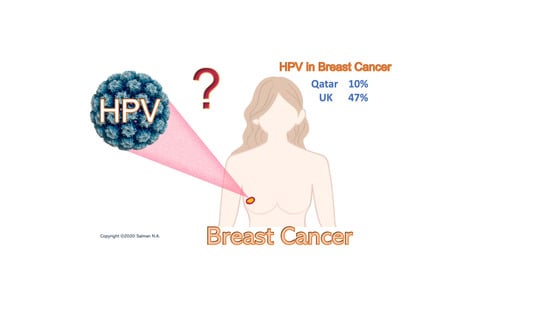
Prevalence and Type Distribution of High-Risk Human Papillomavirus (HPV) in Breast Cancer: A Qatar Based Study
Abstract
:1. Introduction
2. Results
2.1. Histopathological Diagnosis
2.2. PCR Results
2.3. Prevalence and Type Distribution of HR-HPV
3. Discussion
4. Materials and Methods
4.1. Subjects, Enrolment and Breast Tissue Collection
4.2. DNA extraction and Purification
4.3. The Detection and Genotyping of HR-HPV
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qatar National Cancer Registry, Ministry of Public Health, Qatar Cancer Incidence Report, 2015. Doha: 2017. Most Common Cancers, Breast Cancer. pp. 22–23. Available online: https://www.moph.gov.qa/Admin/Lists/PublicationsAttachments/Attachments/53/QNCR-2015-English.pdf (accessed on 9 May 2020).
- Ganguly, N.; Parihar, S.P. Human papillomavirus E6 and E7 oncoproteins as risk factors for tumorigenesis. J. Biosci. 2009, 34, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Bryan, J.T.; Brown, D.R. Transmission of human papillomavirus type 11 infection by desquamated cornified cells. Virology 2001, 281, 35–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baseman, J.G.; Koutsky, L.A. The epidemiology of human papillomavirus infections. J. Clin. Virol. 2005, 32 (Suppl. S1), S16–S24. [Google Scholar] [CrossRef] [PubMed]
- Zur Hausen, H. Papillomaviruses in the causation of human cancers-a brief historical account. Virology 2009, 384, 260–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Cancer Institute (NCI) (2020) HPV and Cancer. Available online: https://www.cancer.gov/about-cancer/causes-prevention/risk/infectious-agents/hpv-and-cancer (accessed on 9 May 2020).
- Ashrafi, G.H.; Salman, N.A. Pathogenesis of Human Papillomavirus–Immunological Responses to HPV Infection. In Human Papillomavirus-Research in a Global Perspective; Rajkumar, R., Ed.; InTech: Rijeka, Croatia, 2016; pp. 243–253. ISBN 978-953-51-2439-9. [Google Scholar]
- Heng, B.; Glenn, W.K.; Ye, Y.; Tran, B.; Delprado, W.; Lutze-Mann, L.; Whitaker, N.J.; Lawson, J.S. Human papilloma virus is associated with breast cancer. Br. J. Cancer 2009, 101, 1345–1350. [Google Scholar] [CrossRef] [PubMed]
- Lawson, J.S.; Glenn, W.K.; Salyakina, D.; Clay, R.; Delprado, W.; Cheerala, B.; Tran, D.D.; Ngan, C.C.; Miyauchi, S.; Karim, M.; et al. Human Papilloma Virus Identification in Breast Cancer Patients with Previous Cervical Neoplasia. Front. Oncol. 2015, 5, 298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allred, D.C.; Mohsin, S.K.; Fuqua, S.A. Histological and biological evolution of human premalignant breast disease. Endocr. Relat. Cancer 2001, 8, 47–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polyak, K. Breast cancer: Origins and evolution. J. Clin. Investig. 2007, 117, 3155–3163. [Google Scholar] [CrossRef] [PubMed]
- De Villiers, E.M.; Sandstrom, R.E.; zur Hausen, H.; Buck, C.E. Presence of papillomavirus sequences in condylomatous lesions of the mamillae and in invasive carcinoma of the breast. Breast Cancer Res. 2005, 7, R1–R11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salman, N.A.; Davies, G.; Majidy, F.; Shakir, F.; Akinrinade, H.; Perumal, D.; Ashrafi, G.H. Association of High Risk Human Papillomavirus and Breast cancer: A UK based Study. Sci. Rep. 2017, 7, 43591. [Google Scholar] [CrossRef] [PubMed]
- Antonsson, A.; Spurr, T.P.; Chen, A.C.; Francis, G.D.; McMillan, N.A.J.; Saunders, N.A.; Law, M.; Bennett, I.C. High prevalence of human papillomaviruses in fresh frozen breast cancer samples. J. Med. Virol. 2011, 83, 2157–2163. [Google Scholar] [CrossRef] [PubMed]
- Divani, S.N.; Giovani, A.M. Detection of human papillomavirus DNA in fine needle aspirates of women with breast cancer. Arch. Oncol. 2012, 20, 12–14. [Google Scholar] [CrossRef]
- Delgado-García, S.; Martínez-Escoriza, J.-C.; Alba, A.; Martín-Bayón, T.-A.; Ballester-Galiana, H.; Peiró, G.; Caballero, P.; Ponce-Lorenzo, J. Presence of human papillomavirus DNA in breast cancer: A Spanish case-control study. BMC Cancer 2017, 17, 320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawson, J.S.; Glenn, W.K.; Heng, B.; Ye, Y.; Tran, B.; Lutze-Mann, L.; Whitaker, N.J. Koilocytes indicate a role for human papilloma virus in breast cancer. Br. J. Cancer 2009, 101, 1351–1356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, N.A.; Castillo, A.; Koriyama, C.; Kijima, Y.; Umekita, Y.; Ohi, Y.; Higashi, M.; Sagara, Y.; Yoshinaka, H.; Tsuji, T.; et al. Human papillomavirus detected in female breast carcinomas in Japan. Br. J. Cancer 2008, 99, 408–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, S.; Dasgupta, H.; Roychowdhury, A.; Bhattacharya, R.; Mukherjee, N.; Roy, A.; Mandal, G.K.; Alam, N.; Biswas, J.; Mandal, S.; et al. Study of association and molecular analysis of human papillomavirus in breast cancer of Indian patients: Clinical and prognostic implication. PLoS ONE 2017, 12, e0172760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fröberg, M.; Östensson, E.; Belkić, K.; Oštrbenk, A.; Poljak, M.; Mints, M.; Arbyn, M.; Andersson, S. Impact of the human papillomavirus status on the development of high-grade cervical intraepithelial neoplasia in women negative for intraepithelial lesions or malignancy at the baseline: A 9-year Swedish nested case-control follow-up study. Cancer 2019, 125, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Malhone, C.; Longatto-Filho, A.; Filassi, J.R. Is Human Papilloma Virus Associated with Breast Cancer? A Review of the Molecular Evidence. Acta Cytol. 2018, 62, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Bansal, D.; Elmi, A.A.; Skariah, S.; Haddad, P.; Abu-Raddad, L.J.; Al Hamadi, A.H.; Mohamed-Nady, N.; Affifi, N.M.; Ghedira, R.; Hassen, E.; et al. Molecular epidemiology and genotype distribution of Human Papillomavirus (HPV) among Arab women in the state of Qatar. J. Transl. Med. 2014, 12, 300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Thani, A.A.; Abu-Rub, A.I.; Al-Ansari, A.; Abushama, M.; Al-Khanji, M.; Al-Lawati, S. Prevalence of human papillomavirus infection in women attending a gynecology/oncology clinic in Qatar. Future Virol. 2010, 5, 513–519. [Google Scholar] [CrossRef]
- Lindel, K.; Forster, A.; Altermatt, H.J.; Greiner, R.; Gruber, G. Breast cancer and human papillomavirus (HPV) infection: No evidence of a viral etiology in a group of Swiss women. Breast 2007, 16, 172–177. [Google Scholar] [CrossRef] [PubMed]
- De Cremoux, P.; Thioux, M.; Lebigot, I.; Sigal-Zafrani, B.; Salmon, R.; Sastre-Garau, X. No evidence of human papillomavirus DNA sequences in invasive breast carcinoma. Breast Cancer Res. Treat. 2008, 109, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Ahangar-Oskouee, M.; Shahmahmoodi, S.; Jalilvand, S.; Mahmoodi, M.; Ziaee, A.A.; Esmaeili, H.A.; Keshtvarz, M.; Pishraft-Sabet, L.; Yousefi, M.; Mollaei-Kandelous, Y.; et al. No Detection of “High-risk” Human Papillomaviruses in a Group of Iranian Women with Breast Cancer. Asian Pac. J. Cancer Prev. 2014, 15, 4061–4065. [Google Scholar] [CrossRef] [PubMed]
- Motoyama, S.; Ladines-Llave, C.A.; Luis Villanueva, S.; Maruo, T. The role of human papilloma virus in the molecular biology of cervical carcinogenesis. Kobe J. Med. Sci. 2004, 50, 9–19. [Google Scholar] [PubMed]
- Muñoz, N.; Bosch, F.X.; de Sanjosé, S.; Herrero, R.; Castellsagué, X.; Shah, K.V.; Snijders, P.J.; Meijer, C.J. Epidemiologic Classification of Human Papillomavirus Types Associated with Cervical Cancer. N. Engl. J. Med. 2003, 348, 518–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Sanjosé, S.; Diaz, M.; Castellsagué, X.; Clifford, G.; Bruni, L.; Muñoz, N.; Bosch, F.X. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: A meta-analysis. Lancet Infect. Dis. 2007, 7, 453–459. [Google Scholar] [CrossRef]
- Wang, R.; Guo, X.L.; Wisman, G.B.; Schuuring, E.; Wang, W.F.; Zeng, Z.Y.; Zhu, H.; Wu, S.W. Nationwide prevalence of human papillomavirus infection and viral genotype distribution in 37 cities in China. BMC Infect. Dis. 2015, 15, 257. [Google Scholar] [CrossRef] [Green Version]


| Total Samples N (%) | HPV + ve N (%) | |||
|---|---|---|---|---|
| Total Number of Samples N | 150 (100) | 13/150 (8.7) | ||
| Male Female | 10 140 | |||
| Age (Year) (15–84) | ||||
| <50 | 116/150 (77) | |||
| >50 | 34/150 (23) | |||
| Pathological Status | Single HPV Infection N (%) | HPV Co-Infection N (%) | ||
| Cancerous Cases | 50/50 (100) | 5/50 (10) | 3/50 (6) | 2/50 (4) |
| In Situ Cases | ||||
| Ductal Carcinoma in Situ (DCIS) | 15/50 (30) | 1/15 (6.6) | - | 1/2 (50) |
| Lobular Carcinoma in Situ (LCIS) | 0/50 (0) | - | - | - |
| Invasive Cases | ||||
| Invasive Ductal Carcinoma (IDC) | 33/50 (66) | 4/33 (12.12) | 3/3 (100) | 1/2 (50) |
| Invasive Lobular Carcinoma (ILC) | 1/50 (2) | - | - | - |
| Invasive and In Situ Cases | ||||
| Invasive and In Situ Ductal Carcinoma | 0/50 (0) | - | - | - |
| Invasive and In Situ Lobular Carcinoma | 1/50 (2) | - | - | - |
| Non- Cancerous Cases | 100/100 (100) | 8/100 (8) | 6/100 (6) | 2/100 (2) |
| Benign Fibroadenoma | 23/100 (23) | 3/23 (13) | 3/6 (50) | - |
| Benign Phyllodes Tumor | 4/100 (4) | 1/4 (25) | 1/6 (16.7) | - |
| Benign Breast Tissue | 13/100 (13) | - | - | - |
| Gynecomastia | 5/100 (5) | - | - | - |
| Papillomatosis | 5/100 (5) | - | - | - |
| Normal | 50/100 (50) | 4/50 (8) | 2/6 (33.3) | 2/2 (100) |
| ID | Pap Smear Status | HPV Status | Breast Histopathology Status | Nationality |
|---|---|---|---|---|
| 18 | 0 | HPV 58 | Benign/Fibroadenoma | Indian |
| 19 | 0 | HPV 16 and 58 | Malignant/IDC | British |
| 63 | 0 | HPV 35 | Malignant/IDC | Afghani |
| 65 | 0 | HPV 59 | Benign/fibrous histiocytoma | Filipino |
| 67 | 0 | HPV 35 | Benign/phyllodes tumor | Filipino |
| 104 | 0 | HPV 35 | Malignant/IDC | Syrian |
| 107 | 0 | HPV 16 and 35 | Malignant/DCIS | Filipino |
| 110 | 0 | HPV 16 | Malignant/IDC | Yemeni |
| 113 | 0 | HPV 35 | Benign/ Fibroadenoma | Ethiopian |
| 129 | 0 | HPV 33 and 35 | Normal | Filipino |
| 130 | 0 | HPV 39 | Normal | Qatari |
| 139 | * | HPV 52 | Normal | Lebanese |
| 145 | 0 | HPV 16, 35, 18, 52 | Normal | Filipino |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sher, G.; Salman, N.A.; Kulinski, M.; Fadel, R.A.; Gupta, V.K.; Anand, A.; Gehani, S.; Abayazeed, S.; Al-Yahri, O.; Shahid, F.; et al. Prevalence and Type Distribution of High-Risk Human Papillomavirus (HPV) in Breast Cancer: A Qatar Based Study. Cancers 2020, 12, 1528. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers12061528
Sher G, Salman NA, Kulinski M, Fadel RA, Gupta VK, Anand A, Gehani S, Abayazeed S, Al-Yahri O, Shahid F, et al. Prevalence and Type Distribution of High-Risk Human Papillomavirus (HPV) in Breast Cancer: A Qatar Based Study. Cancers. 2020; 12(6):1528. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers12061528
Chicago/Turabian StyleSher, Gulab, Nadia Aziz Salman, Michal Kulinski, Rayyan Abdulaziz Fadel, Vinod Kumar Gupta, Ambika Anand, Salahddin Gehani, Sheraz Abayazeed, Omer Al-Yahri, Fakhar Shahid, and et al. 2020. "Prevalence and Type Distribution of High-Risk Human Papillomavirus (HPV) in Breast Cancer: A Qatar Based Study" Cancers 12, no. 6: 1528. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers12061528