Liquid Biopsy Approach for Pancreatic Ductal Adenocarcinoma
Abstract
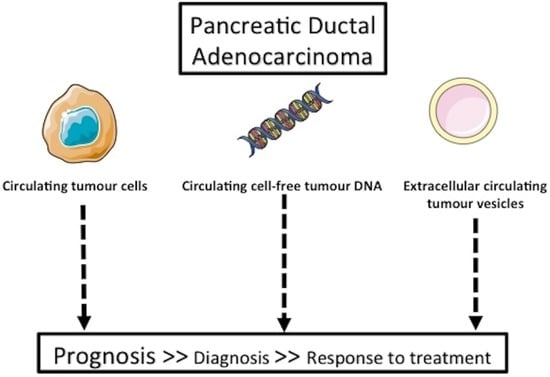
:1. Introduction
2. Current Diagnosis for Pancreatic Cancer
3. Circulating Tumour Cell-Based Diagnosis of Pancreatic Cancer
4. Circulating Tumour DNA for Diagnosis and Prognosis of Pancreatic Cancer
5. Exosome-Based Diagnostic for Pancreatic Cancer
6. Tumour-Educated Platelets
7. Blood-Based Protein and Metabolite Markers
8. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef]
- Bouvier, A.-M.; Uhry, Z.; Jooste, V.; Drouillard, A.; Remontet, L.; Launoy, G.; Leone, N. French Network of Cancer Registries (FRANCIM) Focus on an unusual rise in pancreatic cancer incidence in France. Int. J. Epidemiol. 2017, 46, 1764–1772. [Google Scholar] [CrossRef]
- Ryan, D.P.; Hong, T.S.; Bardeesy, N. Pancreatic adenocarcinoma. N. Engl. J. Med. 2014, 371, 1039–1049. [Google Scholar] [CrossRef]
- Neoptolemos, J.P.; Kleeff, J.; Michl, P.; Costello, E.; Greenhalf, W.; Palmer, D.H. Therapeutic developments in pancreatic cancer: Current and future perspectives. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 333–348. [Google Scholar] [CrossRef]
- Conroy, T.; Hammel, P.; Hebbar, M.; Ben Abdelghani, M.; Wei, A.C.; Raoul, J.-L.; Choné, L.; Francois, E.; Artru, P.; Biagi, J.J.; et al. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N. Engl. J. Med. 2018, 379, 2395–2406. [Google Scholar] [CrossRef]
- Buscail, L. Commentary: Pancreatic cancer: Is the worst to come? Int. J. Epidemiol. 2017, 46, 1774–1775. [Google Scholar] [CrossRef]
- Imamura, T.; Komatsu, S.; Ichikawa, D.; Kawaguchi, T.; Miyamae, M.; Okajima, W.; Ohashi, T.; Arita, T.; Konishi, H.; Shiozaki, A.; et al. Liquid biopsy in patients with pancreatic cancer: Circulating tumor cells and cell-free nucleic acids. World J. Gastroenterol. 2016, 22, 5627–5641. [Google Scholar] [CrossRef]
- Zhang, R.; Peng, R.; Li, Z.; Gao, P.; Jia, S.; Yang, X.; Ding, J.; Han, Y.; Xie, J.; Li, J. Synthetic Circulating Cell-free DNA as Quality Control Materials for Somatic Mutation Detection in Liquid Biopsy for Cancer. Clin. Chem. 2017, 63, 1465–1475. [Google Scholar] [CrossRef]
- Pantel, K.; Alix-Panabières, C. Liquid biopsy and minimal residual disease—Latest advances and implications for cure. Nat. Rev. Clin. Oncol. 2019. [Google Scholar] [CrossRef]
- Alix-Panabières, C.; Pantel, K. Circulating tumor cells: Liquid biopsy of cancer. Clin. Chem. 2013, 59, 110–118. [Google Scholar] [CrossRef]
- Chudasama, D.; Katopodis, P.; Stone, N.; Haskell, J.; Sheridan, H.; Gardner, B.; Urnovitz, H.; Schuetz, E.; Beck, J.; Hall, M.; et al. Liquid Biopsies in Lung Cancer: Four Emerging Technologies and Potential Clinical Applications. Cancers 2019, 11, 311. [Google Scholar] [CrossRef]
- Stefanovic, S.; Deutsch, T.M.; Wirtz, R.; Hartkopf, A.; Sinn, P.; Schuetz, F.; Sohn, C.; Bohlmann, M.K.; Sütterlin, M.; Schneeweiss, A.; et al. Molecular Subtype Conversion between Primary and Metastatic Breast Cancer Corresponding to the Dynamics of Apoptotic and Intact Circulating Tumor Cells. Cancers 2019, 11, 342. [Google Scholar] [CrossRef]
- Zhang, L.; Sanagapalli, S.; Stoita, A. Challenges in diagnosis of pancreatic cancer. World J. Gastroenterol. 2018, 24, 2047–2060. [Google Scholar] [CrossRef]
- Lee, E.S.; Lee, J.M. Imaging diagnosis of pancreatic cancer: A state-of-the-art review. World J. Gastroenterol. 2014, 20, 7864–7877. [Google Scholar] [CrossRef]
- Puli, S.R.; Bechtold, M.L.; Buxbaum, J.L.; Eloubeidi, M.A. How good is endoscopic ultrasound-guided fine-needle aspiration in diagnosing the correct etiology for a solid pancreatic mass?: A meta-analysis and systematic review. Pancreas 2013, 42, 20–26. [Google Scholar] [CrossRef]
- Buscail, L.; Faure, P.; Bournet, B.; Selves, J.; Escourrou, J. Interventional endoscopic ultrasound in pancreatic diseases. Pancreatology 2006, 6, 7–16. [Google Scholar] [CrossRef]
- Savides, T.J.; Donohue, M.; Hunt, G.; Al-Haddad, M.; Aslanian, H.; Ben-Menachem, T.; Chen, V.K.; Coyle, W.; Deutsch, J.; DeWitt, J.; et al. EUS-guided FNA diagnostic yield of malignancy in solid pancreatic masses: A benchmark for quality performance measurement. Gastrointest. Endosc. 2007, 66, 277–282. [Google Scholar] [CrossRef]
- Yoshinaga, S.; Suzuki, H.; Oda, I.; Saito, Y. Role of endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) for diagnosis of solid pancreatic masses. Dig. Endosc. 2011, 23 (Suppl. 1), 29–33. [Google Scholar] [CrossRef]
- Bournet, B.; Buscail, C.; Muscari, F.; Cordelier, P.; Buscail, L. Targeting KRAS for diagnosis, prognosis, and treatment of pancreatic cancer: Hopes and realities. Eur. J. Cancer 2016, 54, 75–83. [Google Scholar] [CrossRef]
- Fuccio, L.; Hassan, C.; Laterza, L.; Correale, L.; Pagano, N.; Bocus, P.; Fabbri, C.; Maimone, A.; Cennamo, V.; Repici, A.; et al. The role of K-ras gene mutation analysis in EUS-guided FNA cytology specimens for the differential diagnosis of pancreatic solid masses: A meta-analysis of prospective studies. Gastrointest. Endosc. 2013, 78, 596–608. [Google Scholar] [CrossRef]
- Fusaroli, P.; Spada, A.; Mancino, M.G.; Caletti, G. Contrast harmonic echo-endoscopic ultrasound improves accuracy in diagnosis of solid pancreatic masses. Clin. Gastroenterol. Hepatol. 2010, 8, 629–634.e1–2. [Google Scholar] [CrossRef]
- Sanjeevi, S.; Ivanics, T.; Lundell, L.; Kartalis, N.; Andrén-Sandberg, Å.; Blomberg, J.; Del Chiaro, M.; Ansorge, C. Impact of delay between imaging and treatment in patients with potentially curable pancreatic cancer. Br. J. Surg. 2016, 103, 267–275. [Google Scholar] [CrossRef]
- Bournet, B.; Pointreau, A.; Delpu, Y.; Selves, J.; Torrisani, J.; Buscail, L.; Cordelier, P. Molecular endoscopic ultrasound for diagnosis of pancreatic cancer. Cancers 2011, 3, 872–882. [Google Scholar] [CrossRef]
- Bournet, B.; Selves, J.; Grand, D.; Danjoux, M.; Hanoun, N.; Cordelier, P.; Buscail, L. Endoscopic ultrasound-guided fine-needle aspiration biopsy coupled with a KRAS mutation assay using allelic discrimination improves the diagnosis of pancreatic cancer. J. Clin. Gastroenterol. 2015, 49, 50–56. [Google Scholar] [CrossRef]
- Trisolini, E.; Armellini, E.; Paganotti, A.; Veggiani, C.; Bozzola, C.; Frattini, M.; Pizio, C.; Mancuso, G.; Andorno, S.; Boldorini, R. KRAS mutation testing on all non-malignant diagnosis of pancreatic endoscopic ultrasound-guided fine-needle aspiration biopsies improves diagnostic accuracy. Pathology 2017, 49, 379–386. [Google Scholar] [CrossRef]
- Sekita-Hatakeyama, Y.; Nishikawa, T.; Takeuchi, M.; Morita, K.; Takeda, M.; Hatakeyama, K.; Nakai, T.; Uchiyama, T.; Itami, H.; Fujii, T.; et al. K-ras mutation analysis of residual liquid-based cytology specimens from endoscopic ultrasound-guided fine needle aspiration improves cell block diagnosis of pancreatic ductal adenocarcinoma. PLoS ONE 2018, 13, e0193692. [Google Scholar] [CrossRef]
- Alix-Panabières, C.; Pantel, K. Challenges in circulating tumour cell research. Nat. Rev. Cancer 2014, 14, 623–631. [Google Scholar] [CrossRef]
- Samandari, M.; Julia, M.G.; Rice, A.; Chronopoulos, A.; Del Rio Hernandez, A.E. Liquid biopsies for management of pancreatic cancer. Transl. Res. 2018, 201, 98–127. [Google Scholar] [CrossRef]
- Mataki, Y.; Takao, S.; Maemura, K.; Mori, S.; Shinchi, H.; Natsugoe, S.; Aikou, T. Carcinoembryonic antigen messenger RNA expression using nested reverse transcription-PCR in the peripheral blood during follow-up period of patients who underwent curative surgery for biliary-pancreatic cancer: Longitudinal analyses. Clin. Cancer Res. 2004, 10, 3807–3814. [Google Scholar] [CrossRef]
- Soeth, E.; Grigoleit, U.; Moellmann, B.; Röder, C.; Schniewind, B.; Kremer, B.; Kalthoff, H.; Vogel, I. Detection of tumor cell dissemination in pancreatic ductal carcinoma patients by CK 20 RT-PCR indicates poor survival. J. Cancer Res. Clin. Oncol. 2005, 131, 669–676. [Google Scholar] [CrossRef]
- Sergeant, G.; Roskams, T.; van Pelt, J.; Houtmeyers, F.; Aerts, R.; Topal, B. Perioperative cancer cell dissemination detected with a real-time RT-PCR assay for EpCAM is not associated with worse prognosis in pancreatic ductal adenocarcinoma. BMC Cancer 2011, 11, 47. [Google Scholar] [CrossRef]
- Zhou, J.; Hu, L.; Yu, Z.; Zheng, J.; Yang, D.; Bouvet, M.; Hoffman, R.M. Marker expression in circulating cancer cells of pancreatic cancer patients. J. Surg. Res. 2011, 171, 631–636. [Google Scholar] [CrossRef]
- Ren, C.; Han, C.; Zhang, J.; He, P.; Wang, D.; Wang, B.; Zhao, P.; Zhao, X. Detection of apoptotic circulating tumor cells in advanced pancreatic cancer following 5-fluorouracil chemotherapy. Cancer Biol. Ther. 2011, 12, 700–706. [Google Scholar] [CrossRef] [Green Version]
- Khoja, L.; Backen, A.; Sloane, R.; Menasce, L.; Ryder, D.; Krebs, M.; Board, R.; Clack, G.; Hughes, A.; Blackhall, F.; et al. A pilot study to explore circulating tumour cells in pancreatic cancer as a novel biomarker. Br. J. Cancer 2012, 106, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Ankeny, J.S.; Court, C.M.; Hou, S.; Li, Q.; Song, M.; Wu, D.; Chen, J.F.; Lee, T.; Lin, M.; Sho, S.; et al. Circulating tumour cells as a biomarker for diagnosis and staging in pancreatic cancer. Br. J. Cancer 2016, 114, 1367–1375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dotan, E.; Alpaugh, R.K.; Ruth, K.; Negin, B.P.; Denlinger, C.S.; Hall, M.J.; Astsaturov, I.; McAleer, C.; Fittipaldi, P.; Thrash-Bingham, C.; et al. Prognostic Significance of MUC-1 in Circulating Tumor Cells in Patients With Metastatic Pancreatic Adenocarcinoma. Pancreas 2016, 45, 1131–1135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poruk, K.E.; Blackford, A.L.; Weiss, M.J.; Cameron, J.L.; He, J.; Goggins, M.; Rasheed, Z.A.; Wolfgang, C.L.; Wood, L.D. Circulating Tumor Cells Expressing Markers of Tumor-Initiating Cells Predict Poor Survival and Cancer Recurrence in Patients with Pancreatic Ductal Adenocarcinoma. Clin. Cancer Res. 2017, 23, 2681–2690. [Google Scholar] [CrossRef] [PubMed]
- Kulemann, B.; Rösch, S.; Seifert, S.; Timme, S.; Bronsert, P.; Seifert, G.; Martini, V.; Kuvendjiska, J.; Glatz, T.; Hussung, S.; et al. Pancreatic cancer: Circulating Tumor Cells and Primary Tumors show Heterogeneous KRAS Mutations. Sci. Rep. 2017, 7, 4510. [Google Scholar] [CrossRef] [PubMed]
- Okubo, K.; Uenosono, Y.; Arigami, T.; Mataki, Y.; Matsushita, D.; Yanagita, S.; Kurahara, H.; Sakoda, M.; Kijima, Y.; Maemura, K.; et al. Clinical impact of circulating tumor cells and therapy response in pancreatic cancer. Eur. J. Surg. Oncol. 2017, 43, 1050–1055. [Google Scholar] [CrossRef]
- Court, C.M.; Ankeny, J.S.; Sho, S.; Winograd, P.; Hou, S.; Song, M.; Wainberg, Z.A.; Girgis, M.D.; Graeber, T.G.; Agopian, V.G.; et al. Circulating Tumor Cells Predict Occult Metastatic Disease and Prognosis in Pancreatic Cancer. Ann. Surg. Oncol. 2018, 25, 1000–1008. [Google Scholar] [CrossRef]
- Effenberger, K.E.; Schroeder, C.; Hanssen, A.; Wolter, S.; Eulenburg, C.; Tachezy, M.; Gebauer, F.; Izbicki, J.R.; Pantel, K.; Bockhorn, M. Improved Risk Stratification by Circulating Tumor Cell Counts in Pancreatic Cancer. Clin. Cancer Res. 2018, 24, 2844–2850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hugenschmidt, H.; Labori, K.J.; Brunborg, C.; Verbeke, C.S.; Seeberg, L.T.; Schirmer, C.B.; Renolen, A.; Borgen, E.F.; Naume, B.; Wiedswang, G. Circulating Tumor Cells are an Independent Predictor of Shorter Survival in Patients Undergoing Resection for Pancreatic and Periampullary Adenocarcinoma. Ann. Surg. 2018. [Google Scholar] [CrossRef] [PubMed]
- Sefrioui, D.; Blanchard, F.; Toure, E.; Basile, P.; Beaussire, L.; Dolfus, C.; Perdrix, A.; Paresy, M.; Antonietti, M.; Iwanicki-Caron, I.; et al. Diagnostic value of CA19.9, circulating tumour DNA and circulating tumour cells in patients with solid pancreatic tumours. Br. J. Cancer 2017, 117, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Varillas, J.I.; Zhang, J.; Chen, K.; Barnes, I.I.; Liu, C.; George, T.J.; Fan, Z.H. Microfluidic Isolation of Circulating Tumor Cells and Cancer Stem-Like Cells from Patients with Pancreatic Ductal Adenocarcinoma. Theranostics 2019, 9, 1417–1425. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Zhang, X.; Zhang, Q.; Yang, J.; Chen, Q.; Wang, J.; Li, X.; Chen, J.; Ma, T.; Li, G.; et al. Vimentin-positive circulating tumor cells as a biomarker for diagnosis and treatment monitoring in patients with pancreatic cancer. Cancer Lett. 2019, 452, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Tam, W.L.; Weinberg, R.A. The epigenetics of epithelial-mesenchymal plasticity in cancer. Nat. Med. 2013, 19, 1438–1449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaffer, C.L.; Weinberg, R.A. A perspective on cancer cell metastasis. Science 2011, 331, 1559–1564. [Google Scholar] [CrossRef] [PubMed]
- Alix-Panabières, C.; Mader, S.; Pantel, K. Epithelial-mesenchymal plasticity in circulating tumor cells. J. Mol. Med. 2017, 95, 133–142. [Google Scholar] [CrossRef]
- Vona, G.; Sabile, A.; Louha, M.; Sitruk, V.; Romana, S.; Schütze, K.; Capron, F.; Franco, D.; Pazzagli, M.; Vekemans, M.; et al. Isolation by size of epithelial tumor cells: A new method for the immunomorphological and molecular characterization of circulatingtumor cells. Am. J. Pathol. 2000, 156, 57–63. [Google Scholar] [CrossRef]
- Rosenbaum, M.W.; Cauley, C.E.; Kulemann, B.; Liss, A.S.; Castillo, C.F.-D.; Warshaw, A.L.; Lillemoe, K.D.; Thayer, S.P.; Pitman, M.B. Cytologic characteristics of circulating epithelioid cells in pancreatic disease. Cancer Cytopathol. 2017, 125, 332–340. [Google Scholar] [CrossRef] [Green Version]
- Pantel, K.; Denève, E.; Nocca, D.; Coffy, A.; Vendrell, J.-P.; Maudelonde, T.; Riethdorf, S.; Alix-Panabières, C. Circulating epithelial cells in patients with benign colon diseases. Clin. Chem. 2012, 58, 936–940. [Google Scholar] [CrossRef]
- Bidard, F.C.; Huguet, F.; Louvet, C.; Mineur, L.; Bouché, O.; Chibaudel, B.; Artru, P.; Desseigne, F.; Bachet, J.B.; Mathiot, C.; et al. Circulating tumor cells in locally advanced pancreatic adenocarcinoma: The ancillary CirCe 07 study to the LAP 07 trial. Ann. Oncol. 2013, 24, 2057–2061. [Google Scholar] [CrossRef]
- Chin, R.-I.; Chen, K.; Usmani, A.; Chua, C.; Harris, P.K.; Binkley, M.S.; Azad, T.D.; Dudley, J.C.; Chaudhuri, A.A. Detection of Solid Tumor Molecular Residual Disease (MRD) Using Circulating Tumor DNA (ctDNA). Mol. Diagn. Ther. 2019, 23, 311–331. [Google Scholar] [CrossRef]
- Lewis, A.R.; Valle, J.W.; McNamara, M.G. Pancreatic cancer: Are “liquid biopsies” ready for prime-time? World J. Gastroenterol. 2016, 22, 7175–7185. [Google Scholar] [CrossRef]
- Castells, A.; Puig, P.; Móra, J.; Boadas, J.; Boix, L.; Urgell, E.; Solé, M.; Capellà, G.; Lluís, F.; Fernández-Cruz, L.; et al. K-ras mutations in DNA extracted from the plasma of patients with pancreatic carcinoma: Diagnostic utility and prognostic significance. J. Clin. Oncol. 1999, 17, 578–584. [Google Scholar] [CrossRef]
- Maire, F.; Micard, S.; Hammel, P.; Voitot, H.; Lévy, P.; Cugnenc, P.-H.; Ruszniewski, P.; Puig, P.L. Differential diagnosis between chronic pancreatitis and pancreatic cancer: Value of the detection of KRAS2 mutations in circulating DNA. Br. J. Cancer 2002, 87, 551–554. [Google Scholar] [CrossRef]
- Däbritz, J.; Preston, R.; Hänfler, J.; Oettle, H. Follow-up study of K-ras mutations in the plasma of patients with pancreatic cancer: Correlation with clinical features and carbohydrate antigen 19-9. Pancreas 2009, 38, 534–541. [Google Scholar] [CrossRef]
- Chen, H.; Tu, H.; Meng, Z.Q.; Chen, Z.; Wang, P.; Liu, L.M. K-ras mutational status predicts poor prognosis in unresectable pancreatic cancer. Eur. J. Surg. Oncol. 2010, 36, 657–662. [Google Scholar] [CrossRef]
- Wu, J.; Zhou, Y.; Zhang, C.-Y.; Song, B.-B.; Wang, B.-L.; Pan, B.-S.; Lou, W.-H.; Guo, W. Co-amplification at lower denaturation-temperature PCR combined with unlabled-probe high-resolution melting to detect KRAS codon 12 and 13 mutations in plasma-circulating DNA of pancreatic adenocarcinoma cases. Asian Pac. J. Cancer Prev. 2014, 15, 10647–10652. [Google Scholar] [CrossRef]
- Semrad, T.; Barzi, A.; Lenz, H.-J.; Hutchins, I.M.; Kim, E.J.; Gong, I.-Y.; Tanaka, M.; Beckett, L.; Holland, W.; Burich, R.A.; et al. Pharmacodynamic separation of gemcitabine and erlotinib in locally advanced or metastatic pancreatic cancer: Therapeutic and biomarker results. Int. J. Clin. Oncol. 2015, 20, 518–524. [Google Scholar] [CrossRef]
- Sausen, M.; Phallen, J.; Adleff, V.; Jones, S.; Leary, R.J.; Barrett, M.T.; Anagnostou, V.; Parpart-Li, S.; Murphy, D.; Kay Li, Q.; et al. Clinical implications of genomic alterations in the tumour and circulation of pancreatic cancer patients. Nat. Commun. 2015, 6, 7686. [Google Scholar] [CrossRef]
- Earl, J.; Garcia-Nieto, S.; Martinez-Avila, J.C.; Montans, J.; Sanjuanbenito, A.; Rodríguez-Garrote, M.; Lisa, E.; Mendía, E.; Lobo, E.; Malats, N.; et al. Circulating tumor cells (Ctc) and kras mutant circulating free DNA (cfdna) detection in peripheral blood as biomarkers in patients diagnosed with exocrine pancreatic cancer. BMC Cancer 2015, 15, 797. [Google Scholar] [CrossRef]
- Singh, N.; Gupta, S.; Pandey, R.M.; Chauhan, S.S.; Saraya, A. High levels of cell-free circulating nucleic acids in pancreatic cancer are associated with vascular encasement, metastasis and poor survival. Cancer Investig. 2015, 33, 78–85. [Google Scholar] [CrossRef]
- Kinugasa, H.; Nouso, K.; Miyahara, K.; Morimoto, Y.; Dohi, C.; Tsutsumi, K.; Kato, H.; Matsubara, T.; Okada, H.; Yamamoto, K. Detection of K-ras gene mutation by liquid biopsy in patients with pancreatic cancer. Cancer 2015, 121, 2271–2280. [Google Scholar] [CrossRef]
- Takai, E.; Totoki, Y.; Nakamura, H.; Morizane, C.; Nara, S.; Hama, N.; Suzuki, M.; Furukawa, E.; Kato, M.; Hayashi, H.; et al. Clinical utility of circulating tumor DNA for molecular assessment in pancreatic cancer. Sci. Rep. 2015, 5, 18425. [Google Scholar] [CrossRef]
- Adamo, P.; Cowley, C.M.; Neal, C.P.; Mistry, V.; Page, K.; Dennison, A.R.; Isherwood, J.; Hastings, R.; Luo, J.; Moore, D.A.; et al. Profiling tumour heterogeneity through circulating tumour DNA in patients with pancreatic cancer. Oncotarget 2017, 8, 87221–87233. [Google Scholar] [CrossRef] [Green Version]
- Ako, S.; Nouso, K.; Kinugasa, H.; Dohi, C.; Matushita, H.; Mizukawa, S.; Muro, S.; Akimoto, Y.; Uchida, D.; Tomoda, T.; et al. Utility of serum DNA as a marker for KRAS mutations in pancreatic cancer tissue. Pancreatology 2017, 17, 285–290. [Google Scholar] [CrossRef]
- Cheng, H.; Liu, C.; Jiang, J.; Luo, G.; Lu, Y.; Jin, K.; Guo, M.; Zhang, Z.; Xu, J.; Liu, L.; et al. Analysis of ctDNA to predict prognosis and monitor treatment responses in metastatic pancreatic cancer patients. Int. J. Cancer 2017, 140, 2344–2350. [Google Scholar] [CrossRef]
- Pietrasz, D.; Pécuchet, N.; Garlan, F.; Didelot, A.; Dubreuil, O.; Doat, S.; Imbert-Bismut, F.; Karoui, M.; Vaillant, J.-C.; Taly, V.; et al. Plasma Circulating Tumor DNA in Pancreatic Cancer Patients Is a Prognostic Marker. Clin. Cancer Res. 2017, 23, 116–123. [Google Scholar] [CrossRef]
- Van Laethem, J.-L.; Riess, H.; Jassem, J.; Haas, M.; Martens, U.M.; Weekes, C.; Peeters, M.; Ross, P.; Bridgewater, J.; Melichar, B.; et al. Phase I/II Study of Refametinib (BAY 86-9766) in Combination with Gemcitabine in Advanced Pancreatic cancer. Target. Oncol. 2017, 12, 97–109. [Google Scholar] [CrossRef]
- Hadano, N.; Murakami, Y.; Uemura, K.; Hashimoto, Y.; Kondo, N.; Nakagawa, N.; Sueda, T.; Hiyama, E. Prognostic value of circulating tumour DNA in patients undergoing curative resection for pancreatic cancer. Br. J. Cancer 2016, 115, 59–65. [Google Scholar] [CrossRef]
- Del Re, M.; Vivaldi, C.; Rofi, E.; Vasile, E.; Miccoli, M.; Caparello, C.; d’Arienzo, P.D.; Fornaro, L.; Falcone, A.; Danesi, R. Early changes in plasma DNA levels of mutant KRAS as a sensitive marker of response to chemotherapy in pancreatic cancer. Sci. Rep. 2017, 7, 7931. [Google Scholar] [CrossRef]
- Cohen, J.D.; Javed, A.A.; Thoburn, C.; Wong, F.; Tie, J.; Gibbs, P.; Schmidt, C.M.; Yip-Schneider, M.T.; Allen, P.J.; Schattner, M.; et al. Combined circulating tumor DNA and protein biomarker-based liquid biopsy for the earlier detection of pancreatic cancers. Proc. Natl. Acad. Sci. USA 2017, 114, 10202–10207. [Google Scholar] [CrossRef] [Green Version]
- Pishvaian, M.J.; Joseph Bender, R.; Matrisian, L.M.; Rahib, L.; Hendifar, A.; Hoos, W.A.; Mikhail, S.; Chung, V.; Picozzi, V.; Heartwell, C.; et al. A pilot study evaluating concordance between blood-based and patient-matched tumor molecular testing within pancreatic cancer patients participating in the Know Your Tumor (KYT) initiative. Oncotarget 2017, 8, 83446–83456. [Google Scholar] [CrossRef]
- Kim, M.K.; Woo, S.M.; Park, B.; Yoon, K.-A.; Kim, Y.-H.; Joo, J.; Lee, W.J.; Han, S.-S.; Park, S.-J.; Kong, S.-Y. Prognostic Implications of Multiplex Detection of KRAS Mutations in Cell-Free DNA from Patients with Pancreatic Ductal Adenocarcinoma. Clin. Chem. 2018, 64, 726–734. [Google Scholar] [CrossRef] [Green Version]
- Nakano, Y.; Kitago, M.; Matsuda, S.; Nakamura, Y.; Fujita, Y.; Imai, S.; Shinoda, M.; Yagi, H.; Abe, Y.; Hibi, T.; et al. KRAS mutations in cell-free DNA from preoperative and postoperative sera as a pancreatic cancer marker: A retrospective study. Br. J. Cancer 2018, 118, 662–669. [Google Scholar] [CrossRef]
- Lin, M.; Alnaggar, M.; Liang, S.; Chen, J.; Xu, K.; Dong, S.; Du, D.; Niu, L. Circulating Tumor DNA as a Sensitive Marker in Patients Undergoing Irreversible Electroporation for Pancreatic Cancer. Cell. Physiol. Biochem. 2018, 47, 1556–1564. [Google Scholar] [CrossRef]
- Gall, T.M.H.; Belete, S.; Khanderia, E.; Frampton, A.E.; Jiao, L.R. Circulating Tumor Cells and Cell-Free DNA in Pancreatic Ductal Adenocarcinoma. Am. J. Pathol. 2019, 189, 71–81. [Google Scholar] [CrossRef] [Green Version]
- Bernard, V.; Kim, D.U.; San Lucas, F.A.; Castillo, J.; Allenson, K.; Mulu, F.C.; Stephens, B.M.; Huang, J.; Semaan, A.; Guerrero, P.A.; et al. Circulating Nucleic Acids Are Associated With Outcomes of Patients with Pancreatic Cancer. Gastroenterology 2019, 156, 108–118.e4. [Google Scholar] [CrossRef]
- Anderson, S.M. Laboratory methods for KRAS mutation analysis. Expert Rev. Mol. Diagn. 2011, 11, 635–642. [Google Scholar] [CrossRef]
- Pritchard, C.C.; Akagi, L.; Reddy, P.L.; Joseph, L.; Tait, J.F. COLD-PCR enhanced melting curve analysis improves diagnostic accuracy for KRAS mutations in colorectal carcinoma. BMC Clin. Pathol. 2010, 10, 6. [Google Scholar] [CrossRef]
- Oliner, K.; Juan, T.; Suggs, S.; Wolf, M.; Sarosi, I.; Freeman, D.J.; Gyuris, T.; Baron, W.; Bakker, A.; Parker, A.; et al. A comparability study of 5 commercial KRAS tests. Diagn. Pathol. 2010, 5, 23. [Google Scholar] [CrossRef]
- Boulaiz, H.; Ramos, M.C.; Griñán-Lisón, C.; García-Rubiño, M.E.; Vicente, F.; Marchal, J.A. What’s new in the diagnosis of pancreatic cancer: A patent review (2011-present). Expert Opin. Ther. Pat. 2017, 27, 1319–1328. [Google Scholar] [CrossRef]
- Sho, S.; Court, C.M.; Kim, S.; Braxton, D.R.; Hou, S.; Muthusamy, V.R.; Watson, R.R.; Sedarat, A.; Tseng, H.-R.; Tomlinson, J.S. Digital PCR Improves Mutation Analysis in Pancreas Fine Needle Aspiration Biopsy Specimens. PLoS ONE 2017, 12, e0170897. [Google Scholar] [CrossRef]
- Henriksen, S.D.; Madsen, P.H.; Larsen, A.C.; Johansen, M.B.; Pedersen, I.S.; Krarup, H.; Thorlacius-Ussing, O. Cell-free DNA promoter hypermethylation in plasma as a predictive marker for survival of patients with pancreatic adenocarcinoma. Oncotarget 2017, 8, 93942–93956. [Google Scholar] [CrossRef]
- Kawesha, A.; Ghaneh, P.; Andrén-Sandberg, A.; Ograed, D.; Skar, R.; Dawiskiba, S.; Evans, J.D.; Campbell, F.; Lemoine, N.; Neoptolemos, J.P. K-ras oncogene subtype mutations are associated with survival but not expression of p53, p16(INK4A), p21(WAF-1), cyclin D1, erbB-2 and erbB-3 in resected pancreatic ductal adenocarcinoma. Int. J. Cancer 2000, 89, 469–474. [Google Scholar] [CrossRef]
- Kim, S.T.; Lim, D.H.; Jang, K.-T.; Lim, T.; Lee, J.; Choi, Y.-L.; Jang, H.-L.; Yi, J.H.; Baek, K.K.; Park, S.H.; et al. Impact of KRAS mutations on clinical outcomes in pancreatic cancer patients treated with first-line gemcitabine-based chemotherapy. Mol. Cancer Ther. 2011, 10, 1993–1999. [Google Scholar] [CrossRef]
- Ogura, T.; Yamao, K.; Hara, K.; Mizuno, N.; Hijioka, S.; Imaoka, H.; Sawaki, A.; Niwa, Y.; Tajika, M.; Kondo, S.; et al. Prognostic value of K-ras mutation status and subtypes in endoscopic ultrasound-guided fine-needle aspiration specimens from patients with unresectable pancreatic cancer. J. Gastroenterol. 2013, 48, 640–646. [Google Scholar] [CrossRef]
- Bournet, B.; Muscari, F.; Buscail, C.; Assenat, E.; Barthet, M.; Hammel, P.; Selves, J.; Guimbaud, R.; Cordelier, P.; Buscail, L. KRAS G12D Mutation Subtype Is A Prognostic Factor for Advanced Pancreatic Adenocarcinoma. Clin. Transl. Gastroenterol. 2016, 7, e157. [Google Scholar] [CrossRef]
- Qian, Z.R.; Rubinson, D.A.; Nowak, J.A.; Morales-Oyarvide, V.; Dunne, R.F.; Kozak, M.M.; Welch, M.W.; Brais, L.K.; Da Silva, A.; Li, T.; et al. Association of Alterations in Main Driver Genes With Outcomes of Patients With Resected Pancreatic Ductal Adenocarcinoma. JAMA Oncol. 2018, 4, e173420. [Google Scholar] [CrossRef]
- Haigis, K.M. KRAS Alleles: The Devil Is in the Detail. Trends Cancer 2017, 3, 686–697. [Google Scholar] [CrossRef]
- Ihle, N.T.; Byers, L.A.; Kim, E.S.; Saintigny, P.; Lee, J.J.; Blumenschein, G.R.; Tsao, A.; Liu, S.; Larsen, J.E.; Wang, J.; et al. Effect of KRAS oncogene substitutions on protein behavior: Implications for signaling and clinical outcome. J. Natl. Cancer Inst. 2012, 104, 228–239. [Google Scholar] [CrossRef]
- Pantsar, T.; Rissanen, S.; Dauch, D.; Laitinen, T.; Vattulainen, I.; Poso, A. Assessment of mutation probabilities of KRAS G12 missense mutants and their long-timescale dynamics by atomistic molecular simulations and Markov state modeling. PLoS Comput. Biol. 2018, 14, e1006458. [Google Scholar] [CrossRef]
- Cristiano, S.; Leal, A.; Phallen, J.; Fiksel, J.; Adleff, V.; Bruhm, D.C.; Jensen, S.Ø.; Medina, J.E.; Hruban, C.; White, J.R.; et al. Genome-wide cell-free DNA fragmentation in patients with cancer. Nature 2019. [Google Scholar] [CrossRef]
- Shen, S.Y.; Singhania, R.; Fehringer, G.; Chakravarthy, A.; Roehrl, M.H.A.; Chadwick, D.; Zuzarte, P.C.; Borgida, A.; Wang, T.T.; Li, T.; et al. Sensitive tumour detection and classification using plasma cell-free DNA methylomes. Nature 2018, 563, 579–583. [Google Scholar] [CrossRef]
- Barault, L.; Amatu, A.; Siravegna, G.; Ponzetti, A.; Moran, S.; Cassingena, A.; Mussolin, B.; Falcomatà, C.; Binder, A.M.; Cristiano, C.; et al. Discovery of methylated circulating DNA biomarkers for comprehensive non-invasive monitoring of treatment response in metastatic colorectal cancer. Gut 2018, 67, 1995–2005. [Google Scholar] [CrossRef]
- Cheon, H.; Paik, J.H.; Choi, M.; Yang, H.-J.; Son, J.-H. Detection and manipulation of methylation in blood cancer DNA using terahertz radiation. Sci. Rep. 2019, 9, 6413. [Google Scholar] [CrossRef]
- Couto, N.; Caja, S.; Maia, J.; Strano Moraes, M.C.; Costa-Silva, B. Exosomes as emerging players in cancer biology. Biochimie 2018, 155, 2–10. [Google Scholar] [CrossRef]
- Capello, M.; Vykoukal, J.V.; Katayama, H.; Bantis, L.E.; Wang, H.; Kundnani, D.L.; Aguilar-Bonavides, C.; Aguilar, M.; Tripathi, S.C.; Dhillon, D.S.; et al. Exosomes harbor B cell targets in pancreatic adenocarcinoma and exert decoy function against complement-mediated cytotoxicity. Nat. Commun. 2019, 10, 254. [Google Scholar] [CrossRef]
- Nazarenko, I.; Rana, S.; Baumann, A.; McAlear, J.; Hellwig, A.; Trendelenburg, M.; Lochnit, G.; Preissner, K.T.; Zöller, M. Cell surface tetraspanin Tspan8 contributes to molecular pathways of exosome-induced endothelial cell activation. Cancer Res. 2010, 70, 1668–1678. [Google Scholar] [CrossRef]
- Costa-Silva, B.; Aiello, N.M.; Ocean, A.J.; Singh, S.; Zhang, H.; Thakur, B.K.; Becker, A.; Hoshino, A.; Mark, M.T.; Molina, H.; et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat. Cell Biol. 2015, 17, 816–826. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Becker, A.; Thakur, B.K.; Weiss, J.M.; Kim, H.S.; Peinado, H.; Lyden, D. Extracellular Vesicles in Cancer: Cell-to-Cell Mediators of Metastasis. Cancer Cell 2016, 30, 836–848. [Google Scholar] [CrossRef] [Green Version]
- Que, R.; Ding, G.; Chen, J.; Cao, L. Analysis of serum exosomal microRNAs and clinicopathologic features of patients with pancreatic adenocarcinoma. World J. Surg. Oncol. 2013, 11, 219. [Google Scholar] [CrossRef]
- Goto, T.; Fujiya, M.; Konishi, H.; Sasajima, J.; Fujibayashi, S.; Hayashi, A.; Utsumi, T.; Sato, H.; Iwama, T.; Ijiri, M.; et al. An elevated expression of serum exosomal microRNA-191, - 21, -451a of pancreatic neoplasm is considered to be efficient diagnostic marker. BMC Cancer 2018, 18, 116. [Google Scholar] [CrossRef]
- Madhavan, B.; Yue, S.; Galli, U.; Rana, S.; Gross, W.; Müller, M.; Giese, N.A.; Kalthoff, H.; Becker, T.; Büchler, M.W.; et al. Combined evaluation of a panel of protein and miRNA serum-exosome biomarkers for pancreatic cancer diagnosis increases sensitivity and specificity. Int. J. Cancer 2015, 136, 2616–2627. [Google Scholar] [CrossRef]
- Melo, S.A.; Luecke, L.B.; Kahlert, C.; Fernandez, A.F.; Gammon, S.T.; Kaye, J.; LeBleu, V.S.; Mittendorf, E.A.; Weitz, J.; Rahbari, N.; et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature 2015, 523, 177–182. [Google Scholar] [CrossRef] [Green Version]
- Lai, X.; Wang, M.; McElyea, S.D.; Sherman, S.; House, M.; Korc, M. A microRNA signature in circulating exosomes is superior to exosomal glypican-1 levels for diagnosing pancreatic cancer. Cancer Lett. 2017, 393, 86–93. [Google Scholar] [CrossRef] [Green Version]
- Allenson, K.; Castillo, J.; San Lucas, F.A.; Scelo, G.; Kim, D.U.; Bernard, V.; Davis, G.; Kumar, T.; Katz, M.; Overman, M.J.; et al. High prevalence of mutant KRAS in circulating exosome-derived DNA from early-stage pancreatic cancer patients. Ann. Oncol. 2017, 28, 741–747. [Google Scholar] [CrossRef]
- Xu, Y.-F.; Hannafon, B.N.; Zhao, Y.D.; Postier, R.G.; Ding, W.-Q. Plasma exosome miR-196a and miR-1246 are potential indicators of localized pancreatic cancer. Oncotarget 2017, 8, 77028–77040. [Google Scholar] [CrossRef]
- Yang, K.S.; Im, H.; Hong, S.; Pergolini, I.; Del Castillo, A.F.; Wang, R.; Clardy, S.; Huang, C.-H.; Pille, C.; Ferrone, S.; et al. Multiparametric plasma EV profiling facilitates diagnosis of pancreatic malignancy. Sci. Transl. Med. 2017, 9, eaal3226. [Google Scholar] [CrossRef] [Green Version]
- Lewis, J.M.; Vyas, A.D.; Qiu, Y.; Messer, K.S.; White, R.; Heller, M.J. Integrated Analysis of Exosomal Protein Biomarkers on Alternating Current Electrokinetic Chips Enables Rapid Detection of Pancreatic Cancer in Patient Blood. ACS Nano 2018, 12, 3311–3320. [Google Scholar] [CrossRef]
- Jin, H.; Liu, P.; Wu, Y.; Meng, X.; Wu, M.; Han, J.; Tan, X. Exosomal zinc transporter ZIP4 promotes cancer growth and is a novel diagnostic biomarker for pancreatic cancer. Cancer Sci. 2018, 109, 2946–2956. [Google Scholar] [CrossRef] [Green Version]
- European Study Group on Cystic Tumours of the Pancreas. European evidence-based guidelines on pancreatic cystic neoplasms. Gut 2018, 67, 789–804. [Google Scholar] [CrossRef]
- Karasek, P.; Gablo, N.; Hlavsa, J.; Kiss, I.; Vychytilova-Faltejskova, P.; Hermanova, M.; Kala, Z.; Slaby, O.; Prochazka, V. Pre-operative Plasma miR-21-5p Is a Sensitive Biomarker and Independent Prognostic Factor in Patients with Pancreatic Ductal Adenocarcinoma Undergoing Surgical Resection. Cancer Genom. Proteom. 2018, 15, 321–327. [Google Scholar] [CrossRef] [Green Version]
- Humeau, M.; Vignolle-Vidoni, A.; Sicard, F.; Martins, F.; Bournet, B.; Buscail, L.; Torrisani, J.; Cordelier, P. Salivary MicroRNA in Pancreatic Cancer Patients. PLoS ONE 2015, 10, e0130996. [Google Scholar] [CrossRef]
- Buscail, L.; Bournet, B.; Vernejoul, F.; Cambois, G.; Lulka, H.; Hanoun, N.; Dufresne, M.; Meulle, A.; Vignolle-Vidoni, A.; Ligat, L.; et al. First-in-man Phase 1 Clinical Trial of Gene Therapy for Advanced Pancreatic Cancer: Safety, Biodistribution, and Preliminary Clinical Findings. Mol. Ther. 2015, 23, 779–789. [Google Scholar] [CrossRef] [Green Version]
- Cacheux, J.; Brut, M.; Bancaud, A.; Cordelier, P.; Leïchlé, T. Spatial Analysis of Nanofluidic-Embedded Biosensors for Wash-Free Single-Nucleotide Difference Discrimination. ACS Sens. 2018, 3, 606–611. [Google Scholar] [CrossRef]
- Thakur, B.K.; Zhang, H.; Becker, A.; Matei, I.; Huang, Y.; Costa-Silva, B.; Zheng, Y.; Hoshino, A.; Brazier, H.; Xiang, J.; et al. Double-stranded DNA in exosomes: A novel biomarker in cancer detection. Cell Res. 2014, 24, 766–769. [Google Scholar] [CrossRef]
- Kahlert, C.; Melo, S.A.; Protopopov, A.; Tang, J.; Seth, S.; Koch, M.; Zhang, J.; Weitz, J.; Chin, L.; Futreal, A.; et al. Identification of double-stranded genomic DNA spanning all chromosomes with mutated KRAS and p53 DNA in the serum exosomes of patients with pancreatic cancer. J. Biol. Chem. 2014, 289, 3869–3875. [Google Scholar] [CrossRef]
- Le Calvez-Kelm, F.; Foll, M.; Wozniak, M.B.; Delhomme, T.M.; Durand, G.; Chopard, P.; Pertesi, M.; Fabianova, E.; Adamcakova, Z.; Holcatova, I.; et al. KRAS mutations in blood circulating cell-free DNA: A pancreatic cancer case-control. Oncotarget 2016, 7, 78827–78840. [Google Scholar] [CrossRef]
- Lu, H.; Niu, F.; Liu, F.; Gao, J.; Sun, Y.; Zhao, X. Elevated glypican-1 expression is associated with an unfavorable prognosis in pancreatic ductal adenocarcinoma. Cancer Med. 2017, 6, 1181–1191. [Google Scholar] [CrossRef]
- Kalluri, R. The biology and function of exosomes in cancer. J. Clin. Investig. 2016, 126, 1208–1215. [Google Scholar] [CrossRef]
- Qian, L.; Yu, S.; Chen, Z.; Meng, Z.; Huang, S.; Wang, P. Functions and clinical implications of exosomes in pancreatic cancer. Biochim. Biophys. Acta Rev. Cancer 2018, 1871, 75–84. [Google Scholar] [CrossRef]
- Sol, N.; Wurdinger, T. Platelet RNA signatures for the detection of cancer. Cancer Metastasis Rev. 2017, 36, 263–272. [Google Scholar] [CrossRef]
- Best, M.G.; Sol, N.; Kooi, I.; Tannous, J.; Westerman, B.A.; Rustenburg, F.; Schellen, P.; Verschueren, H.; Post, E.; Koster, J.; et al. RNA-Seq of Tumor-Educated Platelets Enables Blood-Based Pan-Cancer, Multiclass, and Molecular Pathway Cancer Diagnostics. Cancer Cell 2015, 28, 666–676. [Google Scholar] [CrossRef] [Green Version]
- Kuznetsov, H.S.; Marsh, T.; Markens, B.A.; Castaño, Z.; Greene-Colozzi, A.; Hay, S.A.; Brown, V.E.; Richardson, A.L.; Signoretti, S.; Battinelli, E.M.; et al. Identification of luminal breast cancers that establish a tumor-supportive macroenvironment defined by proangiogenic platelets and bone marrow-derived cells. Cancer Discov. 2012, 2, 1150–1165. [Google Scholar] [CrossRef]
- Kim, J.; Bamlet, W.R.; Oberg, A.L.; Chaffee, K.G.; Donahue, G.; Cao, X.-J.; Chari, S.; Garcia, B.A.; Petersen, G.M.; Zaret, K.S. Detection of early pancreatic ductal adenocarcinoma with thrombospondin-2 and CA19-9 blood markers. Sci. Transl. Med. 2017, 9, eaah5583. [Google Scholar] [CrossRef]
- Berger, A.W.; Schwerdel, D.; Reinacher-Schick, A.; Uhl, W.; Algül, H.; Friess, H.; Janssen, K.-P.; König, A.; Ghadimi, M.; Gallmeier, E.; et al. A Blood-Based Multi Marker Assay Supports the Differential Diagnosis of Early-Stage Pancreatic Cancer. Theranostics 2019, 9, 1280–1287. [Google Scholar] [CrossRef]
- Capello, M.; Bantis, L.E.; Scelo, G.; Zhao, Y.; Li, P.; Dhillon, D.S.; Patel, N.J.; Kundnani, D.L.; Wang, H.; Abbruzzese, J.L.; et al. Sequential Validation of Blood-Based Protein Biomarker Candidates for Early-Stage Pancreatic Cancer. J. Natl. Cancer Inst. 2017, 109. [Google Scholar] [CrossRef]
- Fahrmann, J.F.; Bantis, L.E.; Capello, M.; Scelo, G.; Dennison, J.B.; Patel, N.; Murage, E.; Vykoukal, J.; Kundnani, D.L.; Foretova, L.; et al. A Plasma-Derived Protein-Metabolite Multiplexed Panel for Early-Stage Pancreatic Cancer. J. Natl. Cancer Inst. 2019, 111, 372–379. [Google Scholar] [CrossRef]
- Mayerle, J.; Kalthoff, H.; Reszka, R.; Kamlage, B.; Peter, E.; Schniewind, B.; González Maldonado, S.; Pilarsky, C.; Heidecke, C.-D.; Schatz, P.; et al. Metabolic biomarker signature to differentiate pancreatic ductal adenocarcinoma from chronic pancreatitis. Gut 2018, 67, 128–137. [Google Scholar] [CrossRef]
- Bian, B.; Fanale, D.; Dusetti, N.; Roque, J.; Pastor, S.; Chretien, A.-S.; Incorvaia, L.; Russo, A.; Olive, D.; Iovanna, J. Prognostic significance of circulating PD-1, PD-L1, pan-BTN3As, BTN3A1 and BTLA in patients with pancreatic adenocarcinoma. Oncoimmunology 2019, 8, e1561120. [Google Scholar] [CrossRef]

| PDAC Patient Number (Control) | Type of Tumour: Resected, Locally Advanced, Metastatic, All | CTC Enrichment | CTC Detection | CTC Count | CTC Detection Rate in PDAC Patients | Prognosis Value of CTCs | Reference |
|---|---|---|---|---|---|---|---|
| 20 (15 benign diseases) | All (Samples before treatment) | Density centrifugation | RT-PCR CEA | NA | 26% | Positive correlation with recurrence | Mataki et al., 2004 [29] |
| 154 (68 benign diseases) | All (Samples before treatment) | Density centrifugation | RT-PCR CK20 | NA | 34% | Shorter OS (meta.) (p = 0.05) | Soeth et al., 2005 [30] |
| 25 (15 benign diseases) | All (Samples before treatment) | Immunomagnetic (EpCAM) | RT-PCR: cMET, hTERT, CK20, CEA | NA | 80–100% (sensitivity 100%; specificity 96%) | Not studied | Zhou et al., 2009 [32] |
| 41 (20 HC) | All (Sample before and post treatment) | Immunomagnetic (leukocytes CD45+ depletion) | ICC: CK8/CK18+, CA19-9+, CD45 | 16 | 80% before and 20% after chemotherapy | Not studied | Ren et al., 2011 [33] |
| 48 (10 CP) | All (Samples before and after treatment) | None | Real-time RT-PCR mRNA EpCAM | NA | 25% pre-operative 65% post-operative | No correlation with any outcome | Sergeant et al., 2011 [31] |
| 54 (No) | All (Sample time: NA) | Immunomagnetic: ISET and CellSearch® | ISET: Cytology, CD45− ICC: CK+, DAPI+, CD45− | - ISET: 26 - CellSearch®:6 | ISET:93% CellSearch®:40% | No correlation with any outcome | Khoja et al., 2012 [34] |
| 79 (No) | LA (Samples before and after chemotherapy) | Immunomagnetic: CellSearch® | ICC: CK+, DAPI+, CD45− | 1 to 15 (only 1 or 2 patients) | 11% | Poor differentiation and shorter OS (p = 0.01) | Bidard et al., 2013 [52] |
| 72 (28 benign diseases) | All (Samples before treatment) | Microfluidic (NanoVelcro) | ICC: CK+, DAPI+, CD45− KRAS mutation | 0 to ≥5 (*) | 75% | ≥3 CTCs: discriminate metastatic disease (p < 0.001) | Ankeny et al., 2016 [35] |
| 48 (No) | Metastatic (Samples before treatment) | Immunomagnetic: CellSearch® | ICC: CK+, DAPI+, CD45−, MUC-1+ | 23 patients: ≥1 9 patients: ≥2 | 48% | CTC MUC-1+ correlate with a shorter OS (p = 0.044) | Dotan et al., 2016 [36] |
| 60 (no) | All (40% of the samples performed after neo-adjuvant therapy) | Size based ISET | ICC: CK+, ALDH+, CD133+, CD44+ | Mean: 7.1 Median: 4 | 78% | CK+/ALDH+: shorter OS and DFS CK+/CD133+/CD 44+: shorter DFS | Poruk et al., 2017 [37] |
| 58 PDAC (10 HC) | All (samples time NA) | Size based: Screencell© | Cytology KRAS mutation | Range 0–13 | 67% | >3 CTC+: shorter OS | Kuleman et al., 2017 [38] |
| 52 (10 benign diseases) | All (samples time NA) | Size based Screencell© | Cytology | Median 4 Range 0–151 | 67% | No correlation | Sefrioui et al., 2017 [43] |
| 65 (15 HC) | LA and Meta. (Samples before treatment) | Immunomagnetic CellSearch® | ICC: CK+, DAPI+, CD45− | 4.9 | 32.3% | Independent predictor of shorter OS | Okubo et al., 2017 [39] |
| 100 (26 benign diseases) | All (32% of the samples after neo-adjuvant therapy) | Microfluidic Nano-velcro | ICC: CK+, DAPI+, CD45− | NA | 78% | Correlated with presence of occult metastasis, shorter PFS and OS | Court et al., 2018 [40] |
| 69 (9 benign diseases) | All (10% of the samples after neo-adjuvant therapy) | Immunomagnetic MACS and CellSearch® (n = 20) | ICC: CK+, DAPI+, CD45− | 17 patients >1 13 patients >2 | 33.3% | Independent predictor of shorter PFS and OS | Effenberger et al., 2018 [41] |
| 242 (No) | All (sample time NA) | Immunomagnetic CellSearch® | ICC: CK+, DAPI+, CD45− | Median 1 Range 1–33 | 78.5% | Shorter PFS (p < 0.001) | Hugenschmidt et al., 2018 [42] |
| 24 (no) | Metastatic (Samples before and after chemotherapy) | Microfluidic | ICC: CK+, DAPI+, CD133, EpCAM+, CD45- | Mean 3.87 CTCs/mL | 84.4% | No correlation | Varillas et al., 2019 [44] |
| 100 (16 benign disease, 30 HC) | All (Samples before and after treatment) | Microfluidic | ICC: Vimentin+, EpCAM+, CD45- | Median 3 Range 0–23 | 76% | ≥2 CTCs vimentin+: correlate with a shorter PFS | Wei et al., 2019 [45] |
| PDAC Patient Number (Control) | Type of Tumour: Resected, Locally Advanced, Metastatic, All | Site | Target for ctDNA | % of Mutations or Genetic Alterations in PDAC Patients | Diagnosis Performances | Positive Correlation with a Poor Prognosis (OS) (p) * | Reference |
|---|---|---|---|---|---|---|---|
| 44 (60: 37 CP and 23 miscellaneous) | All | Plasma | KRAS mutation Amplified PCR | 27 | Sensitivity: 27% Specificity: 100% | Yes—<0.005 | Castells et al., 1999 [55] |
| 47 (31: CP) | All | Serum | KRAS mutation sequencing | 47 | Sensitivity: 47% Specificity: 87% | No—Ns | Maire et al., 2002 [56] |
| 56 (13: CP) | All | Plasma | KRAS mutation PNA-mediated PCR clamping and real-time PCR | 36 | Sensitivity: 36% Specificity: 100% | No—0.10 | Däbritz et al., 2009 [57] |
| 91 (No) | LA + Meta. | Plasma | KRAS mutation sequencing | 33 | - | Yes—<0.001 | Chen et al., 2010 [58] |
| 36 (49: 25 HC and 24 miscellaneous) | All | Plasma | KRAS mutation cold-PCR combined with an unlabelled-probe HRM | 72 | Sensitivity: 81% Specificity: 87.5% | - | Wu et al., 2014 [59] |
| 27 (No) | LA + Meta. | Plasma | KRAS mutation ARMS PCR | 37 | - | Yes—0.003 Yes—0.014 *** | Semrad et al., 2015 [60] |
| 51 (No) | R | Plasma | KRAS mutation dPCR | 43 | Sensitivity: 43% Specificity: >99% | Yes (predictor of disease recurrence)—0.015 | Sausen et al., 2015 [61] |
| 45 (No) | All | Plasma | KRAS mutation dPCR | 26 | - | Yes—0.001 | Earl et al., 2015 [62] |
| 110 (25: HC) | All | Plasma | KRAS mutation RFLP + sequ. Two-step enriched-nested PCR | 31 | - | No—0.36 | Singh et al., 2015 [63] |
| 75 (40: 20 CP and 20 HC) | All | Serum | KRAS mutation dPCR | 63 | - | Yes—0.024 | Kinugasa et al., 2015 [64] |
| 259 (No) | All | Plasma | KRAS mutation dPCR | 8 (R), 18 (LA), 59 (M) | - | Yes—<0.0001 | Takai et al., 2015 [65] |
| 105 (20 HC) | R | Plasma | KRAS mutation dPCR | 31 | - | Yes—<0.0001 Yes—<0.001 ** | Hadano et al., 2016 [71] |
| 40 (10 HC) | All | Plasma and serum | KRAS mutation dPCR | 48 (All) 38 (LA) 63 (LA and Meta. Serum) | - | Yes—<0.01 | Ako et al., 2016 [67] |
| 188 (No) | Met | Plasma | KRAS mutation dPCR | 83 | - | Yes—0.019 | Cheng et al., 2017 [68] |
| 135 (No) | All | Plasma | KRAS mutation NGS/dPCR | 41 (LA and Meta.) | - | LA + Met: Yes—p < 0.001 Resected: Yes—0.027; Yes—0.03 ** | Pietrasz et al., 2017 [69] |
| 60 (No) | LA + Meta. | Plasma | KRAS mutation BEAMing | 65 | - | Yes—0.001 Yes—0.0022 ** | Van Laethem et al., 2017 [70] |
| 95 (No) | All | Plasma | 28 genes Methylation-specific PCR | 27 (>10 hypermethylated genes) | - | Yes | Henriksen et al., 2017 [85] |
| 26 (26: 14 CP and 12 HC) | All | Plasma | KRAS mutation dPCR NGS: KRAS, SMAD4, CDKN2A and TP53 | NGS: 27 dPCR: 23 | - | Yes—0.018 **** | Adamoet al., 2017 [66] |
| 27 (43 HC) | LA + Meta. | Plasma | KRAS mutation dPCR | 70.4 | - | No—0.16—0.24 *** | Del Re et al., 2017 [72] |
| 221 (182 HC) | R | Plasma | KRAS mutation PCR Safe-Sequencing System | 30 | Sensitivity: 30% Specificity: 99.5% | - | Cohen et al., 2017 [73] |
| 34 (No) | All | Plasma | NGS: 25 genes (including KRAS) | 25 genes: 74 KRAS only: 29 | - | Yes—0.045 | Pishvaian et al., 2017 [74] |
| 106 (No) | All | Plasma | KRAS mutation dPCR | 68(R), 72(LA), 87(M) | Sensitivity: 78% Specificity: 33% | Yes—0.008 Yes—0.003 *** | Kim et al., 2018 [75] |
| 65 (20 HC) | All | Plasma | KRAS mutation dPCR | 80 | - | No—0.73 | Lin et al., 2018 [77] |
| 45 (No) | R | Serum | KRAS mutation teal-time quantitative PCR | 55 | - | Pre-operative samples: No—0.258–0.710 ** Post-operative samples: Yes—0.027 ** | Nakano et al., 2018 [76] |
| Patient Number (PDAC) | Type of Tumour: Resected, Locally Advanced, Metastatic, All (Treatment) | Molecular Target(s) | Method of Isolation | Exosomes Detection Rate in PDAC Patients | Exosomes Diagnosis Performances | Exosomes Prognosis Value | Reference |
|---|---|---|---|---|---|---|---|
| 16 (6 HP, 6 CP, 5 cysts, 5 ampullary carcinoma) | All (12 metastatic) | miR-17-5p, -21, -155 | Ultracentrifugation RT qPCR | NA | (**) | miR-17-5p correlated with metastasis | Que et al., 2013 [104] |
| 131 (64 HC) | All | miR-1246, -4644, -3976, -4306; CD44v6, TSPAN8, EpCAM, MET, CD104 | Sucrose gradient, micro-array, RTqPCR, flow cytometry, latex beads | NA | Sens. 100% Spec. 80% | NS | Madhavan et al., 2014 [106] |
| 146 (benign pancreatic diseases 32, 120 HC) | All (Neo-adjuvant: 10) | GPC1 | Latex beads Ultracentrifugation Ac GPC1 | 100% | Sens. 100% Spec. 100% | GPC1+ correlates with worse DFS and OS | Melo et al., 2015 [107] |
| 29 (CP 11) | Resected and locally advanced | GPC1 miR-10b, -21, -30c, -181a, -let7a | GPC1 LC-MS/ML RT qPCR | 100% | Sens. 100% Spec. 100% | NS | Lai et al., 2017 [108] |
| 127 (136 HC) | All | Exo DNA ctDNA | Ultracentrifugation Flowcytometry dPCR | 54% | Sens. 54% Spec. 84% PPV 76% NPV 66% | Worse DFS P = 0.03 RR: 4.68 441 days vs. 127 | Allenson et al., 2017 [109] |
| 15 (15 HC) | All | miR: R196a, 196b and 1246 | ExoKit RTqPCR NGS | Significantly higher for 196a and 1246 | AUC: 196a: 0.81 1246: 0.73 196b 0.71 | NS | Xu et al., 2017 [110] |
| 68 (41 benign pancreatic diseases;18 HC) | All (Neo-adjuvant: 33) | Signature: EGRF, EpCAM, MUC1, GPC1, WNT2 | Ultracentrifugation | 89% | Sens. 86% Spec. 81% | NS | Yang et al., 2018 [111] |
| 20 (20 benign diseases) | Resected and locally advanced | Protein CD63, GPC1 | AC electrokinetics immunofluorescence | Significantly higher in PDAC cohort | Sens. 99 Spec. 82 | NS | Lewis et al., 2018 [112] |
| 32 (IPMN 29, 22 HC) | All | miR-191, -21, -451a | ExoKit Quick NGS RT qPCR | (*) | miR21 worse OS | Goto et al., 2018 [105] | |
| 24 (14 CP, 50 miscellaneous, 46 HC) | NA | Protein ZIP4 | Exo Kit precipitation | Significantly higher in PDAC | AUC ROC curve 0.89 | NS | Jin et al., 2018 [113] |
| 194 (25 cysts, 12 HC) | All (123 metastatic) | Exo DNA KRAS | Ultracentrifugation ddPCR | 61% metastatic 38% resectable | NS | MAF >5% Predictor PFS OS | Bernard et al., 2019 [79] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buscail, E.; Maulat, C.; Muscari, F.; Chiche, L.; Cordelier, P.; Dabernat, S.; Alix-Panabières, C.; Buscail, L. Liquid Biopsy Approach for Pancreatic Ductal Adenocarcinoma. Cancers 2019, 11, 852. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers11060852
Buscail E, Maulat C, Muscari F, Chiche L, Cordelier P, Dabernat S, Alix-Panabières C, Buscail L. Liquid Biopsy Approach for Pancreatic Ductal Adenocarcinoma. Cancers. 2019; 11(6):852. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers11060852
Chicago/Turabian StyleBuscail, Etienne, Charlotte Maulat, Fabrice Muscari, Laurence Chiche, Pierre Cordelier, Sandrine Dabernat, Catherine Alix-Panabières, and Louis Buscail. 2019. "Liquid Biopsy Approach for Pancreatic Ductal Adenocarcinoma" Cancers 11, no. 6: 852. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers11060852