Neural Correlates of Numerical Estimation: The Role of Strategy Use
Abstract
:1. Introduction
1.1. Brain and Calculation
1.2. Brain–Exact vs. Approximate
1.3. Computation Estimation
1.4. Present Study
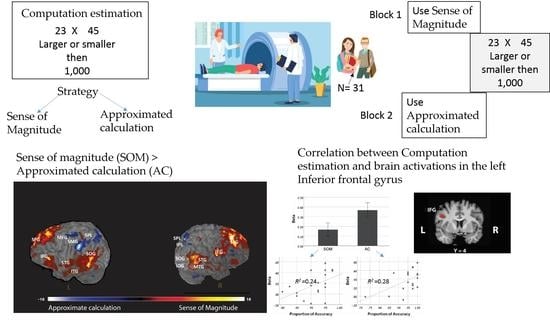
2. Materials and Methods
2.1. Brain Imaging
2.1.1. Procedure and Stimuli
Basic Task
2.1.2. fMRI Data Acquisition
2.1.3. fMRI Preprocessing
2.1.4. Statistical Analysis
3. Results
3.1. Behavioral Analysis
3.2. fMRI Analysis
3.2.1. AC Strategy
3.2.2. SOM Block
3.2.3. Differences between the Strategies
3.2.4. Region of Interest Analysis
4. Discussion
4.1. Distinct Codes Are Involved in SOM and Approximate Calculation
4.2. Left Lateralization of Brain Activity in AC but Not SOM
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chochon, F.; Cohen, L.; van de Moortele, P.F.; Dehaene, S. Differential contributions of the left and right inferior parietal lobules to number processing. J. Cogn. Neurosci. 1999, 11, 617–630. [Google Scholar] [CrossRef] [Green Version]
- Fulbright, R.K.; Molfese, D.L.; Stevens, A.A.; Skudlarski, P.; Lacadie, C.M.; Gore, J.C. Cerebral activation during multiplication: A functional MR imaging study of number processing. Am. J. Neuroradiol. 2000, 21, 1048–1054. [Google Scholar] [PubMed]
- Culham, J.C.; Kanwisher, N.G. Neuroimaging of cognitive functions in human parietal cortex. Curr. Opin. Neurobiol. 2001, 11, 157–163. [Google Scholar] [CrossRef]
- Ashkenazi, S.; Rosenberg-Lee, M.; Tenison, C.; Menon, V. Weak task-related modulation and stimulus representations during arithmetic problem solving in children with developmental dyscalculia. Dev. Cogn. Neurosci. 2012, 2, S152–S166. [Google Scholar] [CrossRef] [Green Version]
- Cantlon, J.F.; Brannon, E.M.; Carter, E.J.; Pelphrey, K.A. Functional imaging of numerical processing in adults and 4-y-old children. PLoS Biol. 2006, 4, e125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cantlon, J.F.; Libertus, M.E.; Pinel, P.; Dehaene, S.; Brannon, E.M.; Pelphrey, K.A. The neural development of an abstract concept of number. J. Cogn. Neurosci. 2009, 21, 2217–2229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadosh, R.C.; Lammertyn, J.; Izard, V. Are numbers special? An overview of chronometric, neuroimaging, developmental and comparative studies of magnitude representation. Prog. Neurobiol. 2008, 84, 132–147. [Google Scholar]
- Taillan, J.; Ardiale, E.; Anton, J.-L.; Nazarian, B.; Félician, O.; Lemaire, P. Processes in arithmetic strategy selection: A fMRI study. Front. Psychol. 2015, 6, 61. [Google Scholar] [CrossRef] [Green Version]
- Dehaene, S.; Spelke, E.; Pinel, P.; Stanescu, R.; Tsivkin, S. Sources of mathematical thinking: Behavioral and brain-imaging evidence. Science 1999, 284, 970–974. [Google Scholar] [CrossRef] [Green Version]
- Ganor-Stern, D.; Gliksman, Y.; Naparstek, S.; Ifergane, G.; Henik, A. Damage to the intraparietal sulcus impairs magnitude representations of results of complex arithmetic problems. Neuroscience 2020, 438, 137–144. [Google Scholar] [CrossRef]
- Stanescu-Cosson, R.; Pinel, P.; van de Moortele, P.F.; Le Bihan, D.; Cohen, L.; Dehaene, S. Understanding dissociations in dyscalculia: A brain imaging study of the impact of number size on the cerebral networks for exact and approximate calculation. Brain 2000, 123, 2240–2255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemer, C.; Dehaene, S.; Spelke, E.; Cohen, L. Approximate quantities and exact number words: Dissociable systems. Neuropsychologia 2003, 41, 1942–1958. [Google Scholar] [CrossRef]
- Li, M.; Tan, Y.; Cui, J.; Chen, C.; Dong, Q.; Zhou, X. The semantic network supports approximate computation. Neuropsychology 2019, 33, 842–854. [Google Scholar] [CrossRef] [PubMed]
- Klein, E.; Nuerk, H.-C.; Wood, G.; Knops, A.; Willmes, K. The exact vs. approximate distinction in numerical cognition may not be exact, but only approximate: How different processes work together in multi-digit addition. Brain Cogn. 2009, 69, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Kucian, K.; von Aster, M.; Loenneker, T.; Dietrich, T.; Martin, E. Development of neural networks for exact and approximate calculation: A FMRI study. Dev. Neuropsychol. 2008, 33, 447–473. [Google Scholar] [CrossRef]
- Lin, J.-F.L.; Imada, T.; Kuhl, P.K. Mental addition in bilinguals: An fMRI study of task-related and performance-related activation. Cereb. Cortex 2011, 22, 1851–1861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dowker, A. Young children’s addition estimates. Math. Cogn. 1997, 3, 140–153. [Google Scholar] [CrossRef]
- Lemaire, P.; Lecacheur, M.; Farioli, F. Children’s strategy use in computational estimation. Can. J. Exp. Psychol. Rev. Can. Psychol. Expérimentale 2000, 54, 141. [Google Scholar] [CrossRef]
- Imbo, I.; LeFevre, J.-A. Cultural differences in strategic behavior: A study in computational estimation. J. Exp. Psychol. Learn. Mem. Cogn. 2011, 37, 1294–1301. [Google Scholar] [CrossRef] [Green Version]
- Lemaire, P.; Lecacheur, M. Children’s strategies in computational estimation. J. Exp. Child Psychol. 2002, 82, 281–304. [Google Scholar] [CrossRef]
- Lemaire, P.; Arnaud, L.; Lecacheur, M. Adults’ Age-Related Differences in Adaptivity of Strategy Choices: Evidence from Computational Estimation. Psychol. Aging 2004, 19, 467–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganor-Stern, D. When you don’t have to be exact: Investigating computational estimation skills with a comparison task. Acta Psychol. 2015, 154, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ganor-Stern, D. Can Dyscalculics Estimate the Results of Arithmetic Problems? J. Learn. Disabil. 2017, 50, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Ganor-Stern, D. Approximation processes in arithmetic in old adulthood. PLoS ONE 2018, 13, e0200136. [Google Scholar] [CrossRef] [PubMed]
- Ganor-Stern, D. Do exact calculation and computation estimation reflect the same skills? Developmental and individual differences perspectives. Front. Psychol. 2018, 9, 1316. [Google Scholar] [CrossRef] [Green Version]
- Duverne, S.; Lemaire, P. Arithmetic split effects reflect strategy selection: An adult age comparative study in addition comparison and verification tasks. Can. J. Exp. Psychol. Rev. Can. Psychol. Expérimentale 2005, 59, 262–278. [Google Scholar] [CrossRef]
- LeFevre, J.-A.; Greenham, S.L.; Waheed, N. The Development of Procedural and Conceptual Knowledge in Computational Estimation. Cogn. Instr. 1993, 11, 95–132. [Google Scholar] [CrossRef]
- Lemaire, P.; Siegler, R.S. Four aspects of strategic change: Contributions to children’s learning of multiplication. J. Exp. Psychol. Gen. 1995, 124, 83. [Google Scholar] [CrossRef]
- Siegler, R.S.; Lemaire, P. Older and younger adults’ strategy choices in multiplication: Testing predictions of ASCM using the choice/no-choice method. J. Exp. Psychol. General 1997, 126, 71. [Google Scholar] [CrossRef]
- Ganor-Stern, D.; Weiss, N. Tracking practice effects in computation estimation. Psychol. Res. 2015, 80, 434–448. [Google Scholar] [CrossRef]
- Desli, D.; Lioliou, A. Relationship between Computational Estimation and Problem Solving. Int. Electron. J. Math. Educ. 2020, 15, em0602. [Google Scholar] [CrossRef]
- Ganor-Stern, D. Solving Math Problems Approximately: A Developmental Perspective. PLoS ONE 2016, 11, e0155515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganor-Stern, D.; Steinhorn, O. ADHD and math—The differential effect on calculation and estimation. Acta Psychol. 2018, 188, 55–64. [Google Scholar] [CrossRef]
- Rubia, K.; Smith, A.B.; Taylor, E.; Brammer, M. Linear age-correlated functional development of right inferior fronto-striato-cerebellar networks during response inhibition and anterior cingulate during error-related processes. Hum. Brain Mapp. 2007, 28, 1163–1177. [Google Scholar] [CrossRef] [PubMed]
- Dehaene, S.; Cohen, L. Cerebral Pathways for Calculation: Double Dissociation between Rote Verbal and Quantitative Knowledge of Arithmetic. Cortex 1997, 33, 219–250. [Google Scholar] [CrossRef]
- Peterson, B.S.; Skudlarski, P.; Gatenby, J.; Zhang, H.; Anderson, A.W.; Gore, J.C. An fMRI study of stroop word-color interference: Evidence for cingulate subregions subserving multiple distributed attentional systems. Biol. Psychiatry 1999, 45, 1237–1258. [Google Scholar] [CrossRef]
- Arsalidou, M.; Taylor, M.J. Is 2 + 2 = 4? Meta-analyses of brain areas needed for numbers and calculations. NeuroImage 2011, 54, 2382–2393. [Google Scholar] [CrossRef]
- Silk, T.J.; Bellgrove, M.; Wrafter, P.; Mattingley, J.; Cunnington, R. Spatial working memory and spatial attention rely on common neural processes in the intraparietal sulcus. NeuroImage 2010, 53, 718–724. [Google Scholar] [CrossRef]
- Zarnhofer, S.; Braunstein, V.; Ebner, F.; Koschutnig, K.; Neuper, C.; Reishofer, G.; Ischebeck, A. The influence of verbalization on the pattern of cortical activation during mental arithmetic. Behav. Brain Funct. 2012, 8, 13. [Google Scholar] [CrossRef] [Green Version]
- Dehaene, S.; Piazza, M.; Pinel, P.; Cohen, L. Three parietal circuits for number processing. Cogn. Neuropsychol. 2003, 20, 487–506. [Google Scholar] [CrossRef] [Green Version]
- Zago, L.; Petit, L.; Turbelin, M.-R.; Andersson, F.; Vigneau, M.; Tzourio-Mazoyer, N. How verbal and spatial manipulation networks contribute to calculation: An fMRI study. Neuropsychologia 2008, 46, 2403–2414. [Google Scholar] [CrossRef] [PubMed]
- Van Harskamp, N.J.; Rudge, P.; Cipolotti, L. Are multiplication facts implemented by the left supramarginal and angular gyri? Neuropsychologia 2002, 40, 1786–1793. [Google Scholar] [CrossRef]
- Venkatraman, V.; Ansari, D.; Chee, M.W. Neural correlates of symbolic and non-symbolic arithmetic. Neuropsychologia 2005, 43, 744–753. [Google Scholar] [CrossRef] [PubMed]
- Hauser, T.U.; Rotzer, S.; Grabner, R.H.; Mérillat, S.; Jäncke, L. Enhancing performance in numerical magnitude processing and mental arithmetic using transcranial Direct Current Stimulation (tDCS). Front. Hum. Neurosci. 2013, 7, 244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cappelletti, M.; Muggleton, N.; Walsh, V. Quantity without numbers and numbers without quantity in the parietal cortex. NeuroImage 2009, 46, 522–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andres, M.; Seron, X.; Olivier, E. Hemispheric lateralization of number comparison. Cogn. Brain Res. 2005, 25, 283–290. [Google Scholar] [CrossRef]



| Region | Hem. | BA | Number of Voxels | Peak Talairach Coordinates | Peak t(18) Score | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| Activations | |||||||
| Middle frontal gyrus | R | 9 | 26,647 | 36 | 35 | 31 | 10.245 |
| Insula | R | 29 | 20 | 8 | 9.544 | ||
| Middle frontal gyrus | L | 9 | 14,114 | −40 | 36 | 26 | 6.980 |
| Inferior frontal gyrus | L | 46 | −39 | 43 | 8 | 6.427 | |
| Inferior frontal gyrus | L | 6 | −35 | 1 | 25 | 6.439 | |
| Intraparietal sulcus | L | 7 | 20,329 | −32 | −66 | 48 | 8.822 |
| Intraparietal sulcus | L | 19 | −29 | −66 | 36 | 8.535 | |
| Cuneus | L | 7 | −9 | −72 | 37 | 8.061 | |
| Intraparietal sulcus | L | 40 | −49 | −43 | 44 | 7.994 | |
| Cuneus | R | 7 | 8 | −77 | 45 | 6.358 | |
| Intraparietal sulcus | R | 7 | 28 | −61 | 30 | 5.255 | |
| Superior frontal gyrus | L | 8 | 5669 | −2 | 15 | 49 | 7.396 |
| Insula | L | 5651 | −28 | 11 | 9 | 4.963 | |
| Extra-nuclear/Corpus clausum | L | 30 | 1367 | −20 | −39 | 8 | 6.480 |
| Fusiform gyrus | R | 37 | 46,314 | 48 | −52 | −14 | 8.160 |
| Fusiform gyrus | L | 18 | −29 | −85 | −14 | 7.571 | |
| Fusiform gyrus | L | 37 | −48 | −49 | −17 | 6.706 | |
| Deactivations | |||||||
| Middle temporal gyrus | R | 22 | 1107 | 51 | −7 | −8 | 6.259 |
| Medial frontal gyrus | L | 10 | 2189 | −2 | 51 | 13 | 5.436 |
| Region | Hem. | BA | Number of Voxels | Peak Talairach Coordinates | Peak t(18) Score | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| Activation | |||||||
| Middle frontal gyrus | R | 10 | 23,252 | 36 | 41 | 25 | 9.477 |
| Middle frontal gyrus | R | 6 | 33 | −1 | 52 | 6.369 | |
| Middle frontal gyrus | L | 10 | 22,750 | −36 | 41 | 22 | 10.062 |
| Superior frontal gyrus | L | 6 | 3684 | −1 | 14 | 46 | 6.098 |
| Supramarginal gyrus | R | 40 | 49,677 | 45 | −46 | 37 | 8.744 |
| Inferior parietal lobule | L | 40 | −42 | −46 | 37 | 6.980 | |
| Inferior parietal lobule | L | 7 | −36 | −64 | 43 | 6.450 | |
| Posterior cingulate cortex | R | 23 | 6 | −10 | 25 | 6.380 | |
| Sub-gyral * | L | −30 | −28 | 28 | 5.850 | ||
| Posterior cingulate cortex * | L | −12 | −13 | 25 | 5.045 | ||
| Insula | R | 13 | 4119 | 36 | 11 | 4 | 6.207 |
| Inferior occipital gyrus | L | 18 | 69,627 | −30 | −87 | −8 | 10.112 |
| Inferior temporal gyrus | L | 21 | −57 | −28 | −14 | 8.490 | |
| Lateral occipital gyrus | L | 37 | −39 | −61 | −14 | 7.360 | |
| Inferior occipital gyrus | R | 18 | 21 | −91 | −13 | 7.183 | |
| Lateral occipital gyrus | R | 37 | 48 | −52 | −11 | 6.394 | |
| Deactivation | |||||||
| None | |||||||
| Region | Hem. | BA | Number of Voxels | Peak Talairach Coordinates | Peak t(18) Score | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| AC strategy > SOM strategy | |||||||
| Inferior frontal gyrus | R | 6 | 13,160 | 40 | 1 | 23 | 8.818 |
| Superior frontal gyrus | R | 6 | 7 | 3 | 49 | 7.542 | |
| Cingulate gyrus | R | 32 | 8 | 16 | 32 | 6.755 | |
| Cingulate gyrus * | L | 24 | −10 | 10 | 32 | 4.430 | |
| Middle frontal gyrus | R | 6 | 28 | −6 | 52 | 4.050 | |
| Insula * | R | 3658 | 29 | 21 | 8 | 5.959 | |
| Supramarginal gyrus | L | 40 | 12,888 | −54 | −31 | 44 | 8.569 |
| Cuneus | R | 7 | 5 | −77 | 45 | 8.358 | |
| Superior parietal lobule | R | 7 | 24 | −73 | 41 | 6.821 | |
| Inferior parietal lobule | R | 7 | 26 | −63 | 27 | 6.810 | |
| Superior parietal lobule | L | 7 | −15 | −73 | 44 | 6.160 | |
| Middle occipital gyrus | R | 17 | 3689 | 21 | −92 | −9 | 7.413 |
| Thalamus | L | 3623 | −7 | −5 | 4 | 8.260 | |
| Thalamus | R | 6 | −5 | 2 | 7.120 | ||
| Inferior frontal gyrus | L | 9 | 2017 | −38 | 7 | 26 | 10.228 |
| Lateral occipitotemporal gyrus | L | 37 | 643 | −43 | −54 | −14 | 6.857 |
| Inferior occipital gyrus | L | 18 | 4054 | −23 | −87 | −9 | 5.952 |
| Orbital Gyrus * | L | 10 | 970 | −30 | 47 | −4 | 5.301 |
| Precentral gyrus | L | 6 | 1561 | −30 | −7 | 59 | 4.813 |
| SOM strategy > AC strategy | |||||||
| Superior frontal gyrus | R | 6 | 33,786 | 2 | 45 | 37 | 11.022 |
| Superior frontal gyrus | R | 8 | 23 | 27 | 52 | 10.350 | |
| Superior frontal gyrus | L | 8 | −20 | 30 | 51 | 7.452 | |
| Postcentral gyrus | R | 4 | 54 | −11 | 38 | 6.225 | |
| Cingulate gyrus | R | 31 | 183,586 | 4 | −37 | 40 | 10.296 |
| Superior occipital gyrus | R | 18 | 14 | −76 | 18 | 10.167 | |
| Middle temporal gyrus | L | 21 | −44 | −7 | −15 | 9.633 | |
| Superior temporal gyrus | L | 42 | −53 | −36 | 12 | 9.229 | |
| Superior occipital gyrus | L | 18 | −18 | −83 | 16 | 9.214 | |
| Cuneus | L | 30 | −19 | −68 | 13 | 9.150 | |
| Lingual gyrus | R | 22 | −42 | −10 | 8.937 | ||
| Lingual gyrus | L | −7 | −67 | −5 | 8.445 | ||
| Precuneus | L | 31 | −13 | −37 | 36 | 8.392 | |
| Inferior temporal gyrus | L | 21 | −48 | −5 | −22 | 7.419 | |
| Inferior occipital gyrus | R | 37 | 51 | −64 | 0 | 6.881 | |
| Supramarginal gyrus | R | 39 | 55 | −55 | 22 | 6.760 | |
| Superior temporal gyrus | R | 22 | 48 | −32 | 1 | 6.728 | |
| Middle temporal gyrus | R | 21 | 50 | −46 | 5 | 5.860 | |
| Inferior frontal gyrus | R | 46 | 1198 | 48 | 31 | 8 | 6.565 |
| Inferior frontal gyrus | L | 45 | 4984 | −48 | 34 | 5 | 9.761 |
| Inferior frontal gyrus | L | 46 | −50 | 31 | 18 | 9.276 | |
| Source | ROI | Talairach Coordinates | Mean Differences (AC Strategy –SOM Strategy) | t (18) Score | p-Value (Right-Tail) | Correlation with Accuracy (and p-Value) | |||
|---|---|---|---|---|---|---|---|---|---|
| X | Y | Z | AC Block | SOM Block | |||||
| Arsalidou and Taylor, 2011 | Right IFG | 46 | 10 | 28 | 0.07 | 1.02 | 0.16 | 0.148 (0.32) | 0.05 (0.56) |
| Left IFG | −42 | 4 | 30 | 0.20 * | 3.30 | 0.002 | 0.53 * (0.02) | 0.49 * (0.03) | |
| Right IPL | 46 | −34 | 46 | −0.02 | −0.34 | 0.36 | 0.18 (0.45) | −0.01 (0.90) | |
| Left IPL | −44 | −40 | 42 | 0.20 * | 2.68 | 0.007 | 0.30 (0.20) | 0.20 (0.40) | |
| Cohen Kadosh et al., 2008 | Right IPS | 36 | −49 | 42 | 0.05 | 0.54 | 0.29 | 0.18 (0.47) | −0.02 (0.92) |
| Left IPS | −32 | −47 | 47 | −0.01 | −0.09 | 0.46 | 0.14 (0.55) | 0.19 (0.41) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashkenazi, S.; Tikochinski, R.; Ganor-Stern, D. Neural Correlates of Numerical Estimation: The Role of Strategy Use. Brain Sci. 2022, 12, 357. https://0-doi-org.brum.beds.ac.uk/10.3390/brainsci12030357
Ashkenazi S, Tikochinski R, Ganor-Stern D. Neural Correlates of Numerical Estimation: The Role of Strategy Use. Brain Sciences. 2022; 12(3):357. https://0-doi-org.brum.beds.ac.uk/10.3390/brainsci12030357
Chicago/Turabian StyleAshkenazi, Sarit, Refael Tikochinski, and Dana Ganor-Stern. 2022. "Neural Correlates of Numerical Estimation: The Role of Strategy Use" Brain Sciences 12, no. 3: 357. https://0-doi-org.brum.beds.ac.uk/10.3390/brainsci12030357