef]
- Safonov, V.A.; Lebedeva, M.A. Automatism or Rhythmogenesis in the Respiratory Center. Fiziol. Cheloveka. 2003, 29, 108–121. [Google Scholar]
- Spyer, K.M.; Gourine, A.V. Chemosensory pathways in the brainstem controlling cardiorespiratory activity. Phil. Trans. R. Soc. B 2009, 364, 2603–2610. [Google Scholar] [CrossRef] [Green Version]
- Feldman, J.L. Chapter 14—Looking forward to breathing. Prog. Brain Res. 2011, 188, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Richter, D.W.; Smith, J.C. Respiratory rhythm generation in vivo. Physiology (Bethesda) 2014, 29, 58–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, R.A.; Berger, A.J. 1975. Neural regulation of respiration. Am. Rev. Respir. Dis. 1975, 111, 206–224. [Google Scholar] [CrossRef] [PubMed]
- Edlow, B.L.; McNab, J.A.; Witzel, T.; Kinney, H.C. The structural connectome of the human central homeostatic network. Brain Connect. 2016, 6, 187–200. [Google Scholar] [CrossRef] [Green Version]
- Arrigo, A.; Mormina, E.; Calamuneri, A.; Gaeta, M.; Marino, S.; Milardi, D.; Anastasi, G.P.; Quartarone, A. Amygdalar and hippocampal connections with brainstem and spinal cord: A diffusion MRI study in human brain. Neuroscience 2017, 343, 346–354. [Google Scholar] [CrossRef]
- Venkatraman, A.; Edlow, B.L.; Immordino-Yang, M.H. The brainstem in emotion: A review. Front. Neuroanat. 2017, 11, 15. [Google Scholar] [CrossRef]
- Pais-Roldán, P.; Edlow, B.L.; Jiang, Y.; Stelzer, J.; Zou, M.; Yu, X. Multimodal Assessment of Recovery From Coma in a Rat Model of Diffuse Brainstem Tegmentum Injury. Neuroimage 2019, 189, 615–630. [Google Scholar] [CrossRef]
- Araki, T.; Kato, H.; Fujiwara, T.; Kogure, K.; Itoyama, Y. Alteration of [3H]hemicholinium-3 binding in the post-ischaemic gerbil brain. Neuroreport 1995, 6, 561–564. [Google Scholar] [CrossRef]
- Dick, T.E.; Dutschmann, M.; Feldman, J.L.; Fong, A.Y.; Hülsmann, S.; Morris, K.M.; Ramirez, J.M.; Smith, J.C. Respiratory neurobiology consortium. Facts and challenges in respiratory neurobiology. Resp. Physiol. Neurobiol. 2018, 258, 104–107. [Google Scholar] [CrossRef]
- Zakharova, E.I.; Storozheva, Z.I.; Proshin, A.T.; Monakov, M.Y.; Dudchenko, A.M. Hypoxic Preconditioning: The multiplicity of central neurotransmitter mechanisms and method of predicting its efficiency. In Hypoxia and Anoxia; Das, K.K., Biradar, M.S., Eds.; InTechOPEN: London, UK, 2018; Chapter 6; pp. 95–131. [Google Scholar] [CrossRef] [Green Version]
- Das, M.; Das, D.K. Molecular Mechanism of Preconditioning. IUBMB Life 2008, 60, 199–203. [Google Scholar] [CrossRef]
- Lukyanova, L.D.; Germanova, E.L.; Tsibina, T.A.; Kopaladze, R.A.; Dudchenko, A.M. Efficiency and mechanism for different regimens of hypoxic training: The possibility of optimization of hypoxic therapy. Pathogenesis 2008, 6, 32–36. (In Russian) [Google Scholar]
- Kirova, I.I. Effect of hypoxia on dynamics of HIF-1alpha level in the cerebral cortex and development of adaptation in rats with different resistance to hypoxia. Patol. Fiziol. Eksp. Ter. 2012, 56, 51–55. (In Russian) [Google Scholar]
- Mukhin, E.I.; Zakharova, E.I.; Kikteva, E.A. Comparison of the cholinergic system in neocortical field Ep in cats with strong and weak cognitive abilities. Neurosci. Behav. Physiol. 2002, 32, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Zakharova, E.I.; Dudchenko, A.M.; Svinov, M.M.; Fedorova, M.M.; Germanova, E.L. Cholinergic Systems of the Rat Brain and Neuronal Reorganization under Conditions of Acute Hypoxia. Neurochem. J. 2010, 4, 290–303. [Google Scholar] [CrossRef]
- Zakharova, E.I.; Germanova, E.L.; Kopaladze, R.A.; Dudchenko, A.M. Central Cholinergic Systems in the Mechanisms of Hypoxic Preconditioning: Diverse Pathways of Synaptic Reorganization in Vivo. Neurochem. J. 2013, 7, 45–55. [Google Scholar] [CrossRef]
- Zakharova, E.I.; Storozheva, Z.I.; Dudchenko, A.M.; Kubatiev, A.A. Chronic cereral ischemia forms news mechanisms of learning and memory. Int. J. Alzheimers Dis. 2010, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Zakharova, E.I.; Dudchenko, A.M. Synaptic soluble and membrane-bound choline acetyltransferase as a marker of cholinergic function in vitro and in vivo. In Neurochemistry; Heinbockel, T., Ed.; InTechOpen: Rijeka, Croatia, 2014; Chapter 5; pp. 143–178. [Google Scholar] [CrossRef] [Green Version]
- Carrol, P.T.; Badamchian, M.; Craig, P.; Lyness, W.H. Veratridine-induced breakdown of 11 cytosolic acetylcholine in rat hippocampal minces: An intraterminal form of acetyl-12 cholinesterase or choline O-acetyltransferase? Brain Res. 1986, 383, 83–99. [Google Scholar] [CrossRef]
- Rylett, R.J.; Schmidt, B.M. Regulation of the synthesis of acetylcholine. Prog. Braim Res. 1993, 98, 161–166. [Google Scholar] [CrossRef]
- Carroll, P.T. Evidence to suggest that extracellular acetate is accumulated by rat hippocampal cholinergic nerve terminals for acetylcholine formation and release. Brain Res. 1997, 753, 47–55. [Google Scholar] [CrossRef]
- Bloc, A.; Bugnard, E.; Dunant, Y.; Falk-Vairant, J.; Israël, M.; Loctin, F.; Roulet, E. Acetylcho—14 line synthesis and quantal release reconstituted by transfection of mediatophore and 15 choline acetyltranferase cDNAs. Eur. J. Neurosci. 1999, 11, 1523–1534. [Google Scholar] [CrossRef]
- Sha, D.; Jin, H.; Kopke, R.D.; Wu, J.Y. Choline acetyltransferase: Regulation and coupling with protein kinase and vesicular acetylcholine transporter on synaptic vesicles. Neurochem. Res. 2004, 29, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Zakharova, E.I.; Dudchenko, A.M. Hypoxic Preconditioning Eliminates Differences in the Innate Resistance of Rats to Severe Hypoxia. J. Biomed. Sci. Eng. 2016, 9, 563–575. [Google Scholar] [CrossRef] [Green Version]
- Dudchenko, A.M.; Zakharova, E.I.; Storozheva, Z.I. Method for Predicting the Limit of Resistance of Animals to Severe Hypoxia after Hypoxic Preconditioning. RU Patent 2571603, 4 July 2014. [Google Scholar]
- Swerdlow, N.R.; Weber, M.; Qu, Y.; Light, G.A.; Braff, D.L. Realistic expectations of prepulse inhibition in translational models for schizophrenia research. Psychopharmacology 2008, 199, 331–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, A.H.; Megalou, E.V.; Wang, J.; Frost, W.N. Axonal conduction block as a novel mechanism of prepulse inhibition. J. Neurosci. 2012, 32, 15262–15270. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Nieto, R.; Hormigo, S.; López, D.E. Prepulse Inhibition of the Auditory Startle Reflex Assessment as a Hallmark of Sensorimotor Gating Mechanisms. Brain Sci. 2020, 10, E639. [Google Scholar] [CrossRef]
- Krause, E.; Benke, C.; Koenig, J.; Thayer, J.F.; Hamm, A.O.; Pané-Farré, C.A. Dynamics of Defensive Response Mobilization to Approaching External Versus Interoceptive Threat. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2018, 3, 525–538. [Google Scholar] [CrossRef]
- Shimohama, S.; Greenwald, D.L.; Shafron, D.H.; Akaika, A.; Maeda, T.; Kaneko, S.; Kimura, J.; Simpkins, C.E.; Day, A.; Meyer, E. Nicotinic alpha 7 receptors protect against glutamate neurotoxicity and neuronal ischemic damage. Brain Res. 1998, 779, 359–363. [Google Scholar] [CrossRef]
- van Rensburg, R.; Errington, D.R.; Ennaceur, A.; Lees, G.; Obrenovitch, T.P.; Chazot, P.L. A new model for the study of high-K(+)-induced preconditioning in cultured neurones: Role of N-methyl-d-aspartate and alpha7-nicotinic acetylcholine receptors. J. Neurosci. Methods 2009, 177, 311–316. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, L.; Tan, X.; Liu, B.; Zhang, Y.; Li, C. miR-210 Mediates Vagus Nerve Stimulation-Induced Antioxidant Stress and Anti-Apoptosis Reactions Following Cerebral Ischemia/Reperfusion Injury in Rats. J. Neurochem. 2015, 134, 173–181. [Google Scholar] [CrossRef] [Green Version]
- Jiang, T.; Wu, M.L.; Zhang, Z.L.; Yan, C.; Ma, Z.; He, S.; Yuan, W.; Pu, K.; Wang, Q. Electroacupuncture attenuated cerebral ischemic injury and neuroinflammation through α7nAChR-mediated inhibition of NLRP3 inflammasome in stroke rats. Mol. Med. 2019, 25, 22. Available online: https://0-molmed-biomedcentral-com.brum.beds.ac.uk/articles/10.1186/s10020-019-0091-4 (accessed on 22 May 2019). [CrossRef] [Green Version]
- Sun, F.; Johnson, S.R.; Jin, K.; Uteshev, V.V. Boosting Endogenous Resistance of Brain to Ischemia. Mol. Neurobiol. 2017, 54, 2045–2059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zakharova, E.I.; Storozheva, E.I.; Proshin, A.T.; Monakov, M.Y.; Dudchenko, A.M. The Acoustic Sensorimotor Gating Predicts the Efficiency of Hypoxic Preconditioning. Participation of the Cholinergic System in This Phenomenon. J. Biomed. Sci. Eng. 2018, 11, 10–25. [Google Scholar] [CrossRef] [Green Version]
- Sams, W.M., Jr.; Carroll, N.V. Cholinesterase inhibitory property of dimethyl sulphoxide. Nature 1966, 212, 405. [Google Scholar] [CrossRef] [PubMed]
- Alkondon, M.; Albuquerque, E.X. Diversity of nicotinic acetylcholine receptors in rat hippocampal neurons: I. Pharmacological and functional evidence for distinct structural subtypes. J. Pharmacol. Exp. Ther. 1993, 265, 1455–1473. [Google Scholar]
- Hajós, M.; Hurst, R.S.; Hoffmann, W.E.; Krause, M.; Wall, T.M.; Higdon, N.R.; Groppi, V.E. The selective alpha7 nicotinic acetylcholine receptor agonist PNU-282987 [N-[(3R)-1-Azabicyclo [2.2.2]oct-3-yl]-4-chlorobenzamide hydrochloride] enhances GABAergic synaptic activity in brain slices and restores auditory gating deficits in anesthetized rats. J. Pharmacol. Exp. Ther. 2005, 312, 1213–1222. [Google Scholar] [CrossRef]
- Stoiljkovic, M.; Leventhal, L.; Chen, A.; Chen, T.; Driscoll, R.; Flood, D.; Hodgdon, H.; Hurst, R.; Nagy, D.; Piser, T.; et al. Concentration-response relationship of the α7 nicotinic acetylcholine receptor agonist FRM-17874 across multiple in vitro and in vivo assays. Biochem. Pharmacol. 2015, 97, 576–589. [Google Scholar] [CrossRef]
- Fabian-Fine, R.; Skehel, P.; Errington, M.L.; Davies, H.A.; Sher, E.; Stewart, M.G.; Fine, A. Ultrastructural distribution of the alpha7 nicotinic acetylcholine receptor subunit in rat hippocampus. J. Neurosci. 2001, 21, 7993–8003. [Google Scholar] [CrossRef]
- Baddick, C.G.; Marks, M.J. An autoradiographic survey of mouse brain nicotinic acetylcholine receptors defined by null mutants. Biochem. Pharmacol. 2011, 82, 828–841. [Google Scholar] [CrossRef] [Green Version]
- Pevtsov, E.F.; Storozheva, Z.I.; Proshin, A.T.; Pevtsova, E.I. A Hardware-and-Software System for Experimental Studies of the Acoustic Startle Response in Laboratory Rodents. Bull. Exp. Biol. Med. 2016, 160, 410–413. [Google Scholar] [CrossRef]
- De Robertis, E.; De Pellegrino Iraldi, A.; Rodrigues De Lores Arnaiz, G.; Salganicoff, L. Cholinergic and non-cholinergic nerve endings in rat brain. I. Isolation and subcellular distribution of acetylcholine and acetylcholinesterase. J. Neurochem. 1962, 9, 23–35. [Google Scholar] [CrossRef]
- Cotman, C.W.; Matthews, D.A. Synaptic plasma membranes from rat brain synaptosomes: Isolation and partial characterization. Biochim. Biophys. Acta 1971, 249, 380–394. [Google Scholar] [CrossRef]
- Bogolepov, N.N.; Dovedova, E.L.; Orlova, E.I.; Yakovleva, N.I. Ultrastructural and biochemical characterization of the subfractions of synaptic membranes isolated from light and heavy synaptosomes of the brain. Arkh. Anat. Gistol. Embriol. 1985, 89, 88–94. (In Russian) [Google Scholar] [PubMed]
- Fonnum, F. Radiochemical microassays for the determination of choline acetyltransferase and acetylcholinesterase activities. Biochem. J. 1969, 115, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Kobzar, A.I. Applied Mathematical Statistics. For Engineers and Scientists; FIZMATLIT: Moscow, Russian, 2006. [Google Scholar]
- Ballmaier, M.; Casamenti, F.; Zoli, M.; Pepeu, G.; Spano, P. Selective Immunolesioning of Cholinergic Neurons in Nucleus Basalis Magnocellularis Impairs Prepulse Inhibition of Acoustic Startle. Neuroscience 2001, 108, 299–305. [Google Scholar] [CrossRef]
- Alkondon, M.; Pereira, E.F.; Potter, M.C.; Kauffman, F.C.; Schwarcz, R.; Albuquerque, E.X. Strain-specific nicotinic modulation of glutamatergic transmission in the CA1 field of the rat hippocampus: August Copenhagen Irish versus Sprague-Dawley. J. Neurophysiol. 2007, 97, 1163–1170. [Google Scholar] [CrossRef] [Green Version]
- Machold, R.P. Loss of rostral brainstem cholinergic activity results in decreased ultrasonic vocalization behavior and altered sensorimotor gating. Behav. Brain Res. 2013, 256, 51–55. [Google Scholar] [CrossRef]
- Azzopardi, E.; Louttit, A.G.; DeOliveira, C.; Laviolette, S.R.; Schmid, S. The Role of Cholinergic Midbrain Neurons in Startle and Prepulse Inhibition. J. Neurosci. 2018, 38, 8798–8808. [Google Scholar] [CrossRef]
- Schreiber, R.; Dalmus, M.; De Vry, J. Effects of alpha 4/beta 2- and alpha 7-nicotine acetylcholine receptor agonists on prepulse inhibition of the acoustic startle response in rats and mice. Psychopharmacology 2002, 159, 248–257. [Google Scholar] [CrossRef]
- Goktalay, T.; Buyukuysal, S.; Uslu, G.; Coskun, A.S.; Yorgancioglu, A.; Kayir, H.; Uzbay, T.; Goktalay, G. Varenicline disrupts prepulse inhibition only in high-inhibitory rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 2014, 53, 54–60. [Google Scholar] [CrossRef]
- Uslu, G.; Savci, V.; Buyukuysal, L.R.; Goktalay, G. CDP-choline attenuates scopolamine induced disruption of prepulse inhibition in rats: Involvement of central nicotinic mechanism. Neurosci. Lett. 2014, 569, 153–157. [Google Scholar] [CrossRef]
- Carroll, P.T. Veratridine-induced activation of choline-O-acetyltransferase activity in rat hippocampal tissue: Relationship to the veratridine-induced release of acetylcholine. Brain Res. 1987, 414, 401–404. [Google Scholar] [CrossRef]
- Schmidt, B.M.; Rylett, R.J. Phosphorylation of rat brain choline acetyltransferase and its relationship to enzyme activity. J. Nerochem. 1993, 61, 1774–1781. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.K.; Carroll, P.T. Membrane-bound choline-O-acetyltransferase in rat hippocampal tissue is anchored by glycosyl-phosphatidylinositol. Brain Res. 1993, 605, 155–163. [Google Scholar] [CrossRef]
- Cordeiro, J.M.; Gonçalves, P.P.; Dunant, Y. Synaptic vesicles control the time course of neurotransmitter secretion via a Ca²+/H+ antiport. J. Physiol. 2011, 589, 149–167. [Google Scholar] [CrossRef]
- North, P.E.; Mrak, R.E. Synaptosomal uptake of choline and of gamma-aminobutyric acid: Effects of ethanol and of dimethylsulfoxide. Neurotoxicology 1989, 10, 569–576. [Google Scholar]
- Nakahiro, M.; Arakawa, O.; Narahashi, T.; Ukai, S.; Kato, Y.; Nishinuma, K.; Nishimura, T. Dimethyl sulfoxide (DMSO) blocks GABA-induced current in rat dorsal root ganglion neurons. Neurosci. Lett. 1992, 138, 5–8. [Google Scholar] [CrossRef]
- Eaton, M.J.; Pagán, O.R.; Hann, R.M.; Eterović, V.A. Differential Effects of Dimethyl Sulfoxide on Nicotinic Acetylcholine Receptors from Mouse Muscle and Torpedo Electrocytes. Neurosci. Lett. 1997, 230, 163–166. [Google Scholar] [CrossRef]
- Soltani, N.; Mohammadi, E.; Allahtavakoli, M.; Shamsizadeh, A.; Roohbakhsh, A.; Haghparast, A. Effects of Dimethyl Sulfoxide on Neuronal Response Characteristics in Deep Layers of Rat Barrel Cortex. Basic Clin. Neurosci. 2016, 7, 213–220. [Google Scholar] [CrossRef]
- Neves, G.A.; Grace, A.A. Nicotinic receptor-modulating agents reverse the hyperdopaminergic tone in the MAM model of schizophrenia. Neuropsychopharmacology 2018, 43, 1712–1720. [Google Scholar] [CrossRef]
- Galvez, B.; Gross, N.; Sumikawa, K. Activation of α7 nicotinic acetylcholine receptors protects potentiated synapses from depotentiation during theta pattern stimulation in the hippocampal CA1 region of rats. Neuropharmacology 2016, 105, 378–387. [Google Scholar] [CrossRef] [Green Version]
- Vinogradova, O.S. Hippocampus as comparator: Role of the two input and two output systems of the hippocampus in selection and registration of information. Hippocampus 2001, 11, 578–598. [Google Scholar] [CrossRef] [PubMed]
- Lisman, J.E.; Grace, A.A. The hippocampal-VTA loop: Controlling the entry of information into long-term memory. Neuron 2005, 46, 703–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.H.; Redondo, R.L.; Morris, R.G. Relevance of synaptic tagging and capture to the persistence of long-term potentiation and everyday spatial memory. PNAS 2010, 107, 19537–19542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeuchi, T.; Duszkiewicz, A.J.; Sonneborn, A.; Spooner, P.A.; Yamasaki, M.; Watanabe, M.; Smith, C.C.; Fernández, G.; Deisseroth, K.; Greene, R.W.; et al. Locus coeruleus and dopaminergic consolidation of everyday memory. Nature 2016, 537, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Sanders, H.; Ji, D.; Sasaki, T.; Leutgeb, J.K.; Wilson, M.A.; Lisman, J.E. Temporal coding and rate remapping: Representation of nonspatial information in the hippocampus. Hippocampus 2019, 29, 111–127. [Google Scholar] [CrossRef] [Green Version]
- Kamiński, J. Intermediate-Term Memory as a Bridge between Working and Long-Term Memory. J. Neurosci. 2017, 17, 5045–5047. [Google Scholar] [CrossRef] [Green Version]
- Arkhipenko, Y.V.; Sazontova, T.G.; Zhukova, A.G. Adaptation to periodic hypoxia and hyperoxia improves resistance of membrane structures in heart, liver, and brain. Bull. Exp. Biol. Med. 2005, 140, 278–281. [Google Scholar] [CrossRef]
- Manukhina, E.B.; Terekhina, O.L.; Belkina, L.M.; Abramochkin, D.V.; Budanova, O.P.; Mashina, S.Y.; Smirin, B.V.; Yakunina, E.B.; Downey, H.F. Vasoprotective effect of adaptation to hypoxia in myocardial ischemia and reperfusion injury. Patol. Fiziol. Eksp. Ter. 2013, 57, 26–31. (In Russian) [Google Scholar]
- Rybnikova, E.; Samoilov, M. Current insights into the molecular mechanisms of hypoxic pre- and postconditioning using hypobaric hypoxia. Front. Neurosci. 2015, 9, 388. [Google Scholar] [CrossRef] [Green Version]
- Serebrovskaya, T.V.; Xi, L. Intermittent hypoxia training as non-pharmacologic therapy for cardiovascular diseases: Practical analysis on methods and equipment. Exp. Biol. Med. 2016, 241, 1708–1723. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.-J.; Yuan, Y.-J.; Liu, Y.-X.; Zhang, M.-Y.; Zhang, J.-G.; Wang, T.-C.; Zhang, L.-N.; Hu, Y.-Y.; Li, L.; Xian, X.-H.; et al. Nitric Oxide Participates in the Brain Ischemic Tolerance Induced by Intermittent Hypobaric Hypoxia in the Hippocampal CA1 Subfield in Rats. Neurochem. Res. 2018, 43, 1779–1790. [Google Scholar] [CrossRef] [PubMed]
- Nalivaeva, N.N.; Rybnikova, E.A. Editorial: Brain Hypoxia and Ischemia: New Insights Into Neurodegeneration and Neuroprotection. Front. Neurosci. 2019, 13, 770. [Google Scholar] [CrossRef] [PubMed]
- Ajayi, I.E.; McGovern, A.E.; Driessen, A.K.; Kerr, N.F.; Mills, P.C.; Stuart, B.; Mazzone, S.B. Hippocampal modulation of cardiorespiratory function. Respir. Physiol. Neurobiol. 2018, 252–253, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Vinogradova, O.S.; Brazhnik, E.S.; Kitchigina, V.F.; Stafekhina, V.S. Acetylcholine, theta-rhythm and activity of hippocampal neurons in the rabbit-IV Sensorystimulation. Neuroscience 1993, 53, 993–1007. [Google Scholar] [CrossRef]
- Vandecasteele, M.; Varga, V.; Berényi, A.; Papp, E.; Barthó, P.; Venance, L.; Freund, T.F.; Buzsáki, G. Optogenetic activation of septal cholinergic neurons suppresses sharp wave ripples and enhances theta oscillations in the hippocampus. PNAS 2014, 111, 13535–13540. [Google Scholar] [CrossRef] [Green Version]
- Müller, C.; Remy, S. Septo-hippocampal interaction. Cell Tissue Res. 2018, 373, 565–575. [Google Scholar] [CrossRef] [Green Version]
- Bassant, M.H.; Jouvenceau, A.; Apartis, E.; Poindessous-Jazat, F.; Dutar, P.; Billard, J.M. Immunolesion of the cholinergic basal forebrain: Effects on functional properties of hippocampal and septal neurons. Int. J. Dev. Neurosci. 1998, 16, 613–632. [Google Scholar] [CrossRef]
- Magno, L.; Barry, C.; Schmidt-Hieber, C.; Theodotou, P.; Häusser, M.; Kessaris, N. NKX2-1 Is Required in the Embryonic Septum for Cholinergic System Development, Learning, and Memory. Cell Rep. 2017, 20, 1572–1584. [Google Scholar] [CrossRef] [Green Version]
- Liu, A.K.L.; Lim, E.; Ahmed, I.; Chang, R.C.-C.; Pearce, R.K.B.; Gentleman, S.M. Revisiting the human cholinergic nucleus of the diagonal band of Broca. Neuropathol. Appl. Neurobiol. 2018, 44, 647–662. [Google Scholar] [CrossRef] [Green Version]
- Solari, N.; Hangya, B. Cholinergic modulation of spatial learning, memory and navigation. Eur. J. Neurosci. 2018, 48, 2199–2230. [Google Scholar] [CrossRef] [Green Version]
- Bland, B.H.; Bird, J.; Jackson, J.; Natsume, K. Medial septal modulation of the ascending brainstem hippocampal synchronizing pathways in the freely moving rat. Hippocampus 2006, 16, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, E.X.; Pereira, E.F.; Alkondon, M.; Rogers, S.W. Mammalian nicotinic acetylcholine receptors: From structure to function. Physiol. Rev. 2009, 89, 73–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, D.; Lape, R.; Dani, J.A. Timing and location of nicotinic activity enhances or depresses hippocampal synaptic plasticity. Neuron 2001, 31, 131–141. [Google Scholar] [CrossRef] [Green Version]
- Udakis, M.; Wright, V.L.; Wonnacott, S.; Bailey, C.P. Integration of inhibitory and excitatory effects of α7 nicotinic acetylcholine receptor activation in the prelimbic cortex regulates network activity and plasticity. Neuropharmacology 2016, 105, 618–629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hummos, A.; Nair, S.S. An integrative model of the intrinsic hippocampal theta rhythm. PLoS ONE 2017, 12, e0182648. [Google Scholar] [CrossRef] [Green Version]
- Lewis, A.S.; Pittenger, S.T.; Mineur, Y.S.; Stout, D.; Smith, P.H.; Picciotto, M.R. Bidirectional Regulation of Aggression in Mice by Hippocampal Alpha- 7 Nicotinic Acetylcholine Receptors. Neuropsychopharmacology 2018, 43, 1267–1275. [Google Scholar] [CrossRef]
- Benfante, R.; Di Lascio, S.; Cardani, S.; Fornasari, D. Acetylcholinesterase inhibitors targeting the cholinergic anti-inflammatory pathway: A new therapeutic perspective in aging-related disorders. Aging Clin. Exp. Res. 2019, 1–12. [Google Scholar] [CrossRef]
- Kupferschmidt, D.A.; Gordon, J.A. The dynamics of disordered dialogue: Prefrontal, hippocampal and thalamic miscommunication underlying working memory deficits in schizophrenia. Brain Neurosci. Adv. 2018, 2, 1–29. [Google Scholar] [CrossRef]
- Zakharova, E.I.; Storozheva, Z.I.; Proshin, A.T.; Monakov, M.Y.; Dudchenko, A.M. The specificity of cholinergic mechanisms of learning and memory in rats with different levels of ability for spatial contextual learning in the Morris water maze. Patol. Fiziol. Eksp. Ter. 2018, 62, 13–20. (In Russian) [Google Scholar] [CrossRef]
- Yi, F.; Catudio-Garrett, E.; Gábriel, R.; Wilhelm, M.; Erdelyi, F.; Szabo, G.; Deisseroth, K.; Lawrence, J. Hippocampal “cholinergic interneurons” visualized with the choline acetyltransferase promoter: Anatomical distribution, intrinsic membrane properties, neurochemical characteristics, and capacity for cholinergic modulation. Front. Synaptic Neurosci. 2015, 7, 4. [Google Scholar] [CrossRef] [Green Version]
- Pignatelli, M.; Beyeler, A.; Leinekugel, X. Neural circuits underlying the generation of theta oscillations. J. Physiol. Paris 2012, 106, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Cheng, X.; Lee, K.-W.; Amreen, B.; McCabe, K.A.; Pitcher, C.; Liebmann, T.; Greengard, P.; Flajolet, M. Cholinergic Neurons of the Medial Septum Are Crucial for Sensorimotor Gating. J. Neurosci. 2019, 39, 5234–5242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plowey, E.D.; Kramer, J.M.; Beatty, J.A.; Waldrop, T.G. In vivo electrophysiological responses of pedunculopontine neurons to static muscle contraction. Am. J. Physiol. Regul Integr. Comp. Physiol. 2002, 283, R1008–R1019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woolf, N.J.; Butcher, L.L. Cholinergic systems in the rat brain: III. Projections from the pontomesencephalic tegmentum to the thalamus, tectum, basal ganglia, and basal forebrain. Brain Res. Bull. 1986, 16, 603–637. [Google Scholar] [CrossRef]
- Semba, K.; Reiner, P.B.; McGeer, E.G.; Fibiger, H.C. Brainstem afferents to the magnocellular basal forebrain studied by axonal transport, immunohistochemistry, and electrophysiology in the rat. J. Comp. Neurol. 1988, 267, 433–453. [Google Scholar] [CrossRef]
- Woolf, N.J.; Butcher, L.L. Cholinergic systems in the rat brain: IV. Descending projections of the pontomesencephalic tegmentum. Brain Res. Bull. 1989, 23, 519–540. [Google Scholar] [CrossRef]
- Semba, K.; Reiner, P.B.; McGeer, E.G.; Fibiger, H.C. Brainstem projecting neurons in the rat basal forebrain: Neurochemical, topographical, and physiological distinctions from cortically projecting cholinergic neurons. Brain Res. Bull. 1989, 22, 501–509. [Google Scholar] [CrossRef]
- Río-Álamos, C.; Piludu, M.A.; Gerbolés, C.; Barroso, D.; Oliveras, I.; Sánchez-González, A.; Cañete, T.; Tapias-Espinosa, C.; Sampedro-Viana, D.; Torrubia, R.; et al. Volumetric brain differences between the Roman rat strains: Neonatal handling effects, sensorimotor gating and working memory. Behav. Brain Res. 2019, 361, 74–85. [Google Scholar] [CrossRef]
- Leszkowicz, E.; Kuśmierczak, M.; Matulewicz, P.; Trojniar, W. Modulation of hippocampal theta rhythm by the opioid system of the pedunculopontine tegmental nucleus. Acta Neurobiol. Exp. (Wars) 2007, 67, 447–460. [Google Scholar]





| Brain Structure | Sub-Fraction | Total Control and HBH Groups | ||
|---|---|---|---|---|
| PPI | HBH | PPI × HBH | ||
| Neocortex | C, SM | F(1, 4) = 0.302, p = 0.60 | F(1, 4) = 0.95, p = 0.42 | F(1, 4) = 5.8, p = 0.050 |
| C, Sp | F(1, 4) = 0.004, p = 0.96 | F(1, 4) = 0.058, p = 0.83 | F(1, 4) = 0.93, p = 0.44 | |
| D, SM | F(1, 4) = 3.9, p = 0.12 | F(1, 4) = 3,9, p = 0.11 | F(1, 4) = 2.9, p = 0.14 | |
| D, Sp | F(1, 4) = 0.19, p = 0.69 | F(1, 4) = 12.6 p = 0.013 | F(1, 4) = 0.72, p = 0.43 | |
| Hippocampus | C, SM | F(1, 4) = 4.21, p = 0.075 | F(1, 4) = 10.81, p = 0.010 | F(1, 4) = 63.1, p = 0.0002 |
| C, Sp | F(1, 4) = 51.9, p = 0.0004 | F(1, 4) = 45.88, p = 0.0005 | F(1, 4) = 88.5, p = 0.00008 | |
| D, SM | F(1, 4) = 10.36, p = 0.019 | F(1, 4) = 1.15, p = 0.33 | F(1, 4) = 1.10, p = 0.34 | |
| D, Sp | F(1, 4) = 4.32, p = 0.079 | F(1, 4) = 0.81, p = 0.39 | F(1, 4) = 297, p = 0.13 | |
| Caudal Brainstem | C, SM | F(1, 4) = 9.3, p = 0.023 | F(1, 4) = 1.07, p = 0.34 | F(1, 4) = 3.19, p = 0.12 |
| C, Sp | F(1, 4) = 0.17, p = 0.82 | F(1, 4) = 2.8, p = 0.14 | F(1, 4) = 1.8, p = 0.22 | |
| D, SM | F(1, 4) = 0.22, p = 0.66 | F(1, 4) = 2.4, p = 0.19 | F(1, 4) = 0.08, p = 0.91 | |
| D, Sp | F(1, 4) = 1.09, p = 0.32 | F(1, 4) = 0.11, p = 0.83 | F(1, 4) = 3.15, p = 0.12 | |
| Brain Structure | Neocortex | Hippocampus | Caudal Brainstem | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sub-Fraction | C, SM | C, Sp | D, SM | D, Sp | C, SM | C, Sp | D, SM | D, Sp | C, SM | C, Sp | D, SM | D, Sp | |
| Control Group n = 6 | r | −0.849 | −0.305 | −0.959 | −0.279 | −0.964 | −0.821 | −0.914 | −0.981 | −0.951 | −0.844 | −0.496 | −0.753 |
| p | <0.05 | >0.05 | <0.01 | >0.05 | <0.01 | <0.05 | <0.02 | <0.001 | <0.01 | <0.05 | >0.05 | >0.05 | |
| HBH Group n = 6 | r | +0.219 | −0.096 | −0.134 | −0.190 | +0.920 | +0.892 | −0.871 | −0.349 | −0.455 | +0.246 | −0.177 | +0.259 |
| p | >0.05 | >0.05 | >0.05 | >0.05 | <0.01 | <0.02 | <0.05 | >0.05 | >0.05 | >0.05 | >0.05 | >0.05 | |
| Brain Structure | Cortex | Hippocampus | Caudal Brainstem | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sub-Fraction | C, SM | C, Sp | D, SM | D, Sp | C, SM | C, Sp | D, SM | D, Sp | C, SM | C, Sp | D, SM | D, Sp | |
| PPI < 40% | |||||||||||||
| Control sub-group n = 3 | M | 3.850 | 13.480 | 0.0382 | 0.973 | 1.241 | 12.025 | 0.0611 | 0.787 | 0.273 | 1.868 | 0.0287 | 0.730 |
| ± SEM | 0.251 | 0.637 | 0.004 | 0.059 | 0.016 | 0.595 | 0.008 | 0.021 | 0.014 | 0.081 | 0.004 | 0.052 | |
| PPI > 40% | |||||||||||||
| Control sub-group n = 3 | M | 2.960 | 13.00. | 0.0189 | 0.860 | 0.793 | 8.103 | 0.0451 | 0.580 | 0.182 | 1.391 | 0.0209 | 0.583 |
| ± SEM | 0.205 | 0.613 | 0.0003 | 0.058 | 0.031 | 0.081 | 0.002 | 0.029 | 0.021 | 0.071 | 0.001 | 0.010 | |
| PPIxneuron type | |||||||||||||
| Total Control group n = 6 | PPI | F(1,4) = 0.28, p = 0.24 | F(1,4) = 0.13, p = 0.82 | F(1,4) = 193, p = 0.005 | F(1,4) = 0.7, p = 0.51 | F(1,4) = 33.2, p = 0.028 | F(1,4) = 95.2, p = 0.01 | F(1,4) = 2.56, p = 0.27 | F(1,4) = 14, p = 0.1 | F(1,4) = 13.8, p = 0.1 | F(1,4) = 11.8, p = 0.07 | F(1,4) = 0.22, p = 0.69 | F(1,4) = 2.4, p = 0.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zakharova, E.I.; Storozheva, Z.I.; Proshin, A.T.; Monakov, M.Y.; Dudchenko, A.M. Opposite Pathways of Cholinergic Mechanisms of Hypoxic Preconditioning in the Hippocampus: Participation of Nicotinic α7 Receptors and Their Association with the Baseline Level of Startle Prepulse Inhibition. Brain Sci. 2021, 11, 12. https://0-doi-org.brum.beds.ac.uk/10.3390/brainsci11010012
Zakharova EI, Storozheva ZI, Proshin AT, Monakov MY, Dudchenko AM. Opposite Pathways of Cholinergic Mechanisms of Hypoxic Preconditioning in the Hippocampus: Participation of Nicotinic α7 Receptors and Their Association with the Baseline Level of Startle Prepulse Inhibition. Brain Sciences. 2021; 11(1):12. https://0-doi-org.brum.beds.ac.uk/10.3390/brainsci11010012
Chicago/Turabian StyleZakharova, Elena I., Zinaida I. Storozheva, Andrey T. Proshin, Mikhail Yu. Monakov, and Alexander M. Dudchenko. 2021. "Opposite Pathways of Cholinergic Mechanisms of Hypoxic Preconditioning in the Hippocampus: Participation of Nicotinic α7 Receptors and Their Association with the Baseline Level of Startle Prepulse Inhibition" Brain Sciences 11, no. 1: 12. https://0-doi-org.brum.beds.ac.uk/10.3390/brainsci11010012