Nanodrug Delivery Systems for the Treatment of Ovarian Cancer
Abstract
:1. Introduction
2. Current Nano-Based Drug Delivery Approaches for Ovarian Cancer Theranostic
3. Critical Comparison of Nanosystems to Nanomicelles for OC Treatment
Morphology, Composition and Mechanism of the Formation of Nanomicelles
4. Classification of Nanomicelles
4.1. Amphiphilic Nanomicelles
4.2. Polycharged Composite Nanomicelles
4.3. Noncovalent Connected Polymeric Nanomicelles
5. Surfactants Employed in Nanomicelle Targeted Platforms for Ovarian Cancer
6. Preparation of Drug-Loaded Nanomicelles for Application in Ovarian Cancer
7. Applications of Nanomicelles in Ovarian Cancer
7.1. Diagnosis of Ovarian Cancer Employing Nanomicelles
7.2. Treatment of Ovarian Cancer Using Nanomicelles
7.3. Targeting Strategies of Nanomicelles
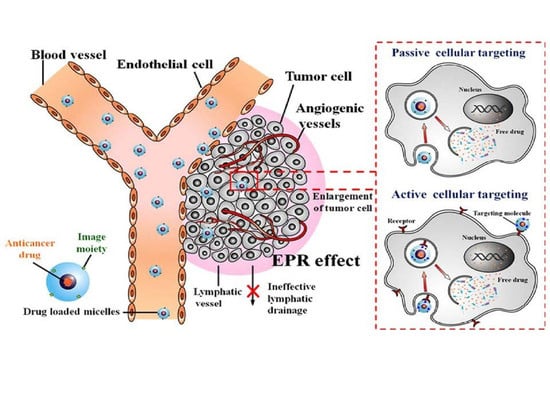
7.3.1. Passive Targeting by Enhanced Permeability Effect of Tumour Tubular Blood Vessels
7.3.2. Specific Active Receptor-Mediated Targeting
8. Mucins as Targets for Antibodies in Chemotherapeutics
9. Stimulus-Responsive Nanomicelles
10. Nanomicelles in Clinical Evaluations
11. Patents in Micellar Technologies for Targeted Chemotherapeutic Drug Delivery
12. Future Recommendations
13. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Loret, N.; Denys, T.; Berx, G. The role of epithelial-to-mesenchymal plasticity in ovarian cancer progression and therapy resistance. Cancers 2019, 11, 838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghisoni, E.; Imbimbo, M.; Zimmermann, S.; Valabrega, G. Ovarian cancer immunotherapy: Turning up the heat. Int. J. Mol. Sci. 2019, 20, 2927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deuster, E.; Mayr, D.; Hester, A.; Kolben, T.; Zeder-Göß, C.; Burges, A. Correlation of the aryl hydrocarbon receptor with fshr in ovarian cancer patients. Int. J. Mol. Sci. 2019, 20, 2862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siu, K.Y.M.; Jiang, Y.; Wang, J.; Leung, T.H.Y.; Han, C.Y.; Benjamin, K. Hexokinase 2 regulates ovarian cancer cell migration, invasion and stemness via FAK/ERK1/2/MMP9/NANOG/SOX9 signaling cascades. Cancers 2019, 11, 813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garziera, M.; Roncato, R.; Montico, M.; De Mattia, E.; Gagno, S.; Poletto, E.; Cecchin, E. New challenges in tumor mutation heterogeneity in advanced ovarian cancer by a targeted next-generation sequencing (NGS) approach. Cells 2019, 8, 584. [Google Scholar] [CrossRef] [Green Version]
- Wieser, V.; Sprung, S.; Tsibulak, I.; Haybaeck, J.; Hackl, H.; Fiegl, H. Clinical impact of RANK signalling in ovarian cancer. Cancers 2019, 11, 791. [Google Scholar] [CrossRef] [Green Version]
- Menyhárt, O.; Fekete, J.T.; Gyorffy, B. Gene expression indicates altered immune modulation and signaling pathway activation in ovarian cancer patients resistant to Topotecan. Int. J. Mol. Sci. 2019, 20, 2750. [Google Scholar] [CrossRef] [Green Version]
- Smith, T.; Guidozzi, F. Epithelial ovarian cancer in Southern Africa. SA J. Gynaecol. Oncol. 2009, 1, 23–27. [Google Scholar] [CrossRef]
- Erol, A.; Niemira, M.; Kretowski, A.D. Novel approaches in ovarian cancer research against heterogeneity, late diagnosis, drug resistance, and transcoelomic metastases. Int. J. Mol. Sci. 2019, 20, 2649. [Google Scholar] [CrossRef] [Green Version]
- Maru, Y.; Hippo, Y. Current status of patient-derived ovarian cancer models. Cells 2019, 8, 505. [Google Scholar] [CrossRef] [Green Version]
- Madariaga, A.; Lheureux, S.; Oza, A.M. Tailoring ovarian cancer treatment: Implications of BRCA1/2 mutations. Cancers 2019, 11, 416. [Google Scholar] [CrossRef] [Green Version]
- Moffitt, L.; Karimnia, N.; Stephens, A.; Bilandzic, M. Therapeutic Targeting of Collective Invasion in Ovarian Cancer. Int. J. Mol. Sci. 2019, 20, 1466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Napoletano, C.; Ruscito, I.; Bellati, F.; Zizzari, I.G. Bevacizumab-based chemotherapy triggers immunological effects in responding multi-treated recurrent ovarian cancer patients by favoring the recruitment of effector t cell subsets. J. Clin. Med. 2019, 8, 380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chishti, N.; Satveer Jagwani, S.; Dhamecha, D.; Jalalpure, S.; Dehghan, M.H. Preparation, optimization, and In Vivo Evaluation of nanoparticle-based formulation for pulmonary delivery of anticancer drug. Medicina 2019, 55, 294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dlamini, N.G.; Basson, A.K.; Pullabhotla, V.S.R. Optimization and application of bioflocculant passivated copper nanoparticles in the wastewater treatment. Int. J. Environ. Res. Public Health 2019, 16, 2185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cagliani, R.; Gatto, F.; Bardi, G. Protein adsorption: A feasible method for nanoparticle functionalization? Materials 2019, 12, 1991. [Google Scholar] [CrossRef] [Green Version]
- Dong, X. Review on current strategies for brain drug delivery. Theranostics 2018, 8, 1481–1493. [Google Scholar] [CrossRef]
- Basso, J.; Miranda, A.; Nunes, S.; Cova, T.; Sousa, J.; Vitorino, C.; Pais, A. Review on hydrogel-based drug delivery nanosystems for the treatment of brain tumors. MDPI J. Gels 2018, 4, 62. [Google Scholar] [CrossRef] [Green Version]
- Blanco, E.; Kessinger, C.W.; Sumer, B.D.; Gao, J. Multifunctional micellar nanomedicine for cancer therapy. Exp. Biol. Med. 2009, 234, 123–131. [Google Scholar] [CrossRef] [Green Version]
- Larraneta, E.; Stewart, S.; Ervine, M.; Al-Kasasbeh, R.; Donnelly, R.F. Hydrogels for hydrophobic drug delivery, classification, synthesis and applications. J. Funct. Biomater. 2018, 9, 13. [Google Scholar] [CrossRef] [Green Version]
- Chirwa, N.; Pillay, V.; Choonara, Y.E.; Kumar, P.; du Toit, L. Pharmaceutical Composition. U.S. Patent 9220773 B2, 29 December 2015. [Google Scholar]
- Vivek, R.; Thangam, R.; Kumar, S.R.; Rejeeth, C.; Kumar, G.S.; Sivasubramanian, S.; Vincent, S.; Gopi, D.; Kannan, S. HER2 targeted breast cancer therapy with switchable “off/on” multifunctional “smart” magnetic polymer core-shell nanocomposites. ACS Appl. Mater. Interfaces 2016, 8, 2262–2279. [Google Scholar] [CrossRef]
- Fanshawe, T.R.; Power, M.; Graziadio, S.; Jones, W.; Ordonez-Mena, J.M.; Simpson, A.J.; Oxford, O. Methods for evaluation of medical prediction models, tests and biomarkers (MEMTAB) symposium. Diagn. Progn. Res. 2018, 2. [Google Scholar] [CrossRef]
- Whitehouse, C.; Solomon, E. Current status of the molecular characterization of the ovarian cancer antigen CA125 and implications for its use in clinical screening. Gynaecol. Oncol. 2003, 88, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Trase, I.; Ren, M.; Duval, K.; Guo, X.; Chen, Z. Design of nanoparticle-based carriers for targeted drug delivery. J. Nanomater. 2016, 2016, 1087250. [Google Scholar] [CrossRef] [PubMed]
- Bhise, K.; Sau, S.; Alsaab, H.; Kashaw, S.K.; Tekade, R.K.; Iyer, A.K. Nanomedicine for cancer diagnosis and therapy, advancement, success and structure–activity relationship. Ther. Deliv. 2017, 8, 1003–1018. [Google Scholar] [CrossRef] [PubMed]
- Luong, D.; Sau, S.; Kesharwani, P.; Iyer, A.K. Polyvalent folate-dendrimer-coated iron oxide theranostic nanoparticles for simultaneous magnetic resonance imaging and precise cancer cell targeting. Biomacromolecules 2017, 18, 1197–1209. [Google Scholar] [CrossRef]
- Sharma, A.K.; Gothwal, A.; Kesharwani, P.; Alsaab, H.; Iyer, A.K.; Gupta, U. Dendrimer nanoarchitectures for cancer diagnosis and anticancer drug delivery. Drug Discov. Today 2017, 22, 314–326. [Google Scholar] [CrossRef]
- Chauhan, S.C.; Singh, A.P.; Ruiz, F.; Johansson, S.L.; Jain, M.; Smith, L.M.; Batra, S.K. Aberrant expression of MUC4 in ovarian modern pathology carcinoma, diagnostic significance alone and in combination with MUC1 and MUC16 (CA125). Mod. Pathol. 2006, 19, 1386–1394. [Google Scholar] [CrossRef] [PubMed]
- Felder, M.; Kapur, A.; Gonzalez-Bosquet, J.; Horibata, S.; Heintz, J.; Albrecht, R.; Whelan, R.J. MUC16 (CA125), tumor biomarker to cancer therapy, a work in progress. J. Mol. Cancer 2014, 13, 129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trucillo, P.; Campardelli, R.; Reverchon, E. Supercritical CO2 assisted liposomes formation: Optimization of the lipidic layer for an efficient hydrophilic drug loading. J. CO2 Util. 2017, 18, 181–188. [Google Scholar] [CrossRef]
- Kue, C.S.; Kamkaew, A.; Burgess, K.; Kiew, L.V.; Chung, L.Y.; Lee, H.B. Small molecules for active targeting in cancer. Med. Res. Rev. 2016, 36, 494–575. [Google Scholar] [CrossRef] [PubMed]
- Fathi, M.; SimaMajidi, S.; Zangabad, P.S.; Barar, J.H.E.; Omidi, Y. Chitosan- based multifunctional nanomedicines and theranostics for targeted therapy of cancer. Med. Res. Rev. 2018, 38, 2110–2136. [Google Scholar] [CrossRef] [PubMed]
- Larsson, M.; Huang, W.C.; Hsiao, M.H.; Wang, Y.J.; Nydén, M.; Chiou, S.H.; Liu, D.M. Biomedical applications and colloidal properties of amphiphilically modified chitosan hybrids. Prog. Polym. Sci. 2013, 38, 1307–1328. [Google Scholar] [CrossRef]
- Chen, H.P.; Chen, M.H.; Tung, F.I.; Liu, T.Y. A novel micelle-forming material used for preparing a theranostic vehicle exhibiting enhanced in vivo therapeutic efficacy. J. Med. Chem. 2015, 58, 3704–3719. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Jiang, F.; Tang, X.; Wang, B. N-octyl-N-arginine-chitosan micelles for gambogic acid intravenous delivery, characterization, cell uptake, pharmacokinetics, and biodistribution. Drug Dev. Ind. Pharm. 2018, 44, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Li, J.; Luo, Y.; Yin, T.; Cai, H.; Wang, Y.; Dong, Z.; Shuai, X.; Li, Z. pH-Sensitive nanomicelles for controlled and efficient drug delivery to human colorectal carcinoma lovo cells. PLoS ONE 2014, 9, e100732. [Google Scholar] [CrossRef]
- Kobayashi, H.; Watanabe, R.; Choyke, P.L. Improving conventional enhanced permeability and retention (EPR) effects; what is the appropriate target? Theranostics 2014, 4, 81–89. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, Y.; Mochida, A.; Choyke, P.L.; Kobayashi, H. Nanodrug delivery, is the enhanced permeability and retention effect sufficient for curing cancer? Bioconjug. Chem. 2016, 27, 2225–2238. [Google Scholar] [CrossRef]
- Din, F.; Aman, W.; Ullah, I.; Qureshi, O.S.; Mustapha, O.; Shafique, S.; Zeb, A. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int. J. Nanomed. 2017, 12, 7291–7309. [Google Scholar] [CrossRef] [Green Version]
- Sutradhar, K.B.S.; Amin, M.L. Nanotechnology in cancer drug delivery and selective targeting. ISRN Nanotechnol. 2014, 939378–939389. [Google Scholar] [CrossRef] [Green Version]
- Bolu, B.S.; Sanyal, M.R.; Sanyal, A. Drug delivery systems from self-assembly of dendron-polymer conjugates. Molecules 2018, 23, 1570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macchione, M.A.; Biglione, C.; Strumia, M. Design, synthesis and architectures of hybrid nanomaterials for therapy and diagnosis applications. Polymers 2018, 10, 527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Target. Ther. 2018, 3, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atkinson, S.P.; Andreu, M.Z.; Vicent, M.J. Polymer therapeutics, biomarkers and new approaches for personalized cancer treatment. J. Pers. Med 2018, 8, 6. [Google Scholar]
- Dou, X.; Wang, H.; Zhang, J.; Wang, F.; Xu, G.; Xu, H.; Xiang, S.; Fu, J.; Song, H. Aptamer—Drug conjugate, targeted delivery of doxorubicin in a HER3 aptamer-functionalized liposomal delivery system reduces cardiotoxicity. Int. J. Nanomed. 2018, 13, 763–776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Y.; Park, K. Polymeric micelles and alternative nanonized delivery vehicles for poorly soluble drugs. Int. J. Pharm. 2012, 8, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Yang, H. Superelastic and pH-responsive degradable dendrimer cryogels prepared by cryo-aza-michael addition reaction. Nat. Sci. Rep. 2018, 8, 7155. [Google Scholar] [CrossRef] [Green Version]
- Miao, T.; Wang, J.; Zeng, Y.; Liu, G.; Chen, X. Polysaccharide-based controlled release systems for therapeutics delivery and tissue engineering, from bench to bedside. Adv. Sci. 2018, 5, 1700513. [Google Scholar] [CrossRef]
- Pelegri-O’Day, E.M.; Lin, E.; Maynard, H.D. Therapeutic protein—Polymer conjugates, advancing beyond PEGylation. J. Am. Chem. Soc. 2014, 136, 14323–14332. [Google Scholar] [CrossRef]
- Brandta, J.V.; Piazzaa, R.D.; dos Santosa, C.C.; Vega-Chacóna, J.; Amantéaa, B.E. Synthesis and colloidal characterization of folic acid-modified PEG-b-PCL micelles for methotrexate delivery. Colloids Surf. B Biointerfaces 2019, 177, 228–234. [Google Scholar] [CrossRef]
- Tong, R.; Yala, L.; Fan, T.M.; Cheng, J. The formulation of aptamer-coated paclitaxel-polylactide nanoconjugates and their targeting to cancer cells. J. Biomater. 2010, 31, 3043–3053. [Google Scholar] [CrossRef] [Green Version]
- Huang, D.; Wu, D. Biodegradable dendrimers for drug delivery. Mater. Sci. Eng. C 2018, 90, 713–727. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, N.; Udayakumar, T.S.; D’Souza, W.D.; Simone, M.I.I.; Charles, B.; Raghavan, S.R.; Mahmood, J.P. Liposomes: Clinical applications and potential for image-guided drug delivery. Molecules 2018, 23, 288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fornaguera, C.; Dols-Perez, A.; Calderó, G.; García-Celma, M.J.; Camarasa, J.; Solans, C. PLGA nanoparticles prepared by nano-emulsion templating using low-energy methods as efficient nanocarriers for drug delivery across the blood-brain barrier. J. Control. Release 2015, 211, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Olusanya, T.O.B.; Ahmad, R.R.H.; Ibegbu, D.M.; Smith, J.R.; Elkordy, A.A. Liposomal drug delivery systems and anticancer drugs. Molecules 2018, 23, 907. [Google Scholar] [CrossRef] [Green Version]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and challenges of liposome assisted drug delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef] [Green Version]
- Lila, A.S.A.; Ishida, T. Liposomal delivery systems, design optimization and current applications. Biol. Pharm. Bull. 2017, 40, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Bhadani, A.; Kafle, A.; Koura, S.; Sakai, K.; Sakai, H.; Masahiko, A.M. Physicochemical evaluation of micellar solution and lyotropic phases formed by self-assembled aggregates of morpholinium geminis. ACS Omega 2017, 2, 5324–5334. [Google Scholar] [CrossRef]
- Vaishya, R.D.; Khurana, V.; Patel, S.; Mitra, A.K. Controlled ocular drug delivery with nanomicelles. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2014, 6, 422–437. [Google Scholar] [CrossRef] [Green Version]
- Bodratti, A.M.; Alexandridis, P. Formulation of Poloxamers for Drug Delivery. J. Funct. Biomater. 2018, 9, 11. [Google Scholar] [CrossRef] [Green Version]
- Aziz, Z.A.A.; Ahmad, A.; Mohd-Setapar, S.H.; Hassan, H.; Lokhat, D.; Kamal, M.A.; Ashraf, M.G. Recent advances in drug delivery of polymeric nano-micelles. Curr. Drug Metab. 2017, 18, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Simões, S.M.N.; Figueiras, A.R.; Veiga, F.; Concheiro, A.; Alvarez-Lorenzo, C. Polymeric micelles for oral drug administration enabling locoregional and systemic treatments. Expert Opin. Drug Deliv. 2014, 12, 297–318. [Google Scholar] [CrossRef] [PubMed]
- Movassaghian, S.; Merkel, O.M.; Torchilin, V.P. Applications of polymer micelles for imaging and drug delivery. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 691–707. [Google Scholar] [CrossRef] [PubMed]
- Vyas, B.; Pillai, S.A.; Bahadur, A.; Bahadur, P. A comparative study on micellar and solubilizing behavior of three eo-po based star block copolymers varying in hydrophobicity and their application for the In Vitro release of anticancer drugs. Polymers 2018, 10, 76. [Google Scholar] [CrossRef] [Green Version]
- Santos, M.S.; Tavares, F.W.; Biscaia, E.C. Molecular Thermodynamics of micellization, micelle size distributions and geometry transitions. Braz. J. Chem. Eng. 2016, 33, 515–523. [Google Scholar] [CrossRef] [Green Version]
- Chidi, O.; Adebayo, I.V. Determination of Critical Micelle Concentration and Thermodynamic Evaluations of Micellization of GMS. Mod. Chem. Appl. 2018, 6, 2. [Google Scholar] [CrossRef]
- Mukherjee, I.; Moulik, S.P.; Rakshit, A.K. Tensiometric determination of Gibbs surface excess and micelle point: A critical revisit. J. Colloid Interface Sci. 2013, 394, 329–336. [Google Scholar] [CrossRef]
- Sutton, D.; Nasongkla, N.; Blanco, E.; Gao, J. Functionalized micellar systems for cancer targeted drug delivery. Pharm. Res. 2007, 24, 1029–1046. [Google Scholar] [CrossRef]
- Nguyen, V.T.A.; De Pauw-Gillet, M.; Sandre, O.; Gauthier, M. Biocompatible polyion complex micelles synthesized from arborescent polymers. langmuir. Am. Chem. Soc. 2016, 32, 13482–13492. [Google Scholar]
- Hussein, Y.H.A.; Youssry, M. Polymeric micelles of biodegradable diblock copolymers, enhanced encapsulation of hydrophobic drugs. Materials 2018, 11, 688. [Google Scholar] [CrossRef] [Green Version]
- Ding, J.; Chen, L.; Xiao, C.; Chen, L.; Zhuang, X.; Chen, X. Noncovalent interaction-assisted polymeric micelles for controlled drug delivery. Chem. Commun 2014, 50, 11274–11290. [Google Scholar] [CrossRef] [PubMed]
- Mandala, A.; Bisht, R.; Rupenthal, I.D.; Mitraa, A.K. Polymeric micelles for ocular drug delivery, From structural frameworks to recent preclinical studies. J. Control. Release 2017, 248, 96–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michalicova, P.; Mravec, F.; Peka, R.M. Fluorescence study of freeze-drying as a method for support the interactions between hyaluronic and hydrophobic species. PLoS ONE 2017, 12, e0184558. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, D.; Josh, N.; Tao, W.; Karp, J.M.; Peer, D. Progress and challenges towards targeted delivery of cancer therapeutics. Nat. Commun. 2018, 9, 1410. [Google Scholar] [CrossRef] [Green Version]
- Desai, K.G.H. Polymeric drug delivery systems for intraoral site-specific chemoprevention of oral cancer. J. Biomed. Mater. Res. Part B 2018, 106, 1383–1413. [Google Scholar] [CrossRef]
- Hekman, M.C.H.; Boerman, O.C.; Bos, D.L.; Massuger, L.F.A.G.; Weil, S.; Grasso, L.; Rybinski, K.A.; Oosterwijk, E.; Mulders, P.F.A.; Rijpkema, M. Improved intraoperative detection of ovarian cancer by folate receptor alpha targeted dual-modality imaging. Mol. Pharm. 2017, 14, 3457–3463. [Google Scholar] [CrossRef]
- Judy, R.P.; Keating, J.J.; DeJesus, E.M.; Jiang, J.X.; Okusanya, O.T.; Nie, S.; Singhal, S. Quantification of tumor fluorescence during intraoperative optical cancer imaging. Sci. Rep. 2015, 5, 16208. [Google Scholar] [CrossRef] [Green Version]
- Jung, K.H.; Lee, K.H. Molecular imaging in the era of personalized medicine. J. Pathol. Transl. Med. 2015, 49, 5–12. [Google Scholar] [CrossRef]
- Kedar, U.; Shidhaye, S.; Kadam, V.U. Advances in polymeric micelles for drug delivery and tumour targeting. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 714–729. [Google Scholar] [CrossRef]
- Chen, Y.; Lo, C.; Hsiue, G. Multifunctional nanomicellar systems for delivering anticancer drugs. J. Biomed. Mater. Res. Part A 2014, 102, 2024–2038. [Google Scholar] [CrossRef]
- Harada, A.; Kataoka, K. Formation of polyion complex micelles in an aqueous milieu from a pair of oppositely-charged block copolymers with poly (ethylene glycol) segments. Macromolecules 1995, 28, 5294–5299. [Google Scholar] [CrossRef]
- Li, Y.; Kwon, G.S. Methotrexate esters of poly (ethylene oxide)-block-poly(2-hydroxyethyl-l-aspartamide). Part I: Effects of the level of methotrexate conjugation on the stability of micelles and on drug release. Pharm. Res. 2000, 17, 607–611. [Google Scholar] [CrossRef] [PubMed]
- Lavasanifar, A.; Samuel, J.; Kwon, G.S. Micelles self-assembled from poly (ethylene oxide)-block-poly (N-hexyl stearate l-aspartamide) by a solvent evaporation method: Effect on the solubilization and haemolytic activity of amphotericin B. J. Control. Release 2001, 77, 155–160. [Google Scholar] [CrossRef]
- Kim, J.H.; Emoto, K.; Iijima, M.; Nagasaki, Y.; Aoyagi, T.; Okano, T.Y. Core-stabilized polymeric micelle as potential drug carrier: Increased solubilization of taxol. Polym. Adv. Technol. 1999, 10, 647–654. [Google Scholar] [CrossRef]
- Zhiang, J.; Wu, M.; Yang, J.; Wu, Q.; Jin, Z. Anionic poly (lactic acid)-polyurethane micelles as potential biodegradable drug delivery carriers. Colloids Surf. A 2009, 337, 200–204. [Google Scholar] [CrossRef]
- Patil, Y.B.; Toti, U.S.; Khdair, A.; Linan, M.; Panyam, J. Single-step surface functionalization of polymeric nanoparticles for targeted drug delivery. Biomaterials 2009, 30, 859–866. [Google Scholar] [CrossRef] [Green Version]
- Chung, J.E.; Yokoyama, M.; Okano, T.; Yamato, M.; Aoyagi, T.; Sakurai, Y. Thermoresponsive drug delivery from polymeric micelles constructed using block copolymer of poly(N-isopropylacrylamide) and poly (butyl methacrylate). J. Control. Release 1999, 62, 115–127. [Google Scholar] [CrossRef]
- Elliott, R.L.; Elliott, M.C.; Wang, F.; Head, J.F. Breast carcinoma and the role of iron metabolism: A cytochemical, tissue culture and ultrastructural study. Ann. N.Y. Acad. Sci. 1993, 698, 159–166. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Nagasaki, Y.; Kato, Y.; Sugiyama, Y.; Kataoka, K. Longcirculating poly (ethylene glycol)-poly (D, L-lactide) block copolymer micelles with modulated surface charge. J. Control. Release 2001, 77, 27–38. [Google Scholar] [CrossRef]
- Guo, J.; Gao, X.; Su, L.; Xia, H.; Gu, G.; Pang, Z.; Jiang, X.; Yao, L.; Chen, J.; Chen, H. Aptamer-functionalized PEG–PLGA nanoparticles for enhanced anti-glioma drug delivery. Biomaterials 2011, 32, 8010–8020. [Google Scholar] [CrossRef]
- Yáñez, J.; Forrest, M.; Ohgami, Y.; Kwon, G.; Davies, N. Pharmacometrics and delivery of novel nanoformulated PEG-b-poly (E-caprolactone) micelles of rapamycin. Cancer Chemother. Pharmacol. 2008, 61, 133–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.; Lee, E.S.; Oh, K.T.; Gao, Z.G.; Bae, Y.H. Doxorubicin-loaded polymeric micelle overcomes multidrug resistance of cancer by double-targeting folate receptor and early endosomal pH. Small 2008, 4, 2043–2050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, R.P.; Jeong, Y.I.; Choi, E.; Chung, C.W.; Kang, D.H.; Oh, S.O.; Suh, H.; Kim, I. Biocompatible poly (2-hydroxyethyl methacrylate)-bpoly (L-histidine) hybrid materials for pH-sensitive intracellular anticancer drug delivery. Adv. Funct. Mater. 2011, 22, 1058–1068. [Google Scholar] [CrossRef]
- Tsai, H.C.; Tsai, C.H.; Lin, S.Y.; Jhang, C.R.; Chiang, Y.S.; Hsiue, G.H. Stimulated release of photosensitizers from graft and diblock micelles for photodynamic therapy. Biomaterials 2012, 33, 1827–1837. [Google Scholar] [CrossRef]
- Lu, P.L.; Chen, Y.C.; Ou, T.W.; Chen, H.H.; Tsai, H.C.; Wen, C.J.; Lo, C.L.; Wey, S.P.; Lin, K.J.; Yen, T.C.; et al. Multifunctional hollow nanoparticles based on graft-diblock copolymers for doxorubicin delivery. Biomaterials 2011, 32, 2213–2221. [Google Scholar] [CrossRef]
- Lin, S.Y.; Hsu, W.H.; Lo, J.M.; Tsai, H.C.; Hsiue, G.H. Novel geometry type of nanocarriers mitigated the phagocytosis for drug delivery. J. Control. Release 2011, 154, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Cholkar, K.; Patel, A.; Vadlapudi, A.D.; Ashim, K.; Mitra, A.K. Novel nanomicellar formulation approaches for anterior and posterior segment ocular drug delivery. Recent Pat. Nanomed. 2012, 2, 82–95. [Google Scholar] [CrossRef]
- Fares, A.R.; ElMeshad, A.N.; Kassem, M.A. Enhancement of dissolution and oral bioavailability of lacidipine via pluronic P123/ F127 mixed polymeric micelles, formulation, optimization using central composite design and in vivo bioavailability study. Drug Deliv. 2018, 25, 132–142. [Google Scholar] [CrossRef] [Green Version]
- Vadlapudi, A.D.; Cholkar, K.; Vadlapatla, R.K.; Mitra, A.K. Aqueous nanomicellar formulation for topical delivery of biotinylated lipid prodrug of acyclovir, formulation development and ocular biocompatibility. J. Ocul. Pharmacol. Ther. 2014, 30, 49–58. [Google Scholar] [CrossRef] [Green Version]
- Lengyel, E. Ovarian cancer development and metastasis. Am. J. Pathol. 2010, 177, 1053–1064. [Google Scholar] [CrossRef]
- Testa, U.; Petrucci, E.; Pasquini, L.; Castelli, G.; Pelosi, E. Ovarian cancers, genetic abnormalities, tumor heterogeneity and progression, clonal evolution and cancer stem cells. Medicines 2018, 5, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorenzo, G.D.; Ricci, G.; Severini, G.M.; Romano, F.; Biffi, S. Imaging and therapy of ovarian cancer, clinical application of nanoparticles and future perspectives. Theranostics 2018, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.A.; Cronin, A.; Milne, D.E.; Bookman, M.A.; Burger, R.A.; Cohn, D.E.; Mantia-Smaldone, G. Use and effectiveness of intraperitoneal chemotherapy for treatment of ovarian cancer. J. Clin. Oncol. 2015, 33, 2841–2847. [Google Scholar] [CrossRef] [PubMed]
- Coward, J.I.G.; Middleton, K.; Murphy, F. New perspectives on targeted therapy in ovarian cancer. Int. J. Women’s Health 2015, 7, 189–203. [Google Scholar] [CrossRef] [Green Version]
- Jahangirian, H.; Lemraski, E.G.; Webster, T.J.; Moghaddam, R.; Abdollahi, Y. A review of drug delivery systems based on nanotechnology and green chemistry, green nanomedicine. Int. J. Nanomed. 2017, 12, 2957–2978. [Google Scholar] [CrossRef] [Green Version]
- Fang, J.; Nakamura, H.; Maeda, H. The EPR effect, unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv. Drug Deliv. Rev. 2011, 63, 136–151. [Google Scholar] [CrossRef]
- Makhmalzade, B.S.; Chavoshy, F. Polymeric micelles as cutaneous drug delivery system in normal skin and dermatological disorders. J. Adv. Pharm. Technol. Res. 2017, 9, 2–8. [Google Scholar]
- Jahan, S.T.; Sam, M.A.; Walliser, M.; Haddadi, A. Targeted therapeutic nanoparticles, an immense promise to fight against cancer. Hindawi J. Drug Deliv. 2017, 2017, 9090325. [Google Scholar] [CrossRef]
- Dai, L.; Liu, J.; Luo, Z.; Li, M.; Cai, K. Tumor therapy, targeted drug delivery systems. J. Mater. Chem. B. 2016, 4, 6758. [Google Scholar] [CrossRef]
- Pillai, G. Nanomedicines for cancer therapy, an update of FDA approved and those under various stages of development. SOJ Pharm Pharm Sci. 2014, 1, 13. [Google Scholar]
- Ljubimova, J.Y.; Sun, T.; Mashouf, L.; Ljubimov, A.V.; Israel, L.L.; Ljubimov, V.A.; Holler, E. Covalent nano delivery systems for selective imaging and treatment of brain tumours. Adv. Drug Deliv. Rev. 2017, 113, 177–200. [Google Scholar] [CrossRef] [PubMed]
- Savla, R.; Minko, T. Nanoparticle design considerations for molecular imaging of apoptosis, diagnostic, prognostic, and therapeutic value. Adv. Drug Deliv. Rev. 2017, 113, 122–140. [Google Scholar] [CrossRef] [PubMed]
- Estelrich, J.; Busquets, M.A.; Morán, M.C. Effect of pegylation on ligand-targeted magnetoliposomes, a missed goal. ACS Omega 2017, 2, 6544–6555. [Google Scholar] [CrossRef] [PubMed]
- Gomes de Castro, M.A.; Ho bartner, C.; Opazo, F. Aptamers provide superior stainings of cellular receptors studied under super resolution microscopy. PLoS ONE 2017, 12, e0173050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cha, H.; Song, K.S. Effect of MUC8 on airway Inflammation: A friend or a foe? J. Clin. Med. 2018, 7, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sudimack, B.A.J.; Lee, R.J. Targeted drug delivery via the folate receptor. J. Adv. Drug Deliv. Rev. 2013, 41, 147–162. [Google Scholar] [CrossRef]
- Liao, C.; Sun, Q.; Liang, B.; Shena, J.; Shuai, X. Targeting EGFR-overexpressing tumour cells using Cetuximab-immunomicelles loaded with doxorubicin and superparamagnetic iron oxide. Eur. J. Radiol. 2010, 80, 699–705. [Google Scholar]
- Das, S.; Batra, S.K. Understanding the unique attributes of MUC16 (CA125), potential implications in targeted therapy. Cancer Res. 2015, 75, 4669–4674. [Google Scholar] [CrossRef] [Green Version]
- Rao, T.D.; Fernández-Tejada, A.; Axelrod, A.; Rosales, N.; Yan, X.; Thapi, S.; Lewis, J.S. Antibodies against Specific MUC16 glycosylation sites inhibit ovarian cancer growth. ACS Chem. Biol. 2017, 12, 2085–2096. [Google Scholar] [CrossRef]
- Schummer, M.; Thorpe, J.; Giraldez, M.; Bergan, L.; Tewari, M.; Urban, N. Evaluating serum markers for hormone receptor-negative breast cancer. PLoS ONE 2015, 10, e0142911. [Google Scholar] [CrossRef]
- Kabel, A.M. Tumour markers of breast cancer: New prospective. J. Oncol. Sci. 2017, 3, 5–11. [Google Scholar]
- Mai, B.T.; Fernandes, S.; Balakrishnan, P.B.; Pellegrino, T. Nanosystems based on magnetic nanoparticles and thermos-or ph-responsive polymers: An update and future perspectives. Acc. Chem. Res. 2018, 51, 999–1013. [Google Scholar] [CrossRef] [PubMed]
- Naseem, S.; Bansal, N.; Logani, L.A. Recent advances in imaging technologies in dentistry. World J. Radiol. 2014, 6, 794–807. [Google Scholar]
- Singh, A.P.; Senapati, S.; Ponnusamy, M.P.; Jain, M.; Lele, S.M.; Davis, J.S.; Batra, S.K. Clinical potential of mucins in diagnosis, prognosis, and therapy of ovarian cancer. Lancet Oncol. 2008, 9, 1076–1085. [Google Scholar] [CrossRef] [Green Version]
- Bansal, K.K.; Gupta, J.; Rosling, A.; Rosenholm, J.M. Renewable poly (δ-decalactone) based block copolymer micelles as drug delivery vehicle: In Vitro and In Vivo evaluation. Saudi Pharm. J. 2018, 26, 358–368. [Google Scholar] [CrossRef]
- Lee, K.S.; Chung, H.C.; Im, S.A.; Park, Y.H.; Kim, C.S.; Kim, S.B. Multicenter phase II trial of genexol-PM, a cremophor-free, polymeric micelle formulation of paclitaxel, in patients with metastatic breast cancer. Breast Cancer Res. Treat. 2008, 108, 241–250. [Google Scholar] [CrossRef]
- Matsumura, Y. Poly (amino acid) micelle nanocarriers in preclinical and clinical studies. Adv. Drug Deliv. Rev. 2008, 60, 899–914. [Google Scholar] [CrossRef]
- Hrkach, J.; Von Hoff, D.; Mukkaram, A.M.; Andrianova, E.; Auer, J.; Campbell, T. Preclinical development and clinical translation of a PSMA-targeted docetaxel nanoparticle with a differentiated pharmacological profile. Sci. Transl. Med. 2012, 4, 128ra39. [Google Scholar] [CrossRef]
- Wilson, R.H.; Plummer, R.; Adam, J.; Eatock, M.; Boddy, A.V.; Griffin, M. Phase I and pharmacokinetic study of NC-6004, a new platinum entity of cisplatin-conjugated polymer forming micelles. J. Clin. Oncol. 2008, 26, 2573. [Google Scholar] [CrossRef]
- Ueno, T.; Endo, K.; Hori, K.; Ozaki, N.; Tsuji, A.; Kondo, S.; Yoshizaki, T. Assessment of antitumor activity and acute peripheral neuropathy of 1,2-diaminocyclohexane platinum (II)-incorporating micelles (NC-4016). Int. J. Nanomed. 2014, 9, 3005–3012. [Google Scholar] [CrossRef] [Green Version]
- Matsumura, Y.; Kataoka, K. Preclinical and clinical studies of anticancer agent-incorporating polymer micelles. Cancer Sci. 2009, 100, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Hamaguchi, T.; Ura, T.; Muro, K.; Yamada, Y.; Shimada, Y.; Watanabe, N. Phase I clinical trial and pharmacokinetic evaluation of NK911, a micelle-encapsulated doxorubicin. Br. J. Cancer 2004, 91, 1775–1781. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Yin, M.; Zhao, L.; Meng, F.; Luo, L. Recent progress on nanoparticle-based drug delivery systems for cancer therapy. Cancer Biol. Med. 2017, 14, 228–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanwal, M.; Ding, X.; Song, X.; Zhou, G.; Cao, Y. MUC16 overexpression induced by gene mutations promotes lung cancer cell growth and invasion. Oncotarget 2018, 9, 12226–12239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, G.S.; Tomoda, K.; Chiang, C.; Kozak, K.R. Examination of gossypol-pluronic micelles as potential radiosensitizers. AAPS J. 2015, 17, 1369–1375. [Google Scholar]
- Siraj, N.; El-Zahab, B.; Hamdan, S.; Karam, T.E.; Haber, L.H.; Li, M.; Patonay, G. Fluorescence, phosphorescence, and chemiluminescence. Anal. Chem. 2016, 88, 170–202. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.; Patel, B.B.; Tiwari, S. Colloidal nanocarriers: a review on formulation technology, types and applications toward targeted drug delivery. Nanomedicine 2010, 6, 9–24. [Google Scholar] [CrossRef]
- Hao, J.; Tong, T.; Jin, K.; Zhuang, Q.; Han, T.; Bi, Y.; Wang, J.; Wang, X. Folic acid-functionalized drug delivery platform of resveratrol based on Pluronic 127/D-α-tocopheryl polyethylene glycol 1000 s uccinate mixed micelles. Int. J. Nanomed. 2017, 12, 2279–2292. [Google Scholar] [CrossRef] [Green Version]
- Rhyner, M.N. Development of Cancer Diagnostics Using Nanoparticles and Amphiphilic Polymers. Ph.D. Thesis, Georgia Institute of Technology, Atlanta, GA, USA, 2008. [Google Scholar]







| Nanosystems | Polymer–Drug Conjugates | Dendrimers | Polymer Micelles | Liposomes | Solid Lipid Nanoparticles |
|---|---|---|---|---|---|
| Size | ≤10 nm | 2–10 nm | 10–100 nm | 100–200 nm | 50–1000 nm |
| Structural characteristics | Macromolecular structure | Macromolecular Tree-like structure | Spherical Supramolecular Core shell structure | Spherical bilayer vesicle structure | Spherical, bilayer-nanocapsular structure |
| Carrier composition | Water-soluble polymer | Hyperbranched polymer chains | Amphiphilic di and tri-block copolymers | Phospholipid, cholesterol membrane lipids | Solid lipid emulsifier water |
| Drug incorporation strategy | Covalent conjugation requiring functional groups on drug and polymer | Covalent conjugation requiring functional groups on drug and polymer | Noncovalent encapsulation/compatible with hydrophobic drugs | Noncovalent encapsulation/compatible with hydrophilic drugs | Noncovalent encapsulation/compatible with hydrophilic drugs |
| PEG-paclitaxel & HPMA copolymer-doxorubicin—phase II trials SMANCS & CDP870 (Cimza)- Approved | Dendrimer- docetaxel & Viva gel- phase II & III trials PSMA-targeted dendrimers & Avidimer- dendrimers- Approved | CRLX- 101&NKTR-102- phase II/III clinical trials Genexol- PM- Approved | SGT53-01& MCC- 46 phase I clinical trials Doxil, Ambisome & DaunoXome- Approved | SLNs with [Gd-DTPA(H2O)]2− and [Gd-DOTA(H2O)]− compounds preclinical trials [31]. Diazemuls & Diprivan- Approved |
| Copolymers | Abbreviation | Repeating Unit Structure |
|---|---|---|
| Corona segment | ||
| Poly (ethylene glycol) | PEG, PEO |  |
| Poly (N-vinyl pyrrolidone) | PVP |  |
| Poly (N-isopropyacrylamide | PNIPAM, NIPAM |  |
| Poly (N-vinyl alcohol) | PVA |  |
| Poly (N-(2-hydroxyproyl methacrylamide) | pHPMAm |  |
| Core segment | ||
| Polyesters | ||
| Poly (propylene oxide) | PPO |  |
| Poly esters | ||
| Poly (L-lactide) | ||
| Poly (D,L-lactide) | PLA, PDLLA |  |
| Poly (lactide-co-gycolide) | PLGA |  |
| Poly (Ɛ-caprolactone) | PCL |  |
| Poly(β-amino ester) |  | |
| Poly(lactic acid) | PLA |  |
| Polymer Structural Formula | Method of Synthesis | Method of Micellization | Delivered Agent | Mode of Delivery | References |
|---|---|---|---|---|---|
| PLGA-b-PPO-b-PLGA and PEG-b-PPO | Ring-opening polymerization | Dialysis method | Doxorubicin (DD) | P | [82] |
| Poly(ε-caprolactone)-b-PEO | Anionic ring opening polymerization | Dialysis method | Pyrene (hydrophobic fluorescent probe) (DA) | P | [83] |
| Poly(lactic acid)-polyurethane | Step condensation | Microphase separation method | Gliclazide (DD) | P | [84] |
| PMPC-b-PBMA | RAFT technology | Self-emulsion evaporation method | Paclitaxel (DD) | P | [85] |
| Poly(ethylene glycol-b-lactide) | Anionic ring opening polymerization | Oil-in-water emulsion method | Taxol (DD) | P | [86] |
| Poly(lactide-b-PEG) | Solvent polymerization | Self-emulsion solvent evaporation method | Paclitaxel (DD) | P | [87] |
| mPEG-b-p(HEMAm-Lacn) | Free-radical polymerization | Rapid heating procedure | Pyrene (DA) | P | [88] |
| ϒ-Benzyl l-glutamate N- Carboxyanhydride | Polymerization | Dialysis | Adriamycin (DD) | P | [89] |
| Acetal-PEG-b-PLA | Ring-opening polymerization | Dialysis method | Docetaxel, 125 I (DD), (DA) | Tyrosine-A, tyrosyl-glutmic acid-A | [90] |
| COOH-PEG-b-PLGA | Polymerization | Dialysis method | Docetaxel, paclitaxel (DD) | RNA aptamer-A DNA aptamer-A | [91] |
| PEG-b-PCL | Free-radical polymerization | Dialysis method | Paclitaxel, rapamycin (DD) | Folate- A | [92] |
| PEG-b-PLLA and P(HEMA)-b-p(His) | Solvent polymerization | Dialysis method | Doxorubicin (DD) | - | [93] |
| P(HEMA)-b-p(His) | Solvent polymerization | Dialysis method | Doxorubicin(DD) | Folate-A | [94] |
| PEG-b-PLA and HEMA-co-his)-g-PLA | Anionic ring opening polymerization | Oil-in-water emulsion method | Doxorubicin, Cy 5.5 (DD), (DA) | Folate-A | [95] |
| PEG-b-PLA and P(NVI-co-NVP)-g-PLA | Anionic ring opening polymerization | Oil-in-water emulsion method | Doxorubicin, 123 I (DD), DA | Folate-A | [96] |
| mPEG-b-PLA and P(NIPAAm-co-MAAc)-g-PLA | Solvent polymerization | Self-emulsion solvent evaporation method | Doxorubicin, FITC (DD), DA | Galactosamine-A | [97] |
| Formulation Trade Name | Incorporated Drug | Purpose | Polymer | Particle Size (nm) | Drug Loading (%) | Phase | References |
|---|---|---|---|---|---|---|---|
| Genexol-PM | Paclitaxel | Solubilization | MPEG-PDLLA | <50 | 16.7 | III, IV | [127] |
| NK-105 | Paclitaxel | Targeting | PEG-P(Asp) | 85 | 23.0 | II, III | [128] |
| SP-1049C | Doxorubicin | Anti-MDR effect | Pluronic L61, F127 | 30 | 8.2 | I, II, III | [69] |
| DTXL-TNP | Doxorubicin | Targeting | PLA-PEG, PLA-PEG-ACUPA | 100 | 10 | I | [129] |
| NC-6004 | Cisplatin | Targeting | PEG-P(Glu)-Cisplatin | 30 | 39 | I, II | [130] |
| NC-4016 | DACH-platin | Targeting | PEG-P(Glu)-DACH-platin | 20–100 | 25 | I | [131] |
| NK 012 | SN-38 | Targeting | PEG-P(Glu)-SN38 | 20 | 20.0 | II | [132] |
| NK911 | Doxorubicin | Targeting | PEG-(Asp)-Dox | 40 | n. a | II | [133] |
| Patent Type | Title | Patent No. | Chemical Formula | Action | Year | Inventor/Assignee |
|---|---|---|---|---|---|---|
| Micelles | C6-c18-acylated derivative of hyaluronic acid | WO2014082609 A1 | (HA)-[0(C:=O)NH-M]p | AC | 2014 | Contipro Biotech S.R.O. |
| Micelles | Polymer conjugated protein micelles | EP 2678001 A2 | PEG-Prolamine | AC | 2014 | South Dakota State University |
| Paclitaxel Micelle (NK105) | Micellar preparation containing sparingly water-soluble anticancer agent and novel block copolymer | 09705599.0 | (poly(ethylene glycol)-copoly (L-aspartic acid) | AC | 2013 | Nanocarrier Co. Ltd. Nippon Kayaku Co., Ltd. |
| Nanoplatin® (NC-6004) | Pharmaceutical composition and combined agent | 098101554 | (poly(ethylene glycol)-copoly (amino acid) | AC | 2013 | TOUDAI TLO Ltd. |
| DACH-Platin Micelle (NC-4016) | Coordination compound composed of diaminocyclohexane platinum (Ii) and block copolymer | 2007-520209 | (poly(ethylene glycol)-copoly (amino acid) | AC | 2013 | The University of Tokyo |
| Protein Micelle | Electrostatic bonding type macromolecular micelle drug carrier | EP2583563 A1 | polyethylene glycol and poly(α,-β -aspartic acid) | AC | 2013 | TOUDAI TLO Ltd. |
| siRNA Micelle | Polyethylene glycol/polycation block copolymer | EP2087912 A1 | PEG-PLys | AC | 2013 | The University of Tokyo |
| Sensor Linked Micelle | Active targeting polymer micelle encapsulating drug, and pharmaceutical composition | 2008-539901 | poly(ethylene glycol)-b-poly(2-aminoethyl methacrylate)-b-poly(styrene) | AC | 2013 | Nanocarrier Co. Ltd. |
| pH-Sensitive Micelle | Novel block copolymer used for preparing pH-responsive polymer micelles | 2009-7007877 | [PEG-p(Asp-Hyd-Adr)] | AC | 2013 | The University of Tokyo |
| Docetaxel Micelle | Docetaxel polymer derivative, method for producing same and use of same | 2009250393 | (mPEG-PDLLA) | AC | 2013 | Nanocarrier Co. Ltd. |
| Bortezomib Micelle | Pharmaceutical composition that includes block copolymer containing boronic acid compound | EP 2692777 A1 | polyethylene glycol-polyglutamic acid | AC | 2013 | Nanocarrier Co. Ltd. |
| Micelles | Micelles for the solubilisation of gossypol | 20120321715 | Poloxamer or PEG-PCL | AC | 2012 | Wisconsin Alumni Research Foundation., US |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pantshwa, J.M.; Kondiah, P.P.D.; Choonara, Y.E.; Marimuthu, T.; Pillay, V. Nanodrug Delivery Systems for the Treatment of Ovarian Cancer. Cancers 2020, 12, 213. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers12010213
Pantshwa JM, Kondiah PPD, Choonara YE, Marimuthu T, Pillay V. Nanodrug Delivery Systems for the Treatment of Ovarian Cancer. Cancers. 2020; 12(1):213. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers12010213
Chicago/Turabian StylePantshwa, Jonathan M., Pierre P. D. Kondiah, Yahya E. Choonara, Thashree Marimuthu, and Viness Pillay. 2020. "Nanodrug Delivery Systems for the Treatment of Ovarian Cancer" Cancers 12, no. 1: 213. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers12010213