Bone Marrow Transplantation as Therapy for Ataxia-Telangiectasia: A Systematic Review
Abstract
:Simple Summary
Abstract
1. Introduction
2. Ataxia-Telangiectasia Syndrome: Diagnosis
3. Ataxia-Telangiectasia Syndrome: Current Treatments and New Emerging Therapies
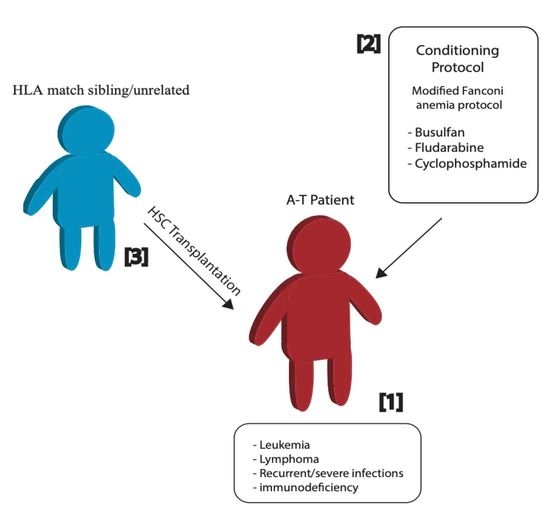
4. Bone Marrow/Hematopoietic Stem Cell Transplantation as a Therapeutic Approach
4.1. Bone Marrow Transplantation in the Mouse Model
4.2. Hematopoietic Stem Cell Transplantation in Human A-T Patients
5. Remarks
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| A-T | Ataxia-Telangiectasia; |
| ATM | Ataxia-Telangiectasia mutated; |
| BMT | bone marrow transplantation; |
| DSBs | double strand breaks; |
| MRN | MRE11/RAD50/NBN; |
| V(D)J | variable (V), diversity (D) and joining (J); |
| AFP | alphafetoprotein; |
| SOD | superoxide dismutase; |
| ROS | reactive oxygen species; |
| IR | irradiation; |
| IGF-1 | insulin-like growth factor 1; |
| SMRT | small molecule read-through; |
| ASO | antisense oligonucleotides; |
| CRISPR/Cas9 | clustered regularly interspaced short palindromic repeats/CRISPR-associated protein-9; |
| iPS cells | induced pluripotent stem cells; |
| HSV-1 | Herpes simplex virus type 1 AAV: adeno-associated virus; |
| HSCs | hematopoietic stem cells; |
| BMDCs | bone marrow-derived cells; |
| GFP | green fluorescent protein; |
| Allo-HSCT | allogenic hematopoietic stem cell transplantation; |
| GvHD | graft-versus-host disease; |
| EBV | Epstein−Barr virus; |
| HLA | human leukocyte antigen; |
| RIC | reduced intensity conditioning. |
References
- Bagley, J.; Cortes, M.L.; Breakefield, X.O.; Iacomini, J. Bone marrow transplantation restores immune system function and prevents lymphoma in Atm-deficient mice. Blood 2004, 104, 572–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chun, H.H.; Gatti, R.A. Ataxia-telangiectasia, an evolving phenotype. DNA Repair 2004, 3, 1187–1196. [Google Scholar] [CrossRef] [PubMed]
- Lange, E.; Borresen, A.-L.; Chen, X.; Chessa, L.; Chiplunkar, S.; Concannon, P.; Dandekar, S.; Gerken, S.; Lange, K.; Liang, T.; et al. Localization of an ataxia-telangiectasia gene to an −500 kb interval on chromosome 11q23.1: Linkage analysis of 176 families by an international consortium. Am. J. Hum. Genet. 1995, 57, 112–119. [Google Scholar] [PubMed]
- Shiloh, Y. ATM and related protein kinases: Safeguarding genome integrity. Nat. Rev. Cancer 2003, 3, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Blackford, A.N.; Jackson, S.P. ATM, ATR, and DNA-PK: The Trinity at the heart of the DNA damage response. Mol. Cell 2017, 66, 801–817. [Google Scholar] [CrossRef] [Green Version]
- Rothblum-Oviatt, C.; Wright, J.; Lefton-Greif, M.A.; McGrath-Morrow, S.A.; Crawford, T.O.; Lederman, H.M. Ataxia telangiectasia: A review. Orphanet J. Rare Dis. 2016, 11, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Duecker, R.; Baer, P.C.; Buecker, A.; Huenecke, S.; Pfeffermann, L.-M.; Modlich, U.; Bakhtiar, S.; Bader, P.; Zielen, S.; Schubert, R. Hematopoietic stem cell transplantation restores naïve t-cell populations in atm-deficient mice and in preemptively treated patients with ataxia-telangiectasia. Front. Immunol. 2019, 10, 2785. [Google Scholar] [CrossRef]
- Nowak-Wegrzyn, A.; Crawford, T.O.; Winkelstein, J.A.; Carson, K.A.; Lederman, H.M. Immunodeficiency and infections in ataxia-telangiectasia. J. Pediatr. 2004, 144, 505–511. [Google Scholar] [CrossRef]
- Perkins, E.J.; Nair, A.; Cowley, D.O.; Van Dyke, T.; Chang, Y.; Ramsden, D.A. Sensing of intermediates in V(D)J recombination by ATM. Genes Dev. 2002, 16, 159–164. [Google Scholar] [CrossRef] [Green Version]
- Pietzner, J.; Baer, P.C.; Duecker, R.P.; Merscher, M.B.; Satzger-Prodinger, C.; Bechmann, I.; Wietelmann, A.; Del Turco, D.; Doering, C.; Kuci, S.; et al. Bone marrow transplantation improves the outcome of Atm-deficient mice through the migration of ATM-competent cells. Hum. Mol. Genet. 2012, 22, 493–507. [Google Scholar] [CrossRef]
- Gatti, R.; Perlman, S. Ataxia-Telangiectasia. In GeneReviews; Adam, M.P., Ardinger, H.H., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Stephens, K., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 2016; pp. 1–25. [Google Scholar]
- Perlman, S.L.; Boder, E.; Sedgewick, R.P.; Gatti, R.A. Ataxia–telangiectasia. In Handbook of Clinical Neurology; Subramony, S.H., Dürr, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; Volume 103, pp. 307–332. [Google Scholar] [CrossRef]
- Schon, K.; Van Os, N.J.; Oscroft, N.; Baxendale, H.; Scoffings, D.; Ray, J.; Suri, M.; Whitehouse, W.P.; Mehta, P.R.; Everett, N.; et al. Genotype, extrapyramidal features and severity of variant Ataxia-Telangiectasia. Ann. Neurol. 2018, 85, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.; Byrd, P.J. Molecular pathology of ataxia telangiectasia. J. Clin. Pathol. 2005, 58, 1009–1015. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.; Lam, Z.; Last, J.; Byrd, P. Ataxia telangiectasia: More variation at clinical and cellular levels. Clin. Genet. 2014, 87, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Van Os, N.; Roeleveld, N.; Weemaes, C.; Jongmans, M.; Janssens, G.O.R.J.; Taylor, A.; Hoogerbrugge, N.; Willemsen, M. Health risks for ataxia-telangiectasia mutated heterozygotes: A systematic review, meta-analysis and evidence-based guideline. Clin. Genet. 2016, 90, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Lavin, M.F.; Gueven, N.; Bottle, S.; Gatti, R.A. Current and potential therapeutic strategies for the treatment of ataxia-telangiectasia. Br. Med Bull. 2007, 81, 129–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Os, N.J.H.; Haaxma, C.A.; Van Der Flier, M.; Merkus, P.J.F.M.; Van Deuren, M.; De Groot, I.J.M.; Loeffen, J.; Van De Warrenburg, B.P.C.; Willemsen, M.A.A.P. The A-T study group Ataxia-telangiectasia: Recommendations for multidisciplinary treatment. Dev. Med. Child Neurol. 2017, 59, 680–689. [Google Scholar] [CrossRef] [Green Version]
- Barzilai, A.; Yamamoto, K.-I. DNA damage responses to oxidative stress. DNA Repair 2004, 3, 1109–1115. [Google Scholar] [CrossRef]
- Reichenbach, J.; Schubert, R.; Schwan, C.; Müller, K.; Böhles, H.J.; Zielen, S. Anti-oxidative capacity in patients with ataxia telangiectasia. Clin. Exp. Immunol. 1999, 117, 535–539. [Google Scholar] [CrossRef]
- Shackelford, R.E.; Manuszak, R.P.; Johnson, C.D.; Hellrung, D.J.; Steele, T.A.; Link, C.J.; Wang, S. Desferrioxamine treatment increases the genomic stability of Ataxia-telangiectasia cells. DNA Repair 2003, 2, 971–981. [Google Scholar] [CrossRef]
- Mongiardi, M.P.; Radice, G.; Piras, M.; Stagni, V.; Pacioni, S.; Re, A.; Putti, S.; Ferrè, F.; Farsetti, A.; Pallini, R.; et al. Axitinib exposure triggers endothelial cells senescence through ROS accumulation and ATM activation. Oncogene 2019, 38, 5413–5424. [Google Scholar] [CrossRef]
- Schubert, R.; Erker, L.; Barlow, C.; Yakushiji, H.; Larson, D.; Russo, A.; Mitchell, J.B.; Wynshaw-Boris, A. Cancer chemoprevention by the antioxidant tempol in Atm-deficient mice. Hum. Mol. Genet. 2004, 13, 1793–1802. [Google Scholar] [CrossRef] [PubMed]
- Ambrose, M.; Gatti, R.A. Pathogenesis of ataxia-telangiectasia: The next generation of ATM functions. Blood 2013, 121, 4036–4045. [Google Scholar] [CrossRef]
- Pollard, J.M.; Rebouças, J.S.; Durazo, A.; Kos, I.; Fike, F.; Panni, M.; Gralla, E.B.; Valentine, J.S.; Batinic-Haberle, I.; Gatti, R.A. Radioprotective effects of manganese-containing superoxide dismutase mimics on ataxia–telangiectasia cells. Free. Radic. Biol. Med. 2009, 47, 250–260. [Google Scholar] [CrossRef] [Green Version]
- Fernandez, A.; Carro, E.; Lopez-Lopez, C.; Torres-Aleman, I. Insulin-like growth factor I treatment for cerebellar ataxia: Addressing a common pathway in the pathological cascade? Brain Res. Rev. 2005, 50, 134–141. [Google Scholar] [CrossRef] [Green Version]
- Woelke, S.; Valesky, E.; Bakhtiar, S.; Pommerening, H.; Pfeffermann, L.M.; Schubert, R.; Zielen, S. Treatment of granulomas in patients with Ataxia Telangiectasia. Front. Immunol. 2018, 9, 2000. [Google Scholar] [CrossRef] [Green Version]
- Giardino, G.; Fusco, A.; Romano, R.; Gallo, V.; Maio, F.; Esposito, T.; Palamaro, L.; Parenti, G.; Salerno, M.C.; Vajro, P.; et al. Betamethasone therapy in ataxia telangiectasia: Unraveling the rationale of this serendipitous observation on the basis of the pathogenesis. Eur. J. Neurol. 2012, 20, 740–747. [Google Scholar] [CrossRef]
- Buoni, S.; Zannolli, R.; Sorrentino, L.; Fois, A. Betamethasone and improvement of neurological symptoms in Ataxia-Telangiectasia. Arch. Neurol. 2006, 63, 1479–1482. [Google Scholar] [CrossRef]
- Chessa, L.; Leuzzi, V.; Plebani, A.; Soresina, A.; Micheli, R.; D’Agnano, D.; Venturi, T.; Molinaro, A.; Fazzi, E.; Marini, M.; et al. Intra-erythrocyte infusion of dexamethasone reduces neurological symptoms in Ataxia Teleangiectasia patients: Results of a phase 2 trial. Orphanet J. Rare Dis. 2014, 9, 5. [Google Scholar] [CrossRef] [Green Version]
- Menotta, M.; Biagiotti, S.; Orazi, S.; Rossi, L.; Chessa, L.; Leuzzi, V.; D’Agnano, D.; Plebani, A.; Soresina, A.; Magnani, M. In vivo effects of dexamethasone on blood gene expression in ataxia telangiectasia. Mol. Cell. Biochem. 2017, 438, 153–166. [Google Scholar] [CrossRef]
- Leuzzi, V.; Micheli, R.; D’Agnano, D.; Molinaro, A.; Venturi, T.; Plebani, A.; Soresina, A.; Marini, M.; Leali, P.F.; Quinti, I.; et al. Positive effect of erythrocyte-delivered dexamethasone in ataxia-telangiectasia. Neurol. -Neuroimmunol. Neuroinflamm. 2015, 2, e98. [Google Scholar] [CrossRef] [Green Version]
- Menotta, M.; Biagiotti, S.; Bianchi, M.; Chessa, L.; Magnani, M. Dexamethasone partially rescues Ataxia Telangiectasia-mutated (ATM) deficiency in Ataxia Telangiectasia by promoting a shortened protein variant retaining kinase activity *. J. Biol. Chem. 2012, 287, 41352–41363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broccoletti, T.; Del Giudice, E.; Amorosi, S.; Russo, I.; Di Bonito, M.; Imperati, F.; Romano, A.; Pignata, C. Steroid-induced improvement of neurological signs in ataxia-telangiectasia patients. Eur. J. Neurol. 2008, 15, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Quek, H.; Luff, J.; Cheung, K.; Kozlov, S.; Gatei, M.; Lee, C.S.; Bellingham, M.C.; Noakes, P.G.; Lim, Y.C.; Barnett, N.L.; et al. A rat model of ataxia-telangiectasia: Evidence for a neurodegenerative phenotype. Hum. Mol. Genet. 2016, 26, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Quek, H.; Luff, J.; Cheung, K.; Kozlov, S.; Gatei, M.; Lee, C.S.; Bellingham, M.C.; Noakes, P.G.; Lim, Y.C.; Barnett, N.L.; et al. Rats with a missense mutation in Atm display neuroinflammation and neurodegeneration subsequent to accumulation of cytosolic DNA following unrepaired DNA damage. J. Leukoc. Biol. 2016, 101, 927–947. [Google Scholar] [CrossRef] [Green Version]
- Di Siena, S.; Campolo, F.; Gimmelli, R.; Di Pietro, C.; Marazziti, D.; Dolci, S.; Lenzi, A.; Nussenzweig, A.; Pellegrini, M. Atm reactivation reverses ataxia telangiectasia phenotypes in vivo. Cell Death Dis. 2018, 9, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Du, L.; Pollard, J.M.; Gatti, R.A. Correction of prototypic ATM splicing mutations and aberrant ATM function with antisense morpholino oligonucleotides. Proc. Natl. Acad. Sci. USA 2007, 104, 6007–6012. [Google Scholar] [CrossRef] [Green Version]
- Lai, C.-H.; Chun, H.H.; Nahas, S.A.; Mitui, M.; Gamo, K.M.; Du, L.; Gatti, R.A. Correction of ATM gene function by aminoglycoside-induced read-through of premature termination codons. Proc. Natl. Acad. Sci. USA 2004, 101, 15676–15681. [Google Scholar] [CrossRef] [Green Version]
- Lee, P.; Martin, N.T.; Nakamura, K.; Azghadi, S.; Amiri, M.; Ben-David, U.; Perlman, S.; Gatti, R.A.; Hu, H.; Lowry, W.E. SMRT compounds abrogate cellular phenotypes of ataxia telangiectasia in neural derivatives of patient-specific hiPSCs. Nat. Commun. 2013, 4, 1824. [Google Scholar] [CrossRef] [Green Version]
- Ovchinnikov, D.A.; Withey, S.L.; Leeson, H.C.; Lei, U.W.; Sundarrajan, A.; Junday, K.; Pewarchuk, M.; Yeo, A.J.; Kijas, A.W.; Lavin, M.F.; et al. Correction of ATM mutations in iPS cells from two ataxia-telangiectasia patients restores DNA damage and oxidative stress responses. Hum. Mol. Genet. 2020, 29, 990–1001. [Google Scholar] [CrossRef]
- Carranza, D.; Torres-Rusillo, S.; Ceballos-Pérez, G.; Blanco-Jimenez, E.; Muñoz-López, M.; Garcia-Perez, J.L.; Molina, I.J. Reconstitution of the Ataxia-Telangiectasia cellular phenotype with lentiviral vectors. Front. Immunol. 2018, 9, 2703. [Google Scholar] [CrossRef]
- Cortés, M.L.; Oehmig, A.; Saydam, O.; Sanford, J.D.; Perry, K.F.; Fraefel, C.; Breakefield, X.O.; Cort, A.O.M.L. Targeted integration of functional human ATM cDNA into genome mediated by HSV/AAV hybrid amplicon vector. Mol. Ther. 2008, 16, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Shackelford, R.; Manuszak, R.; Cheng, D.; Smith, M.; Link, C.; Wang, S.; Jr, C.L. Functional expression of ATM gene carried by HSV amplicon vector in vitro and in vivo. Gene Ther. 2003, 11, 25–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, E.D. Bone marrow transplantation: A review. Semin. Hematol. 1999, 36, 95–103. [Google Scholar] [PubMed]
- Pietzner, J.; Merscher, B.M.; Baer, P.C.; Duecker, R.P.; Eickmeier, O.; Fußbroich, D.; Bader, P.; Del Turco, D.; Henschler, R.; Zielen, S.; et al. Low-dose irradiation prior to bone marrow transplantation results in ATM activation and increased lethality in Atm-deficient mice. Bone Marrow Transplant. 2016, 51, 560–567. [Google Scholar] [CrossRef]
- De la Morena, M.T.; Gatti, R.A. A History of Bone Marrow Transplantation. Immunol. Allergy Clin. N. Am. 2010, 30, 1–15. [Google Scholar] [CrossRef]
- Ayas, M.; Al-Seraihi, A.; El-Solh, H.; Al-Ahmari, A.; Khairy, A.; Aldali, A.; Markiz, S.; Siddiqui, K.; Al-Jefri, A. The Saudi experience in fludarabine-based conditioning regimens in patients with Fanconi anemia undergoing stem cell transplantation: Excellent outcome in recipients of matched related stem cells but not in recipients of unrelated cord blood stem cells. Biol. Blood Marrow Transplant. 2012, 18, 627–632. [Google Scholar] [CrossRef] [Green Version]
- Buckley, R.H. Bone marrow and thymus transplantation in ataxia-telangiectasia. Birth Defects Orig. Artic. Ser. 1975, 11, 421–424. [Google Scholar]
- Ghosh, S.; Schuster, F.R.; Binder, V.; Niehues, T.; Baldus, S.E.; Seiffert, P.; Laws, H.-J.; Borkhardt, A.; Meisel, R. Fatal outcome despite full lympho-hematopoietic reconstitution after allogeneic stem cell transplantation in atypical Ataxia Telangiectasia. J. Clin. Immunol. 2012, 32, 438–440. [Google Scholar] [CrossRef]
- Ussowicz, M.; Musiał, J.; Duszeńko, E.; Haus, O.; Kałwak, K. Long-term survival after allogeneic-matched sibling PBSC transplantation with conditioning consisting of low-dose busilvex and fludarabine in a 3-year-old boy with ataxia-telangiectasia syndrome and ALL. Bone Marrow Transplant. 2012, 48, 740–741. [Google Scholar] [CrossRef]
- Chao, M.M.; Ebell, W.; Bader, P.; Beier, R.; Burkhardt, B.; Feuchtinger, T.; Handgretinger, R.; Hanenberg, H.; Koehl, U.; Kratz, C.; et al. Consensus of German transplant centers on hematopoietic stem cell transplantation in Fanconi anemia. Klin. Pädiatr. 2015, 227, 157–165. [Google Scholar] [CrossRef] [Green Version]
- Ussowicz, M.; Wawrzyniak-Dzierżek, E.; Mielcarek-Siedziuk, M.; Salamonowicz, M.; Frączkiewicz, J.; Rybka, B.; Ryczan-Krawczyk, R.; Kałwak, K. Allogeneic stem cell transplantation after Fanconi anemia conditioning in children with Ataxia-telangiectasia results in stable T cell engraftment and lack of infections despite mixed chimerism. Biol. Blood Marrow Transplant. 2018, 24, 2245–2249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beier, R.; Sykora, K.-W.; Woessmann, W.; Maecker-Kolhoff, B.; Sauer, M.; Kreipe, H.H.; Dörk, T.; Kratz, C.; Lauten, M. Allogeneic-matched sibling stem cell transplantation in a 13-year-old boy with ataxia telangiectasia and EBV-positive non-Hodgkin lymphoma. Bone Marrow Transplant. 2016, 51, 1271–1274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakhtiar, S.; Woelke, S.; Huenecke, S.; Kieslich, M.; Taylor, A.M.; Schubert, R.; Zielen, S.; Bader, P. Pre-emptive allogeneic hematopoietic stem cell transplantation in Ataxia Telangiectasia. Front. Immunol. 2018, 9, 2495. [Google Scholar] [CrossRef]
- Clark, C.A.; Savani, M.; Mohty, M.; Savani, B.N. What do we need to know about allogeneic hematopoietic stem cell transplant survivors? Bone Marrow Transplant. 2016, 51, 1025–1031. [Google Scholar] [CrossRef] [PubMed]

| Patient | Age | Clinical Symptoms | BMT (Year) | Donor | Conditioning Protocol (I.V.) | Follow-Up | Reference |
|---|---|---|---|---|---|---|---|
| 1 | 22 months | Recurrent severe respiratory infections; hepatosplenomegaly; Hyper-IgM phenotype. | N/S | HLA-identical sibling | Treosulfan (3 × 12 g/m2) Fludarabine (5 × 30 mg/m2) ATG- Fresenius (3 × 20 mg/kg) | At the age of 30 months: increasing IgG levels, B cell count increased dramatically, and neutrophils declined; liver enzymes were elevated. Died at the age of 32 months, due to fulminant hepatic failure. | [50] |
| 2 (PT1) | 3 years | Leukemia; scheduled for high-risk chemotherapy with subsequent allo-HSCT | 2009 | HLA-matched sibling | Busilvex (0.5 mg/kg, 2 times/day; total dose 2 mg/kg) Fludarabine (30 mg/m2; total dose 150 mg/m2) ATG-Fresenius (20 mg/kg) | No symptoms of either acute or chronic GvHD; complete hematological remission; Did not worsen the patient’s neurological status. 8 years after transplantation: remains in leukemia remission; attends primary school; coordination deficits that make him wheelchair-bound; does not require intravenous immunoglobulin substitution and did not suffer from serious infections. | [51,53] |
| 3 | 13 years | Cervical lymphadenopathy; prominent Waldeyer’s lymphatic ring; enlarged tracheal and intrapulmonary lymph nodes; cutaneous infiltration of the abdominal wall; infiltration of the right mastoid bones and the right middle ear; EBV-positive non-Hodgkin lymphoma | 2014 | HLA-identical sibling | Fludarabine 180 mg/m2, Busilvex (1.6 mg/kg) Cyclophosphamide (40 mg/kg) Rituximab (2 × 375 mg/m2) | Life-threatening toxicity in several organ systems due to conditioning; CD19+ and CD16+/CD56+ cells reconstituted 10 months post HSCT; total lymphocyte count and CD8+ cells normalized at 18 months; CD4+ cells at 30 months post HSCT. No acute or chronic GvHD was observed. Able to sit and stand without support; walks a few steps with assistance. | [54] |
| 4 (PT2) | 12 months | undefined, radiosensitive, severe combined immunodeficiency disease | N/S | HLA-10/10 matched unrelated | Busulfan (2 × 0.5 mg/kg/d) Fludarabine (6 × 30 mg/m2) Cyclophosphamide (2 × 20 mg/kg/d) MabCampath (1 × 0.25 mg/kg and 3 × 0.5 mg/kg/d) | Stage I acute skin graft-versus-host disease, which resolved completely with methylprednisolone 1 mg/kg/day after 3 days; Readmitted and treated with methylprednisolone for Coombs-positive hemolytic anemia; 8 months after SCT, polyclonal T cell repertoire was observed. Neurologic symptoms were observed in the second year of life, and A-T diagnosis was made 2 years after SCT. | [53] |
| 5 (PT3) | 23 months | Preemptive transplantation was performed | N/S | matched unrelated | Busulfan (2 × 0.5 mg/kg/d) Fludarabine (6 × 30 mg/m2) Cyclophosphamide (2 × 20 mg/kg/d) | post-transplant period was uneventful, and 6 months after SCT cyclosporine was stopped because of decreasing donor chimerism. | [53] |
| 6 | 4 years | Upper respiratory infections; skin and joint granulomas; very low naïve T cells; absence of IgA; low IgG2 and IgG4, alpha fetoprotein (AFP) level of 52 ng/mL (normal range <7); Total serum IgG and IgM normal | 2012 | HLA-identical sibling | Fludarabine (5 × 30 mg/m2/d), Cyclophosphamide (4 × 20 mg/kg/d) ATG-Fresenius (20 mg/kg) | AlloHSCT corrected the T-cell lymphopenia by expansion of naïve and memory CD4+ T-cells, CD19+ cells, and CD8+ T-cells; gain in height and weight; complete remission of skin and joint granulomas; milder progression of ataxic symptoms. | [55] |
| Case | Conditioning Protocol Prior to Allo-HSCT | Outcome |
|---|---|---|
| 1 | N/S | Death due to hepatic failure |
| 2 | Modified GEFA02 | Leukemia remission |
| 3 | GEFA02 | CD8+ and CD4+ T cells normalized with no GvHD |
| 4 | GEFA03 | Poor immunologic recovery and low donor chimerism |
| 5 | Modified GEFA03 | Stable CD4+ and CD8+ T cell counts |
| 6 | N/S | Renewal in peripheral blood cells and ATM protein expression |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabino Pinho de Oliveira, B.; Putti, S.; Naro, F.; Pellegrini, M. Bone Marrow Transplantation as Therapy for Ataxia-Telangiectasia: A Systematic Review. Cancers 2020, 12, 3207. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers12113207
Sabino Pinho de Oliveira B, Putti S, Naro F, Pellegrini M. Bone Marrow Transplantation as Therapy for Ataxia-Telangiectasia: A Systematic Review. Cancers. 2020; 12(11):3207. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers12113207
Chicago/Turabian StyleSabino Pinho de Oliveira, Bruna, Sabrina Putti, Fabio Naro, and Manuela Pellegrini. 2020. "Bone Marrow Transplantation as Therapy for Ataxia-Telangiectasia: A Systematic Review" Cancers 12, no. 11: 3207. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers12113207