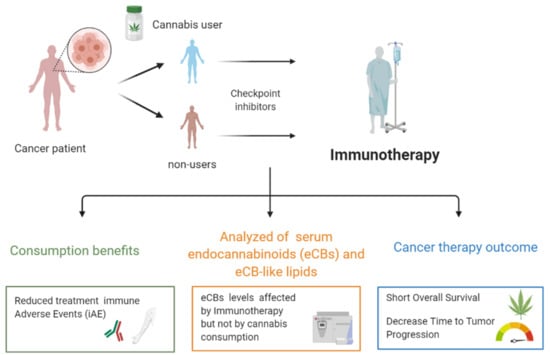
Cannabis Consumption Used by Cancer Patients during Immunotherapy Correlates with Poor Clinical Outcome
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
2.2. Overall Response
2.3. TTP and O.S.
2.4. Adverse Events
2.5. eCB and eCB-Like Levels
3. Discussion
4. Methods
4.1. Patients
4.2. Assessments and Analyses
4.3. Safety
4.4. Statistical Analysis
4.5. Measurement of eCB Serum Levels
5. Conclusions and Study Limitations
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| eCBS | Endocannabinoid system |
| eCBs | Endocannabinoids |
| GPCR | G-protein-coupled receptors |
| CB1 and CB2, respectively | Cannabinoid receptors type 1 and 2 |
| anandamide, AEA | N-arachidonoylethanolamide |
| 2-AG | 2-arachidonoylglycerol |
| ICIs | Immune Checkpoint Inhibitors |
| CTLA4 | Cytotoxic T-lymphocyte-associated protein 4 |
| PD-1 | Programmed cell death protein 1 |
| PD-L1 | Programmed Death-ligand 1 |
| NSCLC | Non-Small Cell Lung Cancer |
| I-G | Immunotherapy Group |
| IC-G | Immunotherapy-Cannabis Group |
| CR | Complete response |
| PR | Partial response |
| SD | Stable disease |
| DEA | Drug Enforcement Administration |
| TTP | Time to tumor progression |
| OS | Overall Survival |
| AEs | Adverse events |
| CTLs | Cytotoxic T Lymphocytes |
References
- Mead, A. Legal and regulatory issues governing cannabis and cannabis-derived products in the United States. Front. Plant Sci. 2019, 10, e697. [Google Scholar] [CrossRef]
- Abrams, D.; Guzman, M. Cannabis in cancer care. Clin. Pharmacol. Ther. 2015, 97, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Bar-Sela, G.; Avisar, A.; Batash, R.; Schaffer, M. Is the Clinical Use of Cannabis by Oncology Patients Advisable? Curr. Med. Chem. 2014, 21, 1923–1930. [Google Scholar] [CrossRef] [PubMed]
- Dzierżanowski, T. Prospects for the Use of Cannabinoids in Oncology and Palliative Care Practice: A Review of the Evidence. Cancers 2019, 11, 129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bar-Sela, G.; Vorobeichik, M.; Drawsheh, S.; Omer, A.; Goldberg, V.; Muller, E. The Medical Necessity for Medicinal Cannabis: Prospective, Observational Study Evaluating the Treatment in Cancer Patients on Supportive or Palliative Care. Evid. Based Complement Altern. Med. 2013, 2013, 1–8. [Google Scholar] [CrossRef]
- Abrams, D.I. Integrating cannabis into clinical cancer care. Curr. Oncol. 2016, 23, S8–S14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagarkatti, P.; Pandey, R.; Rieder, S.A.; Hegde, V.L.; Nagarkatti, M. Cannabinoids as novel anti-inflammatory drugs. Future Med. Chem. 2009, 1, 1333–1349. [Google Scholar] [CrossRef] [Green Version]
- Gallily, R.; Yekhtin, Z.; Hanuš, L.O. The Anti-Inflammatory Properties of Terpenoids from Cannabis. Cannabis Cannabinoid Res. 2018, 3, 282–290. [Google Scholar] [CrossRef] [Green Version]
- Di Marzo, V.; Melck, D.; Bisogno, T.; De Petrocellis, L. Endocannabinoids: Endogenous cannabinoid receptor ligands with neuromodulatory action. Trends Neurosci. 1998, 21, 521–528. [Google Scholar] [CrossRef]
- Di Marzo, V.; Piscitelli, F. The Endocannabinoid System and its Modulation by Phytocannabinoids. Neurotherapeutics 2015, 12, 692–698. [Google Scholar] [CrossRef]
- Pertwee, R.G.; Howlett, A.C.; Abood, M.E.; Alexander, S.P.H.; Di Marzo, V.; Elphick, M.R.; Greasley, P.J.; Hansen, H.S.; Kunos, G.; Mackie, K.; et al. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: Beyond CB1 and CB2. Pharmacol. Rev. 2010, 62, 588–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leishman, E.; Bradshaw, H.B. N-Acyl Amides: Ubiquitous Endogenous Cannabimimetic Lipids That Are in the Right Place at the Right Time. Endocannabinoidome World Endocannabinoids Relat. Mediat. 2015, 33–48. [Google Scholar] [CrossRef]
- Aizpurua-Olaizola, O.; Elezgarai, I.; Rico-Barrio, I.; Zarandona, I.; Etxebarria, N.; Usobiaga, A. Targeting the endocannabinoid system: Future therapeutic strategies. Drug Discov. Today 2017, 22, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.; Mousawy, K.; Nagarkatti, M.; Nagarkatti, P. Endocannabinoids and immune regulation. Pharmacol. Res. 2009, 60, 85–92. [Google Scholar] [CrossRef] [Green Version]
- Schwarz, H.; Blanco, F.J.; Lotz, M. Anandamide, an endogenous cannabinoid receptor agonist inhibits lymphocyte proliferation and induces apoptosis. J. Neuroimmunol. 1994, 55, 107–115. [Google Scholar] [CrossRef]
- Chiurchiù, V.; Leuti, A.; Smoum, R.; Mechoulam, R.; Maccarrone, M. Bioactive lipids ALIAmides differentially modulate inflammatory responses of distinct subsets of primary human T lymphocytes. FASEB J. 2018, 32, 5716–5723. [Google Scholar] [CrossRef] [PubMed]
- Ligresti, A.; De Petrocellis, L.; Di Marzo, V. From phytocannabinoids to cannabinoid receptors and endocannabinoids: Pleiotropic physiological and pathological roles through complex pharmacology. Physiol. Rev. 2016, 96, 1593–1659. [Google Scholar] [CrossRef] [Green Version]
- Bisogno, T.; Hanuš, L.; De Petrocellis, L.; Tchilibon, S.; Ponde, D.E.; Brandi, I.; Moriello, A.S.; Davis, J.B.; Mechoulam, R.; Di Marzo, V. Molecular targets for cannabidiol and its synthetic analogues: Effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br. J. Pharmacol. 2001, 134, 845–852. [Google Scholar] [CrossRef]
- De Petrocellis, L.; Ligresti, A.; Moriello, A.S.; Allarà, M.; Bisogno, T.; Petrosino, S.; Stott, C.G.; Di Marzo, V. Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br. J. Pharmacol. 2011, 163, 1479–1494. [Google Scholar] [CrossRef] [Green Version]
- Morgan, C.J.A.; Page, E.; Schaefer, C.; Chatten, K.; Manocha, A.; Gulati, S.; Curran, H.V.; Brandner, B.; Leweke, F.M. Cerebrospinal fluid anandamide levels, cannabis use, and psychotic-like symptoms. Br. J. Psychiatry 2013, 202, 381–382. [Google Scholar] [CrossRef] [Green Version]
- Leweke, F.M.; Piomelli, D.; Pahlisch, F.; Muhl, D.; Gerth, C.W.; Hoyer, C.; Klosterkötter, J.; Hellmich, M.; Koethe, D. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl. Psychiatry 2012, 2, e94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berman, P.; Sulimani, L.; Gelfand, A.; Amsalem, K.; Lewitus, G.M.; Meiri, D. Cannabinoidomics–An analytical approach to understand the effect of medical Cannabis treatment on the endocannabinoid metabolome. Talanta 2020, 219, e121336. [Google Scholar] [CrossRef]
- Jain, P.; Jain, C.; Velcheti, V. Role of immune-checkpoint inhibitors in lung cancer. Ther. Adv. Respir. Dis. 2018, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahoney, K.M.; Freeman, G.J.; McDermott, D.F. The Next Immune-Checkpoint Inhibitors: PD-1/PD-L1 Blockade in Melanoma. Clin. Ther. 2015, 37, 764–782. [Google Scholar] [CrossRef] [Green Version]
- Ross, K.; Jones, R.J. Immune checkpoint inhibitors in renal cell carcinoma. Clin. Sci. (Lond) 2017, 131, 2627–2642. [Google Scholar] [CrossRef] [Green Version]
- Postow, M.A.; Callahan, M.K.; Wolchok, J.D. Immune Checkpoint Blockade in Cancer Therapy. J. Clin. Oncol. 2015, 33, 1974–1982. [Google Scholar] [CrossRef] [Green Version]
- Wei, S.C.; Duffy, C.R.; Allison, J.P. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov. 2018, 8, 1069–1086. [Google Scholar] [CrossRef] [Green Version]
- Taha, T.; Meiri, D.; Talhamy, S.; Wollner, M.; Peer, A.; Bar-Sela, G. Cannabis Impacts Tumor Response Rate to Nivolumab in Patients with Advanced Malignancies. Oncologist 2019, 24, 549–554. [Google Scholar] [CrossRef] [Green Version]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Sosman, J.A.; Atkins, M.B.; Leming, P.D.; et al. Five-Year Survival and Correlates among Patients with Advanced Melanoma, Renal Cell Carcinoma, or Non-Small Cell Lung Cancer Treated with Nivolumab. JAMA Oncol. 2019, 5, 1411–1420. [Google Scholar] [CrossRef] [Green Version]
- El-Gohary, M.; Eid, M.A. Effect of cannabinoid ingestion (in the form of bhang) on the immune system of high school and university students. Hum. Exp. Toxicol. 2004, 23, 149–156. [Google Scholar] [CrossRef]
- Eisenstein, T.K.; Meissler, J.J. Effects of Cannabinoids on T-cell Function and Resistance to Infection. J. Neuroimmune Pharmacol. 2015, 10, 204–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oláh, A.; Szekanecz, Z.; Bíró, T. Targeting cannabinoid signaling in the immune system: “High”-ly exciting questions, possibilities, and challenges. Front. Immunol. 2017, 8, e1487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Croxford, J.L.; Yamamura, T. Cannabinoids, and the immune system: Potential for the treatment of inflammatory diseases? J. Neuroimmunol. 2005, 166, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Manuzak, J.A.; Gott, T.M.; Kirkwood, J.S.; Coronado, E.; Hensley-Mcbain, T.; Miller, C.; Cheu, R.K.; Collier, A.C.; Funderburg, N.T.; Martin, J.N.; et al. Heavy Cannabis Use Associated with Reduction in Activated and Inflammatory Immune Cell Frequencies in Antiretroviral Therapy-Treated Human Immunodeficiency Virus-Infected Individuals. Clin. Infect. Dis. 2018, 66, 1872–1882. [Google Scholar] [CrossRef] [Green Version]
- Keen, L.; Abbate, A.; Blanden, G.; Priddie, C.; Moeller, F.G.; Rathore, M. Confirmed marijuana use and lymphocyte count in black people living with HIV. Drug Alcohol Depend. 2019, 198, 112–115. [Google Scholar] [CrossRef]
- Yuan, M.; Kiertscher, S.M.; Cheng, Q.; Zoumalan, R.; Tashkin, D.P.; Roth, M.D. Delta 9-Tetrahydrocannabinol regulates Th1/Th2 cytokine balance in activated human T cells. J. Neuroimmunol. 2002, 133, 124–131. [Google Scholar] [CrossRef]
- Börner, C.; Smida, M.; Höllt, V.; Schraven, B.; Kraus, J. Cannabinoid receptor type 1- and 2-mediated increase in cyclic AMP inhibits T cell receptor-triggered signaling. J. Biol. Chem. 2009, 284, 35450–35460. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.D.; Patel, M.K.; Wiederhold, M.D.; Ou, D.W. Effects of cannabinoids and cocaine on the mitogen-induced transformations of lymphocytes of human and mouse origins. Int. J. Immunopharmacol. 1992, 14, 49–56. [Google Scholar] [CrossRef]
- Klein, T.W.; Newton, C.A.; Widen, R.; Friedman, H. The effect of delta-9-tetrahydrocannabinol and 11-hydroxy-delta-9-tetrahydrocannabinol on t-lymphocyte and b-lymphocyte mitogen responses. Immunopharmacol. Immunotoxicol. 1985, 7, 451–466. [Google Scholar] [CrossRef]
- Klein, T.W.; Kawakami, Y.; Newton, C.; Friedman, H. Marijuana components suppress induction and cytolytic function of murine cytotoxic T cells in vitro and in vivo. J. Toxicol. Environ. Health 1991, 32, 465–477. [Google Scholar] [CrossRef]
- Blank, C.U.; Haining, W.N.; Held, W.; Hogan, P.G.; Kallies, A.; Lugli, E.; Lynn, R.C.; Philip, M.; Rao, A.; Restifo, N.P.; et al. Defining ‘T cell exhaustion’. Nat. Rev. Immunol. 2019, 19, 665–674. [Google Scholar] [CrossRef]
- McLane, L.M.; Abdel-Hakeem, M.S.; Wherry, E.J. CD8 T Cell Exhaustion During Chronic Viral Infection and Cancer. Annu. Rev. Immunol. 2019, 37, 457–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. Available online: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm (accessed on 20 June 2020).
- Zoerner, A.A.; Gutzki, F.M.; Batkai, S.; May, M.; Rakers, C.; Engeli, S.; Jordan, J.; Tsikas, D. Quantification of endocannabinoids in biological systems by chromatography and mass spectrometry: A comprehensive review from an analytical and biological perspective. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2011, 1811, 706–723. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Morena, M.; Vecchiarelli, H.A.; Hill, M.N.; Schriemer, D.C. A robust capillary liquid chromatography/tandem mass spectrometry method for quantitation of neuromodulatory endocannabinoids. Rapid Commun. Mass Spectrom. 2015, 29, 1889–1897. [Google Scholar] [CrossRef] [PubMed]




| Characteristics | Cannabis Non-Users N = 68 | Cannabis Users N = 34 | p-Value |
|---|---|---|---|
| Age in years median (range) | 69 (18–92) | 66 (37–85) | |
| Gender—N (%) | |||
| Female | 22 (32.4) | 10 (29.5) | |
| Male | 46 (67.6) | 24 (70.5) | 0.9399 |
| Performance Status Eastern Cooperative Oncology Group (ECOG)—N (%) | |||
| 1≤ | 55 (80.8) | 24 (70.5) | 0.3568 |
| ≥2 | 13 (19.1) | 10 (29.4) | 0.3568 |
| Chronic diseases per patient—N (%) | |||
| 0 | 22 (32.3) | 13 (22.0) | 0.7124 |
| 1 | 16 (23.5) | 7 (20.5) | 0.9332 |
| 2 or more | 30 (44.1) | 14 (41.1) | 0.9437 |
| Background diseases—N (%) | |||
| Chronic heart disease | 18 (26.4) | 5 (14.7) | 0.2762 |
| Diabetes | 17 (25.0) | 6 (17.6) | 0.5576 |
| High blood pressure | 34 (50.0) | 13 (34.1) | 0.3612 |
| Chronic obstructive pulmonary disease (COPD) | 9 (13.2) | 3 (8.8) | 1 |
| Hyperlipidemia | 23 (33.8) | 7 (20.5) | 0.2491 |
| Other | 2 (2.9) | 0 (0.0) | 1 |
| Type of malignancy—N (%) | |||
| Non-small cell lung cancer | 37 (54.4) | 20 (58.8) | 0.8325 |
| Melanoma | 25 (36.7) | 9 (26.4) | 0.414 |
| Renal cell carcinoma | 4 (5.8) | 2 (5.8) | 1 |
| Other | 2 (2.9) | 3 (8.8) | 1 |
| Main site of metastasis—N (%) | |||
| Brain | 12 (17.6) | 8 (13.2) | 0.6593 |
| Lungs | 39 (57.3) | 23 (67.6) | 0.4303 |
| Liver | 13 (19.1) | 11 (32.3) | 0.2157 |
| Immunotherapy given as—N (%) | |||
| First line of treatment | 31 (45.5) | 8 (23.5) | 0.05178 |
| Second line of treatment or more | 37 (54.4) | 26 (76.4) | 0.05178 |
| Checkpoint therapy—N (%) | |||
| Anti PD1: Pembrolizumab or Nivolumab | 47 (69.1) | 29 (85.2) | 0.127 |
| Ipilimumab and Nivolumab | 16 (23.5) | 4 (11.7) | 0.2517 |
| Anti PDL-1: Durvalumab or Atezolizumab | 5 (7.3) | 1 (2.9) | 1 |
| Test | Cannabis: Nonusers n = 68 | Cannabis: Users n = 34 | p-Value |
|---|---|---|---|
| Lymphocytes ≤ 1.5 K/uL—N (%) | 35 (51) | 23 (67) | 0.08 |
| Blood count WBC ≤ 4.5 K/uL—N (%) | 7 (10) | 2 (6) | - |
| Liver Function | |||
| Alanine Aminotransferase (ALT) > 45 | 7 (10) | 3 (9) | - |
| Aspartate Aminotrasferase (AST) > 35 | 8 (12) | 5 (15) | - |
| Alkaline phosphatase level (ALKP) > 120 | 13 (19) | 11 (32) | 0.09 |
| Renal Function—N (%) | |||
| Creatinine > 1.17 mL/min | 12 (17) | 3 (9) | - |
| Side Effects | Cannabis: Nonusers n = 68 | Cannabis: Users n = 34 | p-Value |
|---|---|---|---|
| Any iAE, grade ≥ 2—N (%) | 28 (39) | 7 (21) | 0.057 |
| Skin toxicity—N (%) | 9 (13) | 2 (6) | - |
| Colitis—N (%) | 6 (9) | 0 | - |
| Thyroid disorders—N (%) | 6 (9) | 2 (6) | - |
| Other—N (%) | 3 (4) | 0 | - |
| Arthritis—N (%) | 1 (1.5) | 0 | - |
| Panuveitis—N (%) | 1 (1.5) | 0 | - |
| Hepatitis—N (%) | 1 (1.5) | 1 (3) | - |
| General Deterioration—N (%) | 0 | 1 (3) | - |
| Edema—N (%) | 0 | 1 (3) | - |
| Analyte (ng/mL) | before Immunotherapy Cannabis Nonusers | before Immunotherapy Cannabis Users | after Immunotherapy Cannabis Nonusers | after Immunotherapy Cannabis Users | SMD |
|---|---|---|---|---|---|
| n = 8 | n = 6 | n = 8 | n = 6 | ||
| Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | ||
| LnA | 432.52 (211.53, 522.96) | 240.99 (228.69, 298.71) | 184.35 (155.67, 365.81) | 252.72 (118.91, 312.50) | 0.443 |
| AA | 685.94 (449.35, 831.05) | 627.04 (563.61, 700.79) | 457.61 (412.89, 657.92) | 564.63 (549.41, 583.55) | 0.315 |
| EPEA | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.03) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | - |
| LnEA | 0.00 (0.00, 0.03) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.01) | 0.01 (0.00, 0.03) | 0.351 |
| DHEA | 0.34 (0.29, 0.40) | 0.33 (0.28, 0.37) | 0.29 (0.27, 0.34) | 0.25 (0.22, 0.29) | 0.41 |
| AEA | 0.31 (0.26, 0.41) | 0.35 (0.29, 0.43) | 0.32 (0.25, 0.35) | 0.25 (0.20, 0.34) | 0.559 |
| LEA | 0.36 (0.35, 0.48) | 0.41 (0.33, 0.45) | 0.36 (0.31, 0.39) | 0.28 (0.24, 0.33) | 0.745 |
| PEA | 0.17 (0.11, 0.21) | 0.08 (0.04, 0.21) | 0.11 (0.06, 0.21) | 0.04 (0.01, 0.18) | 0.45 |
| OEA | 0.35 (0.27, 0.38) | 0.35 (0.31, 0.46) | 0.31 (0.26, 0.39) | 0.25 (0.19, 0.30) | 0.412 |
| SEA | 0.13 (0.07, 0.24) | 0.10 (0.03, 0.23) | 0.10 (0.05, 0.17) | 0.11 (0.02, 0.24) | 0.335 |
| DtEA | 0.01 (0.01, 0.02) | 0.02 (0.02, 0.03) | 0.01 (0.00, 0.02) | 0.02 (0.01, 0.03) | 0.582 |
| 2-AG | 3.27 (1.55, 5.12) | 4.02 (2.80, 5.84) | 2.18 (1.78, 3.78) | 3.06 (2.70, 4.58) | 0.472 |
| 2-LG | 48.74 (33.36, 104.28) | 44.91 (33.91, 71.57) | 50.95 (35.25, 73.29) | 73.83 (38.35, 100.75) | 0.352 |
| 2-OG | 71.84 (36.88, 134.98) | 52.58 (38.89, 93.19) | 71.59 (52.16, 95.28) | 77.85 (57.94, 84.19) | 0.338 |
| A-Am | 0.02 (0.02, 0.03) | 0.02 (0.01, 0.02) | 0.02 (0.01, 0.03) | 0.01 (0.00, 0.01) | 0.607 |
| L-Am | 4.65 (1.81, 15.28) | 44.44 (15.32, 55.42) | 52.71 (10.04, 63.00) | 30.45 (9.10, 43.01) | 0.625 |
| O-Am | 12.85 (7.11, 15.89) | 6.68 (3.19, 19.69) | 35.98 (19.39, 58.34) | 13.85 (6.39, 23.43) | 0.384 |
| 2-AGe | 0.49 (0.46, 0.51) | 0.41 (0.40, 0.45) | 0.45 (0.41, 0.48) | 0.44 (0.39, 0.50) | 0.478 |
| O-AEA | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | - |
| A-serine | 0.22 (0.00, 0.23) | 0.22 (0.22, 0.23) | 0.23 (0.22, 0.23) | 0.22 (0.22, 0.23) | 0.647 |
| DH-Gly | 0.00 (0.00, 0.42) | 0.00 (0.00, 0.00) | 0.20 (0.00, 0.41) | 0.00 (0.00, 0.00) | 0.422 |
| A-Gly | 0.07 (0.00, 0.15) | 0.07 (0.00, 0.14) | 0.07 (0.00, 0.14) | 0.00 (0.00, 0.10) | 0.234 |
| L-Gly | 0.37 (0.33, 0.43) | 0.26 (0.24, 0.31) | 0.32 (0.28, 0.38) | 0.26 (0.19, 0.37) | 0.606 |
| P-Gly | 0.92 (0.82, 1.11) | 0.90 (0.80, 1.15) | 0.90 (0.79, 1.04) | 0.78 (0.66, 1.04) | 0.352 |
| O-Gly | 0.64 (0.49, 0.68) | 0.49 (0.40, 0.65) | 0.54 (0.41, 0.60) | 0.31 (0.28, 0.51) | 0.524 |
| A-Ala | 0.00 (0.00, 0.15) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | - |
| O-Ala | 0.40 (0.35, 0.49) | 0.36 (0.35, 0.36) | 0.39 (0.31, 0.44) | 0.34 (0.34, 0.37) | 0.586 |
| A-GABA | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bar-Sela, G.; Cohen, I.; Campisi-Pinto, S.; Lewitus, G.M.; Oz-Ari, L.; Jehassi, A.; Peer, A.; Turgeman, I.; Vernicova, O.; Berman, P.; et al. Cannabis Consumption Used by Cancer Patients during Immunotherapy Correlates with Poor Clinical Outcome. Cancers 2020, 12, 2447. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers12092447
Bar-Sela G, Cohen I, Campisi-Pinto S, Lewitus GM, Oz-Ari L, Jehassi A, Peer A, Turgeman I, Vernicova O, Berman P, et al. Cannabis Consumption Used by Cancer Patients during Immunotherapy Correlates with Poor Clinical Outcome. Cancers. 2020; 12(9):2447. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers12092447
Chicago/Turabian StyleBar-Sela, Gil, Idan Cohen, Salvatore Campisi-Pinto, Gil M. Lewitus, Lanuel Oz-Ari, Ayellet Jehassi, Avivit Peer, Ilit Turgeman, Olga Vernicova, Paula Berman, and et al. 2020. "Cannabis Consumption Used by Cancer Patients during Immunotherapy Correlates with Poor Clinical Outcome" Cancers 12, no. 9: 2447. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers12092447