Genetic Determinants for Prediction of Outcome of Patients with Papillary Thyroid Carcinoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Samples
2.2. Patient Follow-Up and Risk Stratification
2.3. DNA Extraction
2.4. PCR and Sanger Sequencing Analysis
2.5. Statistical Analysis
3. Results
3.1. Description of the Patients
3.2. Primary Tumors’ Genetic Characterization
3.3. Metastatic Lesions’ Genetic Characterization and Molecular Profile Concordance
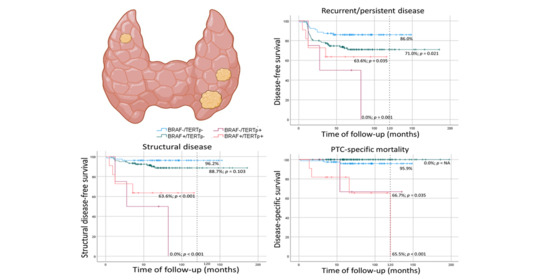
3.4. Molecular Alterations in Recurrent/Persistent Disease
3.5. Molecular Alterations in Structural Disease
3.6. Molecular Alterations in Disease-Specific Mortality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raposo, L.; Morais, S.; Oliveira, M.J.; Marques, A.P.; Bento, M.J.; Lunet, N. Trends in thyroid cancer incidence and mortality in Portugal. Eur. J. Cancer Prev. 2017, 26, 135–143. [Google Scholar] [CrossRef]
- Olson, E.; Wintheiser, G.; Wolfe, K.M.; Droessler, J.; Silberstein, P.T. Epidemiology of Thyroid Cancer: A Review of the National Cancer Database, 2000-2013. Cureus 2019, 11, e4127. [Google Scholar] [CrossRef] [Green Version]
- Maso, L.D.; Tavilla, A.; Pacini, F.; Serraino, D.; Van Dijk, B.; Chirlaque, M.; Capocaccia, R.; Larrañaga, N.; Colonna, M.; Agius, D.; et al. Survival of 86,690 patients with thyroid cancer: A population-based study in 29 European countries from EUROCARE-5. Eur. J. Cancer 2017, 77, 140–152. [Google Scholar] [CrossRef]
- Agrawal, N.; Akbani, R.; Aksoy, B.A.; Ally, A.; Arachchi, H.; Asa, S.L.; Auman, J.T.; Balasundaram, M.; Balu, S.; Baylin, S.B.; et al. Integrated Genomic Characterization of Papillary Thyroid Carcinoma. Cell 2014, 159, 676–690. [Google Scholar] [CrossRef] [Green Version]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Global Cancer Observatory. Available online: https://gco.iarc.fr (accessed on 30 December 2020).
- Enewold, L.; Zhu, K.; Ron, E.; Marrogi, A.J.; Stojadinovic, A.; Peoples, G.E.; Devesa, S.S. Rising Thyroid Cancer Incidence in the United States by Demographic and Tumor Characteristics, 1980-2005. Cancer Epidemiol. Biomark. Prev. 2009, 18, 784–791. [Google Scholar] [CrossRef] [Green Version]
- Kitahara, C.M.; Sosa, J.A. The changing incidence of thyroid cancer. Nat. Rev. Endocrinol. 2016, 12, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Miyauchi, A.; Kihara, M.; Fukushima, M.; Higashiyama, T.; Miya, A. Overall Survival of Papillary Thyroid Carcinoma Patients: A Single-Institution Long-Term Follow-Up of 5897 Patients. World J. Surg. 2018, 42, 615–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melo, M.; Da Rocha, A.G.; Batista, R.; Vinagre, J.; Martins, M.J.; Costa, G.; Ribeiro, C.; Carrilho, F.; Leite, V.; Lobo, C.; et al. TERT, BRAF, and NRAS in Primary Thyroid Cancer and Metastatic Disease. J. Clin. Endocrinol. Metab. 2017, 102, 1898–1907. [Google Scholar] [CrossRef] [PubMed]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [Green Version]
- Sugitani, I.; Fujimoto, Y.; Yamamoto, N. Papillary thyroid carcinoma with distant metastases: Survival predictors and the importance of local control. Surgery 2008, 143, 35–42. [Google Scholar] [CrossRef]
- Giorgenon, T.M.V.; Carrijo, F.T.; Arruda, M.A.; Cerqueira, T.L.O.; Barreto, H.R.; Cabral, J.B.; Da Silva, T.M.; Magalhães, P.K.R.; Maciel, L.M.Z.; Ramos, H.E. Preoperative detection of TERT promoter and BRAFV600E mutations in papillary thyroid carcinoma in high-risk thyroid nodules. Arch. Endocrinol. Metab. 2019, 63, 107–112. [Google Scholar] [CrossRef]
- Nikiforov, Y.E.; Steward, D.L.; Robinson-Smith, T.M.; Haugen, B.R.; Klopper, J.P.; Zhu, Z.; Fagin, J.A.; Falciglia, M.; Weber, K.; Nikiforova, M.N. Molecular Testing for Mutations in Improving the Fine-Needle Aspiration Diagnosis of Thyroid Nodules. J. Clin. Endocrinol. Metab. 2009, 94, 2092–2098. [Google Scholar] [CrossRef] [PubMed]
- Baloch, Z.W.; Sack, M.J.; Yu, G.H.; Livolsi, V.A.; Gupta, P.K. Fine-Needle Aspiration of Thyroid: An Institutional Experience. Thyroid 1998, 8, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Sipos, J.; Mazzaferri, E. Thyroid Cancer Epidemiology and Prognostic Variables. Clin. Oncol. 2010, 22, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Castro, L.; Alves, S.; Chaves, S.R.; Costa, J.L.; Soares, P.; Preto, A. RAF-1 promotes survival of thyroid cancer cells harboring RET/PTC1 rearrangement independently of ERK activation. Mol. Cell. Endocrinol. 2015, 415, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Sobrinho-Simões, M.; Máximo, V.; Rocha, A.S.; Trovisco, V.; Castro, P.; Preto, A.; Lima, J.; Soares, P. Intragenic Mutations in Thyroid Cancer. Endocrinol. Metab. Clin. N. Am. 2008, 37, 333–362. [Google Scholar] [CrossRef]
- Nikiforov, Y.E. Thyroid carcinoma: Molecular pathways and therapeutic targets. Mod. Pathol. 2008, 21, S37–S43. [Google Scholar] [CrossRef] [Green Version]
- Panebianco, F.; Mazzanti, C.; Tomei, S.; Aretini, P.; Franceschi, S.; Lessi, F.; Di Coscio, G.; Bevilacqua, G.; Marchetti, I. The combination of four molecular markers improves thyroid cancer cytologic diagnosis and patient management. Bmc Cancer 2015, 15, 918. [Google Scholar] [CrossRef] [Green Version]
- Melo, M.; Da Rocha, A.G.; Vinagre, J.; Batista, R.; Peixoto, J.; Tavares, C.; Celestino, R.; Almeida, A.; Salgado, C.; Eloy, C.; et al. TERT Promoter Mutations Are a Major Indicator of Poor Outcome in Differentiated Thyroid Carcinomas. J. Clin. Endocrinol. Metab. 2014, 99, E754–E765. [Google Scholar] [CrossRef] [Green Version]
- Penna, G.C.; Pestana, A.; Cameselle, J.M.; Momesso, D.; De Andrade, F.A.; Vidal, A.P.A.; Junior, M.L.A.; Melo, M.; Fernandes, P.V.; Corbo, R.; et al. TERTp mutation is associated with a shorter progression free survival in patients with aggressive histology subtypes of follicular-cell derived thyroid carcinoma. Endocrine 2018, 61, 489–498. [Google Scholar] [CrossRef]
- Censi, S.; Cavedon, E.; Bertazza, L.; Galuppini, F.; Watutantrige-Fernando, S.; De Lazzari, P.; Nacamulli, D.; Pennelli, G.; Fassina, A.; Iacobone, M.; et al. Frequency and Significance of Ras, Tert Promoter, and Braf Mutations in Cytologically Indeterminate Thyroid Nodules: A Monocentric Case Series at a Tertiary-Level Endocrinology Unit. Front. Endocrinol. 2017, 8, 273. [Google Scholar] [CrossRef]
- Xing, M.; Liu, R.; Liu, X.; Murugan, A.K.; Zhu, G.; Zeiger, M.A.; Pai, S.; Bishop, J. BRAF V600E and TERT Promoter Mutations Cooperatively Identify the Most Aggressive Papillary Thyroid Cancer With Highest Recurrence. J. Clin. Oncol. 2014, 32, 2718–2726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cantwell-Dorris, E.R.; O’Leary, J.J.; Sheils, O.M. BRAFV600E: Implications for Carcinogenesis and Molecular Therapy. Mol. Cancer Ther. 2011, 10, 385–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xing, M.; Westra, W.H.; Tufano, R.P.; Cohen, Y.; Rosenbaum, E.; Rhoden, K.J.; Carson, K.A.; Vasko, V.; Larin, A.; Tallini, G.; et al. BRAF Mutation Predicts a Poorer Clinical Prognosis for Papillary Thyroid Cancer. J. Clin. Endocrinol. Metab. 2005, 90, 6373–6379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, X.; Zhu, G.; Liu, R.; Viola, D.; Elisei, R.; Puxeddu, E.; Fugazzola, L.; Colombo, C.; Jarzab, B.; Czarniecka, A.; et al. Patient Age–Associated Mortality Risk Is Differentiated by BRAF V600E Status in Papillary Thyroid Cancer. J. Clin. Oncol. 2018, 36, 438–445. [Google Scholar] [CrossRef] [Green Version]
- Trovisco, V.; Soares, P.; Preto, A.; De Castro, I.V.; Lima, J.; Castro, P.; Máximo, V.; Botelho, T.; Moreira, S.; Meireles, A.M.; et al. Type and prevalence of BRAF mutations are closely associated with papillary thyroid carcinoma histotype and patients’ age but not with tumour aggressiveness. Virchows Arch. 2005, 446, 589–595. [Google Scholar] [CrossRef]
- Xing, M.; Alzahrani, A.S.; Carson, K.A.; Viola, D.; Elisei, R.; Bendlova, B.; Yip, L.; Mian, C.; Vianello, F.; Tuttle, R.M.; et al. Association Between BRAF V600E Mutation and Mortality in Patients With Papillary Thyroid Cancer. JAMA 2013, 309, 1493–1501. [Google Scholar] [CrossRef] [Green Version]
- Xing, M.; Alzahrani, A.S.; Carson, K.A.; Shong, Y.K.; Kim, T.Y.; Viola, D.; Elisei, R.; Bendlová, B.; Yip, L.; Mian, C. Association between BRAF V600E mutation and recurrence of papillary thyroid cancer. J. Clin. Oncol. 2015, 33, 42. [Google Scholar] [CrossRef] [Green Version]
- Lemoine, N.R.M.E.; Wyllie, F.S.; Williams, E.D.; Goyns, M.; Stringer, B.; Wynford-Thomas, D. High frequency of ras oncogene activation in all stages of human thyroid tumorigenesis. Oncogene 1989, 4, 149–164. [Google Scholar]
- Schulten, H.-J.; Salama, S.; Al-Ahmadi, A.; Al-Mansouri, Z.; Mirza, Z.; Al-Ghamdi, K.; Al-Hamour, O.A.; Huwait, E.; Gari, M.; Al-Qahtani, M.H.; et al. Comprehensive survey of HRAS, KRAS, and NRAS mutations in proliferative thyroid lesions from an ethnically diverse population. Anticancer. Res. 2013, 33, 4779–4784. [Google Scholar] [PubMed]
- Medici, M.; Kwong, N.; Angell, T.E.; Marqusee, E.; Kim, M.I.; Frates, M.C.; Benson, C.B.; Cibas, E.S.; Barletta, J.A.; Krane, J.F.; et al. The variable phenotype and low-risk nature of RAS-positive thyroid nodules. BMC Med. 2015, 13, 184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukahori, M.; Yoshida, A.; Hayashi, H.; Yoshihara, M.; Matsukuma, S.; Sakuma, Y.; Koizume, S.; Okamoto, N.; Kondo, T.; Masuda, M.; et al. The association between RAS gene mutations and clinical characteristics in follicular thyroid tumors: New insights from a single center and a large patient cohort. Thyroid 2012, 22, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Jang, E.K.; Song, D.E.; Sim, S.Y.; Kwon, H.; Choi, Y.M.; Jeon, M.J.; Han, J.M.; Kim, W.G.; Kim, T.Y.; Shong, Y.K.; et al. NRAS Codon 61 Mutation Is Associated with Distant Metastasis in Patients with Follicular Thyroid Carcinoma. Thyroid 2014, 24, 1275–1281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, A.; Xu, J.; Wang, Y. The role of TERT promoter mutations in postoperative and preoperative diagnosis and prognosis in thyroid cancer. Medicine 2018, 97, e11548. [Google Scholar] [CrossRef] [PubMed]
- Gandolfi, G.; Ragazzi, M.; Frasoldati, A.; Piana, S.; Ciarrocchi, A.; Sancisi, V. TERT promoter mutations are associated with distant metastases in papillary thyroid carcinoma. Eur. J. Endocrinol. 2015, 172, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Xing, M. TERT promoter mutations in thyroid cancer. Endocr. Relat. Cancer 2016, 23, R143–R155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lloyd, R.V.; Osamura, R.Y.; Klöppel, G.; Rosai, J. WHO Classification of Tumours of Endocrine Organs, 4th ed.; International Agency for Research on Cancer (IARC): Lyon, France, 2017. [Google Scholar]
- Tuttle, R.M.; Haugen, B.; Perrier, N.D. Updated American Joint Committee on Cancer/Tumor-Node-Metastasis Staging System for Differentiated and Anaplastic Thyroid Cancer (Eighth Edition): What Changed and Why? Thyroid 2017, 27, 751–756. [Google Scholar] [CrossRef]
- Momesso, D.P.; Vaisman, F.; Yang, S.P.; Bulzico, D.A.; Corbo, R.; Vaisman, M.; Tuttle, R.M. Dynamic Risk Stratification in Patients with Differentiated Thyroid Cancer Treated Without Radioactive Iodine. J. Clin. Endocrinol. Metab. 2016, 101, 2692–2700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- George, J.R.; Henderson, Y.C.; Williams, M.D.; Roberts, D.B.; Hei, H.; Lai, S.Y.; Clayman, G.L. Association of TERT Promoter Mutation, But Not BRAF Mutation, with Increased Mortality in PTC. J. Clin. Endocrinol. Metab. 2015, 100, E1550–E1559. [Google Scholar] [CrossRef] [Green Version]
- De Castro, T.P.; Penha, R.C.C.; Buexm, L.A.; De Carvalho, F.N.; Oliveira, R.D.V.C.; Agarez, F.V.; Pinto, L.W.; Carvalho, D.P. Molecular Predictors for Advanced Papillary Thyroid Carcinoma Recurrence. Front. Endocrinol. 2019, 10, 839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, C.; Huang, M.L.; Li, X.; Wang, T.; Ling, R. Relationship between BRAFV600E and clinical features in papillary thyroid carcinoma. Endocr. Connect. 2019, 8, 988–996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garre, M.C.; Casares, M.L.D.L.T.; Massare, P.B.; Nevot, M.; Ángel, L.; Del-Moral, J.V.; Pérez, N.M.; Joya, R.V.; Ramírez, R.M.; Elvira, J.M.L. Presencia de la mutación BRAFT1799A en el tumor primario como indicador de riesgo, recidiva o persistencia de carcinoma papilar de tiroides. Endocrinol. Y Nutr. 2011, 58, 175–184. [Google Scholar] [CrossRef]
- Shen, X.; Liu, R.; Xing, M. A six-genotype genetic prognostic model for papillary thyroid cancer. Endocr. Relat. Cancer 2017, 24, 41–52. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Zhao, S.; Shen, X.; Zhu, G.; Liu, R.; Viola, D.; Elisei, R.; Puxeddu, E.; Fugazzola, L.; Colombo, C.; et al. BRAF V600E Confers Male Sex Disease-Specific Mortality Risk in Patients With Papillary Thyroid Cancer. J. Clin. Oncol. 2018, 36, 2787–2795. [Google Scholar] [CrossRef] [Green Version]
- Howell, G.M.; Nikiforova, M.N.; Carty, S.E.; Armstrong, M.J.; Hodak, S.P.; Stang, M.T.; McCoy, K.L.; Nikiforov, Y.E.; Yip, L. BRAF V600E Mutation Independently Predicts Central Compartment Lymph Node Metastasis in Patients with Papillary Thyroid Cancer. Ann. Surg. Oncol. 2012, 20, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-J.; Lee, K.E.; Myong, J.P.; Park, J.-H.; Jeon, Y.K.; Min, H.S.; Park, S.Y.; Jung, K.C.; Koo, D.H.; Youn, Y.-K. BRAFV600E Mutation is Associated with Tumor Aggressiveness in Papillary Thyroid Cancer. World J. Surg. 2012, 36, 310–317. [Google Scholar] [CrossRef]
- O’Neill, C.J.; Bullock, M.; Chou, A.; Sidhu, S.B.; Delbridge, L.W.; Robinson, B.G.; Gill, A.J.; Learoyd, D.L.; Clifton-Bligh, R.; Sywak, M.S. BRAFV600E mutation is associated with an increased risk of nodal recurrence requiring reoperative surgery in patients with papillary thyroid cancer. Surgery 2010, 148, 1139–1146. [Google Scholar] [CrossRef]
- Tufano, R.P.; Bishop, J.; Wu, G. Reoperative central compartment dissection for patients with recurrent/persistent papillary thyroid cancer: Efficacy, safety, and the association of the BRAF mutation. Laryngoscope 2012, 122, 1634–1640. [Google Scholar] [CrossRef]
- Kim, T.Y.; Kim, W.B.; Rhee, Y.S.; Song, J.Y.; Kim, J.M.; Gong, G.; Lee, S.; Kim, S.Y.; Kim, S.C.; Hong, S.J.; et al. The BRAF mutation is useful for prediction of clinical recurrence in low-risk patients with conventional papillary thyroid carcinoma. Clin. Endocrinol. 2006, 65, 364–368. [Google Scholar] [CrossRef]
- Vuong, H.G.; Duong, U.N.; Altibi, A.M.; Ngo, H.T.; Pham, T.Q.; Tran, H.M.; Gandolfi, G.; Hassell, L. A meta-analysis of prognostic roles of molecular markers in papillary thyroid carcinoma. Endocr. Connect. 2017, 6, R8–R17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, X.; Wei, S.; Han, Y.; Li, Y.; Yu, Y.; Yun, X.; Ren, X.; Gao, M. Papillary Microcarcinoma of the Thyroid: Clinical Characteristics and BRAFV600E Mutational Status of 977 Cases. Ann. Surg. Oncol. 2013, 20, 2266–2273. [Google Scholar] [CrossRef] [PubMed]
- Howell, G.M.; Hodak, S.P.; Yip, L. RAS Mutations in Thyroid Cancer. Oncology 2013, 18, 926–932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Rostan, G.; Zhao, H.; Camp, R.L.; Pollan, M.; Herrero, A.; Pardo, J.; Wu, R.; Carcangiu, M.L.; Costa, J.; Tallini, G. ras Mutations Are Associated With Aggressive Tumor Phenotypes and Poor Prognosis in Thyroid Cancer. J. Clin. Oncol. 2003, 21, 3226–3235. [Google Scholar] [CrossRef] [PubMed]
- Bullock, M.; Ren, Y.; Oneill, C.J.; Gill, A.J.; Aniss, A.; Sywak, M.S.; Sidhu, S.B.; Delbridge, L.; Learoyd, D.L.; De Vathaire, F.; et al. TERT promoter mutations are a major indicator of recurrence and death due to papillary thyroid carcinomas. Clin. Endocrinol. 2016, 85, 283–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Liu, Z.; Chen, T.; Zeng, W.; Guo, Y.; Huang, T. TERT promoter Mutation and Its Association with Clinicopathological Features and Prognosis of Papillary Thyroid Cancer: A Meta-analysis. Sci. Rep. 2016, 6, 36990. [Google Scholar] [CrossRef]
- Yin, D.-T.; Mengyuan, L.; Lu, R.-Q.; Li, X.; Xu, J.; Lei, M.; Li, H.; Wang, Y.; Liu, Z. Clinicopathological significance of TERT promoter mutation in papillary thyroid carcinomas: A systematic review and meta-analysis. Clin. Endocrinol. 2016, 85, 299–305. [Google Scholar] [CrossRef]
- Myung, J.K.; Kwak, B.K.; Lim, J.A.; Lee, M.-C.; Kim, M.J. TERT Promoter Mutations and Tumor Persistence/Recurrence in Papillary Thyroid Cancer. Cancer Res. Treat. 2016, 48, 942–947. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.H.; Kim, Y.-E.; Ahn, S.; Kim, J.-Y.; Ki, C.-S.; Oh, Y.L.; Kim, K.; Yun, J.W.; Park, W.-Y.; Choe, J.-H.; et al. TERT promoter mutations and long-term survival in patients with thyroid cancer. Endocr. Relat. Cancer 2016, 23, 813–823. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Gong, Y.; Yan, S.; Chen, H.; Qin, S.; Gong, R. Association between TERT promoter mutations and clinical behaviors in differentiated thyroid carcinoma: A systematic review and meta-analysis. Endocrine 2019, 67, 44–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rusinek, D.; Pfeifer, A.; Krajewska, J.; Oczko-Wojciechowska, M.; Handkiewicz-Junak, D.; Pawlaczek, A.; Zebracka-Gala, J.; Kowalska, M.; Cyplinska, R.; Zembala-Nozynska, E.; et al. Coexistence of TERT Promoter Mutations and the BRAF V600E Alteration and Its Impact on Histopathological Features of Papillary Thyroid Carcinoma in a Selected Series of Polish Patients. Int. J. Mol. Sci. 2018, 19, 2647. [Google Scholar] [CrossRef] [Green Version]
- Jin, L.; Chen, E.; Dong, S.; Cai, Y.; Zhang, X.; Zhou, Y.; Zeng, R.; Yang, F.; Pan, C.; Liu, Y.; et al. BRAF and TERT promoter mutations in the aggressiveness of papillary thyroid carcinoma: A study of 653 patients. Oncotarget 2016, 7, 18346–18355. [Google Scholar] [CrossRef] [Green Version]
- Vinagre, J.; Almeida, A.; Pópulo, H.; Batista, R.; Lyra, J.; Pinto, V.; Coelho, R.; Celestino, R.; Prazeres, H.; Lima, L.; et al. Frequency of TERT promoter mutations in human cancers. Nat. Commun. 2013, 4, 2185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trybek, T.; Walczyk, A.; Gąsior-Perczak, D.; Pałyga, I.; Mikina, E.; Kowalik, A.; Hińcza, K.; Kopczyński, J.; Chrapek, M.; Góźdź, S.; et al. Impact of BRAF V600E and TERT Promoter Mutations on Response to Therapy in Papillary Thyroid Cancer. Endocrinology 2019, 160, 2328–2338. [Google Scholar] [CrossRef]
- Vuong, H.G.; Altibi, A.M.; Duong, U.N.; Hassell, L. Prognostic implication of BRAF and TERT promoter mutation combination in papillary thyroid carcinoma-A meta-analysis. Clin. Endocrinol. 2017, 87, 411–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, R.; Bishop, J.; Zhu, G.; Zhang, T.; Ladenson, P.W.; Xing, M. Mortality Risk Stratification by Combining BRAF V600E and TERT Promoter Mutations in Papillary Thyroid Cancer. Jama Oncol. 2017, 3, 202–208. [Google Scholar] [CrossRef] [PubMed]



| Patient Characteristics | n | (%) | n (Total) | Staging | n | (%) | n (Total) | ||
|---|---|---|---|---|---|---|---|---|---|
| Age | 241 | T stage | 241 | ||||||
| <55 years | 135 | (56.0%) | T1a | 96 | (39.8%) | ||||
| ≥55 years | 106 | (44.0%) | T1b | 97 | (40.2%) | ||||
| Gender | 241 | T2 | 31 | (12.9%) | |||||
| Female | 202 | (83.8%) | T3a | 7 | (2.9%) | ||||
| Male | 39 | (16.2%) | T3b | 9 | (3.7%) | ||||
| Histology | n | (%) | n (total) | T4a | 1 | (0.4%) | |||
| Nodule size | Median ± IR | 12.0 ± 10.00 | 238 a | N stage | 241 | ||||
| Histological subtype | 238 a | N0 | 207 | (85.9%) | |||||
| CPTC | 156 | (65.5%) | N1 | 34 | (14.1%) | ||||
| FVPTC | 55 | (23.1%) | M stage | 241 | |||||
| OVPTC | 18 | (7.6%) | M0 | 237 | (98.3%) | ||||
| AVPTC | 9 | (3.8%) | M1 | 4 | (1.7%) | ||||
| Necrosis | 237 | Stage | 241 | ||||||
| Absent | 232 | (97.9%) | SI | 225 | (93.4%) | ||||
| Present | 5 | (2.1%) | SII | 12 | (5.0%) | ||||
| Psammomas | 237 | SIV | 4 | (1.7%) | |||||
| Absent | 155 | (65.4%) | Lymph node invasion and extranodal extension | n | (%) | n (total) | |||
| Present | 82 | (34.6%) | Lateral compartment | 241 | |||||
| Focality | 241 | Lymph node invasion | Absent | 225 | (93.4%) | ||||
| Unifocal | 151 | (62.7%) | Present | 16 | (6.6%) | ||||
| Multifocal | 90 | (37.3%) | Lateral compartment | 241 | |||||
| Extrathyroidal extension | 240 | Extranodal extension | Absent | 236 | (97.9%) | ||||
| Absent/minimal | 230 | (95.8%) | Present | 5 | (2.1%) | ||||
| Major | 10 | (4.2%) | Patient outcome | n | (%) | n (total) | |||
| Lymphatic invasion | 237 | Recurrent/persistent | 241 | ||||||
| Absent | 190 | (80.2%) | disease | Absent | 184 | (76.3%) | |||
| Present | 47 | (19.8%) | Present | 57 | (23.7%) | ||||
| Venous invasion | 239 | Structural disease | 241 | ||||||
| Absent | 223 | (93.3%) | Absent | 216 | (89.6%) | ||||
| Present | 16 | (6.7%) | Present | 25 | (10.4%) | ||||
| Resection margins | 240 | PTC related death | 241 | ||||||
| R0 | 209 | (87.1%) | Alive or deceased by other causes | 232 | (96.3%) | ||||
| R1 | 31 | (12.9%) | Deceased of PTC | 9 | (3.7%) |
| Gene(s) | Status | All n (%) | Histological Variants of Primary Tumors | |||
|---|---|---|---|---|---|---|
| CPTC | FVPTC | OVPTC | AVPTC | |||
| n = 238 a | n = 156 | n = 55 | n = 18 | n = 9 | ||
| BRAF | wt | 85 | 39 | 33 | 9 | 4 |
| p.Val600Glu | 143 (62.7%) | 110 (73.8%) | 20 (37.7%) | 8 (47.1%) | 5 (55.6%) | |
| TERTp | wt | 202 | 133 | 47 | 14 | 8 |
| −124 G > A | 12 (5.5%) | 8 (5.6%) | 2 (4.0%) | 1 (6.7%) | 1 (11.1%) | |
| −146 G > A | 3 (1.4%) | 3 (2.1%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| −124/-125 G > A | 1 (0.5%) | 0 (0.0%) | 1 (2.0%) | 0 (0.0%) | 0 (0.0%) | |
| BRAF/TERTp | BRAFwt/TERTpwt | 79 | 36 | 31 | 8 | 4 |
| BRAFmut/TERTpwt | 120 (56.1%) | 94 (67.1%) | 16 (32.0%) | 6 (40.0%) | 4 (44.4%) | |
| BRAFwt/TERTpmut | 4 (1.9%) | 2 (1.4%) | 1 (2.0%) | 1 (6.7%) | 0 (0.0%) | |
| BRAFmut/TERTpmut | 11 (5.1%) | 8 (5.7%) | 2 (4.0%) | 0 (0.0%) | 1 (11.1%) | |
| NRAS | wt | 223 | 152 | 47 | 15 | 9 |
| p.Gln61Arg | 8 (3.5%) | 1 (0.7%) | 5 (9.6%) | 2 (11.8%) | 0 (0.0%) | |
| HRAS | wt | 127 | 75 | 36 | 10 | 6 |
| p.Gln61Arg | 3 (2.3%) | 0 (0.0%) | 3 (7.7%) | 0 (0.0%) | 0 (0.0%) | |
| KRAS | wt | 117 | 67 | 35 | 10 | 5 |
| p.Gln61Arg | 2 (1.7%) | 1 (1.5%) | 1 (2.8%) | 0 (0.0%) | 0 (0.0%) | |
| RASb | wt | 218 | 151 | 43 | 15 | 9 |
| RASmut | 13 (5.6%) | 2 (1.3%) | 9 (17.3%) | 2 (11.8%) | 0 (0.0%) | |
| Molecular Status | n | Recurrent/Persistent Disease Absent | Recurrent/Persistent Disease Present | Unadjusted HR (95% CI) | p-Value | Adjusted HR (95% CI) | p-Value * |
|---|---|---|---|---|---|---|---|
| BRAF status | 228 | ||||||
| BRAFwt | 85 | 70 (39.8%) | 15 (28.8%) | 1 (Reference) | 1 (Reference) | ||
| BRAFmut | 143 | 106 (60.2%) | 37 (71.2%) | 1.6 (0.88–2.91) | 0.128 | − | − |
| TERTp status | 218 | ||||||
| TERTpwt | 202 | 157 (94.6%) | 45 (86.5%) | 1 | 1 | ||
| TERTpmut | 16 | 9 (5.4%) | 7 (13.5%) | 2.3 (1.04–5.12) | 0.040 | 2.2 (0.96–5.01) | 0.062 |
| RAS status | 231 | ||||||
| RASwt | 218 | 168 (93.9%) | 50 (96.2%) | 1 | 1 | ||
| RASmut | 13 | 11 (6.1%) | 2 (3.8%) | 0.6 (0.16–2.61) | 0.530 | − | − |
| BRAF and TERTp status | 214 | ||||||
| BRAFwt/TERTpwt | 79 | 68 (41.7%) | 11 (21.6%) | 1 | 1 | ||
| BRAFmut/TERTpwt | 120 | 87 (53.4%) | 33 (64.7%) | 2.2 (1.11–4.34) | 0.024 | 2.2 (1.11–4.36) | 0.023 |
| BRAFwt/TERTpmut | 4 | 1 (0.6%) | 3 (5.9%) | 6.5 (1.82–23.42) | 0.004 | 6.8 (1.89–24.55) | 0.003 |
| BRAFmut/TERTpmut | 11 | 7 (4.3%) | 4 (7.8%) | 3.4 (1.10–10.82) | 0.043 | 3.2 (0.97–10.33) | 0.056 |
| Molecular Status | n | Structural Disease Absent | Structural Disease Present | Unadjusted HR (95% CI) | p-Value | Adjusted HR (95% CI) | p-Value * |
|---|---|---|---|---|---|---|---|
| BRAF status | 228 | ||||||
| BRAFwt | 85 | 79 (38.3%) | 6 (27.3%) | 1 (Reference) | 1 (Reference) | ||
| BRAFmut | 143 | 127 (61.7%) | 16 (72.7%) | 1.7 (0.65–4.26) | 0.286 | − | − |
| TERTp status | 218 | ||||||
| TERTpwt | 202 | 187 (95.4%) | 15 (68.2%) | 1 | 1 | ||
| TERTpmut | 16 | 9 (4.6%) | 7 (31.8%) | 7.4 (3.01–18.23) | <0.001 | 7.0 (2.67–18.54) | <0.001 |
| RAS status | 231 | ||||||
| RASwt | 218 | 198 (94.3%) | 20 (95.2%) | 1 | 1 | ||
| RASmut | 13 | 12 (5.7%) | 1 (4.8%) | 0.9 (0.11–6.35) | 0.875 | − | − |
| BRAF and TERTp status | 214 | ||||||
| BRAFwt/TERTpwt | 79 | 76 (39.6%) | 3 (13.6%) | 1 | 1 | ||
| BRAFmut/TERTpwt | 120 | 108 (56.3%) | 12 (54.5%) | 2.8 (0.79–9.90) | 0.112 | − | − |
| BRAFwt/TERTpmut | 4 | 1 (0.5%) | 3 (13.6%) | 24.3 (4.89–120.80) | <0.001 | 24.2 (4.80–122.05) | <0.001 |
| BRAFmut/TERTpmut | 11 | 7 (3.6%) | 4 (18.2%) | 13.2 (2.93–59.05) | 0.001 | 11.5 (2.40–55.60) | 0.002 |
| Molecular Status | n | Disease-Specific Mortality Absent | Disease-Specific Mortality Present | Unadjusted HR (95% CI) | p-Value | Adjusted HR (95% CI) | p-Value * |
|---|---|---|---|---|---|---|---|
| BRAF status | 228 | ||||||
| BRAFwt | 85 | 81 (36.8%) | 4 (50.0%) | 1 (Reference) | 1 (Reference) | ||
| BRAFmut | 143 | 139 (63.2%) | 4 (50.0%) | 0.6 (0.15–2.39) | 0.466 | - | - |
| TERTp status | 218 | ||||||
| TERTpwt | 202 | 199 (94.8%) | 3 (37.5%) | 1 | 1 | ||
| TERTpmut | 16 | 11 (5.2%) | 5 (62.5%) | 23.9 (5.70–100.23) | <0.001 | 10.1 (1.76–58.41) | 0.010 |
| RAS status | 231 | ||||||
| RASwt | 218 | 212 (94.6%) | 6 (85.7%) | 1 | 1 | ||
| RASmut | 13 | 12 (5.4%) | 1 (14.3%) | 2.4 (0.28–20.36) | 0.429 | - | - |
| BRAF and TERTp status | 214 | ||||||
| BRAFwt/TERTpwt | 79 | 76 (36.9%) | 3 (37.5%) | 1 | 1 | ||
| BRAFmut/TERTpwt | 120 | 120 (58.3%) | 0 (0.0%) | n.a. | n.a. | - | - |
| BRAFwt/TERTpmut | 4 | 3 (1.5%) | 1 (25.0%) | 6.5 (0.67–63.31) | 0.109 | - | - |
| BRAFmut/TERTpmut | 11 | 7 (3.4%) | 4 (50.0%) | 11.6 (2.59–52.09) | 0.001 | n.s. | n.s. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Póvoa, A.A.; Teixeira, E.; Bella-Cueto, M.R.; Batista, R.; Pestana, A.; Melo, M.; Alves, T.; Pinto, M.; Sobrinho-Simões, M.; Maciel, J.; et al. Genetic Determinants for Prediction of Outcome of Patients with Papillary Thyroid Carcinoma. Cancers 2021, 13, 2048. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers13092048
Póvoa AA, Teixeira E, Bella-Cueto MR, Batista R, Pestana A, Melo M, Alves T, Pinto M, Sobrinho-Simões M, Maciel J, et al. Genetic Determinants for Prediction of Outcome of Patients with Papillary Thyroid Carcinoma. Cancers. 2021; 13(9):2048. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers13092048
Chicago/Turabian StylePóvoa, Antónia Afonso, Elisabete Teixeira, Maria Rosa Bella-Cueto, Rui Batista, Ana Pestana, Miguel Melo, Thalita Alves, Mafalda Pinto, Manuel Sobrinho-Simões, Jorge Maciel, and et al. 2021. "Genetic Determinants for Prediction of Outcome of Patients with Papillary Thyroid Carcinoma" Cancers 13, no. 9: 2048. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers13092048