Systematic Analysis of Genetic and Pathway Determinants of Eribulin Sensitivity across 100 Human Cancer Cell Lines from the Cancer Cell Line Encyclopedia (CCLE)
Abstract
:Simple Summary
Abstract
1. Introduction
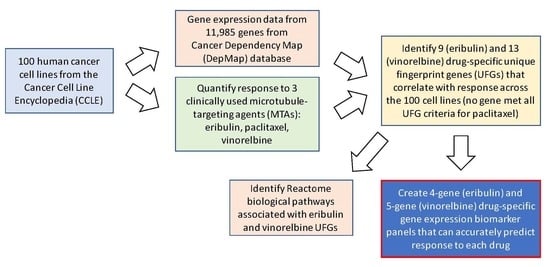
2. Materials and Methods
2.1. Test Agents
2.2. Human Cancer Cell Lines
2.3. In Vitro Cell-Based Antiproliferative Assays
2.4. Gene Expression Data Analysis
2.5. Identification of Drug-Specific UFGs
2.6. Network Propagation and Reactome Pathway Analyses
2.7. Multigene MVR Model Building
3. Results
3.1. Antiproliferative Effects of Eribulin, Paclitaxel and Vinorelbine against 100 CCLE Cell Lines
3.2. Gene Expression Analysis of Most Sensitive versus Least Sensitive Cell Line Quartiles
3.3. Identification of Unique Fingerprint Genes (UFGs) for Eribulin and Vinorelbine
3.4. Molecular Interactions Associated with Eribulin and Vinorelbine UFG Sets
3.5. Reactome Pathways Associated with Eribulin and Vinorelbine Response
3.6. Multivariate Regression (MVR) Model Building to Predict Drug Sensitivities
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Florian, S.; Mitchison, T.J. Anti-microtubule drugs. Methods Mol. Biol. 2016, 1413, 403–421. [Google Scholar] [PubMed]
- Bates, D.; Eastman, A. Microtubule destabilising agents: Far more than just antimitotic anticancer drugs. Br. J. Clin. Pharmacol. 2017, 83, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Kaul, R.; Risinger, A.L.; Mooberry, S.L. Microtubule-targeting drugs: More than antimitotics. J. Nat. Prod. 2019, 82, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Čermák, V.; Dostál, V.; Jelínek, M.; Libusová, L.; Kovář, J.; Rösel, D.; Brábek, J. Microtubule-targeting agents and their impact on cancer treatment. Eur. J. Cell Biol. 2020, 99, 151075. [Google Scholar] [CrossRef]
- Towle, M.J.; Salvato, K.A.; Budrow, J.; Wels, B.F.; Kuznetsov, G.; Aalfs, K.K.; Welsh, S.; Zheng, W.; Seletsky, B.M.; Palme, M.H.; et al. In vitro and in vivo anticancer activities of synthetic macrocyclic ketone analogues of halichondrin B. Cancer Res. 2001, 61, 1013–1021. [Google Scholar]
- Kuznetsov, G.; Towle, M.J.; Cheng, H.; Kawamura, T.; TenDyke, K.; Liu, D.; Kishi, Y.; Yu, M.J.; Littlefield, B.A. Induction of morphological and biochemical apoptosis following prolonged mitotic blockage by halichondrin B macrocyclic ketone analog E7389. Cancer Res. 2004, 64, 5760–5766. [Google Scholar] [CrossRef]
- Jordan, M.A.; Kamath, K.; Manna, T.; Okouneva, T.; Miller, H.P.; Davis, C.; Littlefield, B.A.; Wilson, L. The primary antimitotic mechanism of action of the synthetic halichondrin E7389 is suppression of microtubule growth. Mol. Cancer Ther. 2005, 4, 1086–1095. [Google Scholar] [CrossRef]
- Yoshida, T.; Ozawa, Y.; Kimura, T.; Sato, Y.; Kuznetsov, G.; Xu, S.; Uesugi, M.; Agoulnik, S.; Taylor, N.; Funahashi, Y.; et al. Eribulin mesilate suppresses experimental metastasis of breast cancer cells by reversing phenotype from epithelial-mesenchymal transition (EMT) to mesenchymal-epithelial transition (MET) states. Br. J. Cancer 2014, 110, 1497–1505. [Google Scholar] [CrossRef]
- Funahashi, Y.; Okamoto, K.; Adachi, Y.; Semba, T.; Uesugi, M.; Ozawa, Y.; Tohyama, O.; Uehara, T.; Kimura, T.; Watanabe, H.; et al. Eribulin mesylate reduces tumor microenvironment abnormality by vascular remodeling in preclinical human breast cancer models. Cancer Sci. 2014, 105, 1334–1342. [Google Scholar] [CrossRef]
- Goto, W.; Kashiwagi, S.; Asano, Y.; Takada, K.; Morisaki, T.; Fujita, H.; Takashima, T.; Ohsawa, M.; Hirakawa, K.; Ohira, M. Eribulin promotes antitumor immune responses in patients with locally advanced or metastatic breast cancer. Anticancer Res. 2018, 38, 2929–2938. [Google Scholar]
- Dybdal-Hargreaves, N.F.; Risinger, A.L.; Mooberry, S.L. Eribulin mesylate: Mechanism of action of a unique microtubule-targeting agent. Clin. Cancer Res. 2015, 21, 2445–2452. [Google Scholar] [CrossRef]
- Cortes, J.; Schöffski, P.; Littlefield, B.A. Multiple modes of action of eribulin mesylate: Emerging data and clinical implications. Cancer Treat. Rev. 2018, 70, 190–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Shaughnessy, J.; Kaklamani, V.; Kalinsky, K. Perspectives on the mechanism of action and clinical application of eribulin for metastatic breast cancer. Future Oncol. 2019, 15, 1641–1653. [Google Scholar] [CrossRef] [PubMed]
- Barretina, J.; Caponigro, G.; Stransky, N.; Venkatesan, K.; Margolin, A.A.; Kim, S.; Wilson, C.J.; Lehár, J.; Kryukov, G.V.; Sonkin, D.; et al. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature 2012, 483, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Tsherniak, A.; Vazquez, F.; Montgomery, P.G.; Weir, B.A.; Kryukov, G.; Cowley, G.S.; Gill, S.; Harrington, W.F.; Pantel, S.; Krill-Burger, J.M.; et al. Defining a cancer dependency map. Cell 2017, 170, 564–576. [Google Scholar] [CrossRef]
- Data4Cure, Inc. Data4Cure Biomedical Intelligence Cloud. 2022. Available online: www.data4cure.com (accessed on 3 August 2021).
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Statist. Soc. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Cowen, L.; Ideker, T.; Raphael, B.J.; Sharan, R. Network propagation: A universal amplifier of genetic associations. Nat. Rev. Genet. 2017, 18, 551–562. [Google Scholar] [CrossRef]
- Fabregat, A.; Sidiropoulos, K.; Viteri, G.; Forner, O.; Marin-Garcia, P.; Arnau, V.; D’Eustachio, P.; Stein, L.; Hermjakob, H. Reactome pathway analysis: A high-performance in-memory approach. BMC Bioinform. 2017, 18, 142. [Google Scholar] [CrossRef]
- Jassal, B.; Matthews, L.; Viteri, G.; Gong, C.; Lorente, P.; Fabregat, A.; Sidiropoulos, K.; Cook, J.; Gillespie, M.; Haw, R.; et al. The reactome pathway knowledgebase. Nucleic Acids Res. 2020, 48, D498–D503. [Google Scholar] [CrossRef]
- Kawano, S.; Asano, M.; Adachi, Y.; Matsui, J. Antimitotic and non-mitotic effects of eribulin mesilate in soft tissue sarcoma. Anticancer Res. 2016, 36, 1553–1561. [Google Scholar] [PubMed]
- Kitahara, H.; Hirai, M.; Kato, K.; Bou-Gharios, G.; Nakamura, H.; Kawashiri, S. Eribulin sensitizes oral squamous cell carcinoma cells to cetuximab via induction of mesenchymal-to-epithelial transition. Oncol. Rep. 2016, 36, 3139–3144. [Google Scholar] [CrossRef] [Green Version]
- Bai, R.L.; Paull, K.D.; Herald, C.L.; Malspeis, L.; Pettit, G.R.; Hamel, E. Halichondrin B and homohalichondrin B, marine natural products binding in the vinca domain of tubulin. Discovery of tubulin-based mechanism of action by analysis of differential cytotoxicity data. J. Biol. Chem. 1991, 266, 15882–15889. [Google Scholar] [CrossRef]
- Shoemaker, R.H. The NCI60 human tumour cell line anticancer drug screen. Nat. Rev. Cancer 2006, 6, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Y.; Zhang, Z.; Zhao, Z.; Lv, Q. A four-gene signature predicts the efficacy of paclitaxel-based neoadjuvant therapy in human epidermal growth factor receptor 2-negative breast cancer. J. Cell Biochem. 2019, 120, 6046–6056. [Google Scholar] [CrossRef]
- Bomane, A.; Gonçalves, A.; Ballester, P.J. Paclitaxel response can be predicted with interpretable multi-variate classifiers exploiting DNA-methylation and miRNA data. Front. Genet. 2019, 10, 1041. [Google Scholar] [CrossRef]
- Feng, X.; Wang, E.; Cui, Q. Gene expression-based predictive markers for paclitaxel treatment in ER+ and ER- breast cancer. Front. Genet. 2019, 10, 156. [Google Scholar] [CrossRef]
- Pusztai, L. Markers predicting clinical benefit in breast cancer from microtubule-targeting agents. Ann. Oncol. 2007, 18 (Suppl. 12), xii15–xii20. [Google Scholar] [CrossRef]
- Ben-Hamo, R.; Jacob Berger, A.; Gavert, N.; Miller, M.; Pines, G.; Oren, R.; Pikarsky, E.; Benes, C.H.; Neuman, T.; Zwang, Y.; et al. Predicting and affecting response to cancer therapy based on pathway-level biomarkers. Nat. Commun. 2020, 11, 3296. [Google Scholar] [CrossRef]
- Dorman, S.N.; Baranova, K.; Knoll, J.H.; Urquhart, B.L.; Mariani, G.; Carcangiu, M.L.; Rogan, P.K. Genomic signatures for paclitaxel and gemcitabine resistance in breast cancer derived by machine learning. Mol. Oncol. 2016, 10, 85–100. [Google Scholar] [CrossRef]
- Nogales, E.; Grayer Wolf, S.; Khan, I.A.; Ludueña, R.F.; Downing, K.H. Structure of tubulin at 6.5 Å and location of the taxol-binding site. Nature 1995, 375, 424–427. [Google Scholar] [CrossRef] [PubMed]
- Jordan, M.A.; Wilson, L. Microtubules as a target for anticancer drugs. Nat. Rev. Cancer 2004, 4, 253–265. [Google Scholar] [CrossRef]
- Smith, J.A.; Wilson, L.; Azarenko, O.; Zhu, X.; Lewis, B.M.; Littlefield, B.A.; Jordan, M.A. Eribulin binds at microtubule ends to a single site on tubulin to suppress dynamic instability. Biochemistry 2010, 49, 1331–1337. [Google Scholar] [CrossRef] [PubMed]
- Doodhi, H.; Prota, A.E.; Rodríguez-García, R.; Xiao, H.; Custar, D.W.; Bargsten, K.; Katrukha, E.A.; Hilbert, M.; Hua, S.; Jiang, K.; et al. Termination of protofilament elongation by eribulin induces lattice defects that promote microtubule catastrophes. Curr. Biol. 2016, 26, 1713–1721. [Google Scholar] [CrossRef] [PubMed]
- O’Rourke, B.; Yang, C.P.; Sharp, D.; Horwitz, S.B. Eribulin disrupts EB1-microtubule plus-tip complex formation. Cell Cycle 2014, 13, 3218–3221. [Google Scholar] [CrossRef]
- Chanez, B.; Gonçalves, A.; Badache, A.; Verdier-Pinard, P. Eribulin targets a ch-TOG-dependent directed migration of cancer cells. Oncotarget 2015, 6, 41667–41678. [Google Scholar] [CrossRef]
- Akhmanova, A.; Steinmetz, M.O. Tracking the ends: A dynamic protein network controls the fate of microtubule tips. Nat. Rev. Mol. Cell Biol. 2008, 9, 309–322. [Google Scholar] [CrossRef]
- Leterrier, C.; Vacher, H.; Fache, M.P.; d’Ortoli, S.A.; Castets, F.; Autillo-Touati, A.; Dargent, B. End-binding proteins EB3 and EB1 link microtubules to ankyrin G in the axon initial segment. Proc. Natl. Acad. Sci. USA 2011, 108, 8826–8831. [Google Scholar] [CrossRef]
- Cao, D.; Su, Z.; Wang, W.; Wu, H.; Liu, X.; Akram, S.; Qin, B.; Zhou, J.; Zhuang, X.; Adams, G.; et al. Signaling scaffold protein IQGAP1 interacts with microtubule plus-end tracking protein SKAP and links dynamic microtubule plus-end to steer cell migration. J. Biol. Chem. 2015, 290, 23766–23780. [Google Scholar] [CrossRef]
- Dybdal-Hargreaves, N.F.; Risinger, A.L.; Mooberry, S.L. Regulation of E-cadherin localization by microtubule targeting agents: Rapid promotion of cortical E-cadherin through p130Cas/Src inhibition by eribulin. Oncotarget 2017, 9, 5545–5561. [Google Scholar] [CrossRef]
- Kaul, R.; Risinger, A.L.; Mooberry, S.L. Eribulin rapidly inhibits TGF-β-induced Snail expression and can induce Slug expression in a Smad4-dependent manner. Br. J. Cancer 2019, 121, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Tommerup, N.; Ming Wang, S.; Hansen, L. A novel primate specific gene, CEI, is located in the homeobox gene IRXA2 promoter in Homo sapiens. Gene 2006, 371, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Sato, A.; Khadka, D. Mechanism of activation of the Formin protein Daam1. Proc. Natl. Acad. Sci. USA 2008, 105, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Arroyo Hornero, R.; Georgiadis, C.; Hua, P.; Trzupek, D.; He, L.-Z.; Qasim, W.; Todd, J.A.; Ferreira, R.C.; Wood, K.J.; Issa, F.; et al. CD70 expression determines the therapeutic efficacy of expanded human regulatory T cells. Commun. Biol. 2020, 3, 375. [Google Scholar] [CrossRef]
- Singh, D.R.; Kanvinde, P.; King, C.; Pasquale, E.B.; Hristova, K. The EphA2 receptor is activated through induction of distinct, ligand-dependent oligomeric structures. Commun. Biol. 2018, 1, 15. [Google Scholar] [CrossRef]
- Rodrigues, N.R.; Theodosiou, A.M.; Nesbit, M.A.; Campbell, L.; Tandle, A.T.; Saranath, D.; Davies, K.E. Characterization of Ngef, a novel member of the Dbl family of genes expressed predominantly in the caudate nucleus. Genomics 2000, 65, 53–61. [Google Scholar] [CrossRef]
- Bilban, M.; Heintel, D.; Scharl, T. Deregulated expression of fat and muscle genes in B-cell chronic lymphocytic leukemia with high lipoprotein lipase expression. Leukemia 2006, 20, 1080–1088. [Google Scholar] [CrossRef]
- Hsu, C.C.; Leu, Y.W.; Tseng, M.J.; Lee, K.-D.; Kuo, T.-Y.; Yen, J.-Y.; Lai, Y.-L.; Hung, Y.-C.; Sun, W.-S.; Chen, C.-M.; et al. Functional characterization of Trip10 in cancer cell growth and survival. J. Biomed. Sci. 2011, 18, 12. [Google Scholar] [CrossRef]
- Iftikhar, R.; Penrose, H.M.; King, A.N.; Kim, Y.; Ruiz, E.; Kandil, E.; Machado, H.L.; Savkovic, S.D. FOXO3 expression in macrophages is lowered by a high-fat diet and regulates colonic inflammation and tumorigenesis. Metabolites 2022, 12, 250. [Google Scholar] [CrossRef]








| Compound | Mean, nM | SD | SEM | Median, nM |
|---|---|---|---|---|
| Eribulin 2 | 20.1 | 66.2 | 6.6 | 1.6 |
| Paclitaxel 2 | 31.0 | 106.9 | 10.7 | 10.7 |
| Vinorelbine | 39.9 | 65.2 | 6.5 | 23.9 |
| Eribulin | Vinorelbine |
|---|---|
| Up (7): ARRB1, C5ORF38, DAAM1, GPR157, IRX2, KRT16, OSBPL1A | Up (2): PREX1, SH2B2 |
| Down (2): BICC1, CD70 | Down (11): EPHA2, GSTT2, GSTT2B, NGEF, PEAR1, PRSS3, RAP1GAP2, SEPTIN10, STEAP2, TRIP10, VSIG10 |
| Drug | Reactome Pathway 2 | p-Value | q-Value | Genes in Pathway | Over-Lapping Genes | Shared 3 |
|---|---|---|---|---|---|---|
| Eribulin | RHO GTPase Effectors | 0.000010 | 0.012839 | 251 | 10 | |
| Eribulin | RHO GTPases Activate Formins | 0.000016 | 0.012839 | 114 | 7 | |
| Eribulin | Signaling by Rho GTPases | 0.000045 | 0.019422 | 363 | 11 | |
| Eribulin | Toll Like Receptor 9 (TLR9) Cascade | 0.000047 | 0.019422 | 92 | 6 | |
| Eribulin | MyD88 cascade initiated on plasma membrane | 0.000343 | 0.057053 | 85 | 5 | |
| Eribulin | Toll Like Receptor 10 (TLR10) Cascade | 0.000343 | 0.057053 | 85 | 5 | |
| Eribulin | Toll Like Receptor 5 (TLR5) Cascade | 0.000343 | 0.057053 | 85 | 5 | |
| Eribulin | TRAF6 mediated induction of NFkB and MAP kinases upon TLR7/8 or 9 activation | 0.000363 | 0.057053 | 86 | 5 | |
| Eribulin | MyD88 dependent cascade initiated on endosome | 0.000403 | 0.057053 | 88 | 5 | |
| Eribulin | Toll Like Receptor 7/8 (TLR7/8) Cascade | 0.000403 | 0.057053 | 88 | 5 | |
| Eribulin | Toll-Like Receptors Cascades | 0.000514 | 0.057053 | 142 | 6 | |
| Eribulin | MyD88: Mal cascade initiated on plasma membrane | 0.000574 | 0.057053 | 95 | 5 | |
| Eribulin | Toll Like Receptor 2 (TLR2) Cascade | 0.000574 | 0.057053 | 95 | 5 | |
| Eribulin | Toll Like Receptor TLR1:TLR2 Cascade | 0.000574 | 0.057053 | 95 | 5 | |
| Eribulin | Toll Like Receptor TLR6:TLR2 Cascade | 0.000574 | 0.057053 | 95 | 5 | |
| Eribulin | Immune System | 0.000655 | 0.057053 | 1232 | 19 | X |
| Eribulin | MAP kinase activation in TLR cascade | 0.000674 | 0.057053 | 56 | 4 | |
| Eribulin | MyD88-independent TLR3/TLR4 cascade | 0.000694 | 0.057053 | 99 | 5 | |
| Eribulin | Toll Like Receptor 3 (TLR3) Cascade | 0.000694 | 0.057053 | 99 | 5 | |
| Eribulin | TRIF-mediated TLR3/TLR4 signaling | 0.000694 | 0.057053 | 99 | 5 | |
| Eribulin | Cell Cycle | 0.001075 | 0.083265 | 523 | 11 | |
| Eribulin | Cell Cycle, Mitotic | 0.001134 | 0.083265 | 445 | 10 | |
| Eribulin | Signaling by Interleukins | 0.001164 | 0.083265 | 111 | 5 | X |
| Eribulin | Activated TLR4 signalling | 0.001312 | 0.086320 | 114 | 5 | |
| Eribulin | G2/M Transition | 0.001312 | 0.086320 | 114 | 5 | |
| Eribulin | Mitotic G2-G2/M phases | 0.001418 | 0.089698 | 116 | 5 | |
| Vinorelbine | Signalling by NGF | 4.383506 × 10−12 | 7.210868 × 10−9 | 288 | 18 | |
| Vinorelbine | Signaling by SCF-KIT | 6.752605 × 10−11 | 5.554018 × 10−8 | 144 | 13 | |
| Vinorelbine | Signaling by FGFR3 | 3.228850 × 10−10 | 8.992552 × 10−8 | 163 | 13 | |
| Vinorelbine | Signaling by FGFR4 | 3.228850 × 10−10 | 8.992552 × 10−8 | 163 | 13 | |
| Vinorelbine | Signaling by FGFR1 | 3.485981 × 10−10 | 8.992552 × 10−8 | 164 | 13 | |
| Vinorelbine | DAP12 signaling | 3.761623 × 10−10 | 8.992552 × 10−8 | 165 | 13 | |
| Vinorelbine | Signaling by FGFR2 | 4.056969 × 10−10 | 8.992552 × 10−8 | 166 | 13 | |
| Vinorelbine | Signaling by FGFR | 4.373277 × 10−10 | 8.992552 × 10−8 | 167 | 13 | |
| Vinorelbine | NGF signalling via TRKA from the plasma membrane | 5.528565 × 10−10 | 1.010499 × 10−7 | 207 | 14 | |
| Vinorelbine | DAP12 interactions | 1.111051 × 10−9 | 1.661526 × 10−7 | 180 | 13 | |
| Vinorelbine | Signaling by EGFR | 1.111051 × 10−9 | 1.661526 × 10−7 | 180 | 13 | |
| Vinorelbine | Signaling by the B Cell Receptor (BCR) | 1.309948 × 10−9 | 1.673749 × 10−7 | 221 | 14 | |
| Vinorelbine | Downstream signaling of activated FGFR1 | 1.627962 × 10−9 | 1.673749 × 10−7 | 150 | 12 | |
| Vinorelbine | Downstream signaling of activated FGFR2 | 1.627962 × 10−9 | 1.673749 × 10−7 | 150 | 12 | |
| Vinorelbine | Downstream signaling of activated FGFR3 | 1.627962 × 10−9 | 1.673749 × 10−7 | 150 | 12 | |
| Vinorelbine | Downstream signaling of activated FGFR4 | 1.627962 × 10−9 | 1.673749 × 10−7 | 150 | 12 | |
| Vinorelbine | Interleukin-3, 5 and GM-CSF signaling | 1.767887 × 10−9 | 1.710691 × 10−7 | 45 | 8 | |
| Vinorelbine | Signaling by ERBB4 | 2.205662 × 10−9 | 2.015730 × 10−7 | 154 | 12 | |
| Vinorelbine | Downstream signal transduction | 3.945920 × 10−9 | 3.416336 × 10−7 | 162 | 12 | |
| Vinorelbine | Signaling by ERBB2 | 4.540573 × 10−9 | 3.734621 × 10−7 | 164 | 12 | |
| Vinorelbine | PI3K/AKT activation | 6.543594 × 10−9 | 5.125815 × 10−7 | 103 | 10 | |
| Vinorelbine | Diseases of signal transduction | 1.515872 × 10−8 | 1.133459 × 10−6 | 267 | 14 | |
| Vinorelbine | Signaling by PDGF | 1.780926 × 10−8 | 1.273749 × 10−6 | 185 | 12 | |
| Vinorelbine | Role of LAT2/NTAL/LAB on calcium mobilization | 2.254580 × 10−8 | 1.545327 × 10−6 | 151 | 11 | |
| Vinorelbine | Downstream signaling events of B Cell Receptor (BCR) | 7.714714 × 10−8 | 4.001226 × 10−6 | 170 | 11 | |
| Vinorelbine | PI-3K cascade:FGFR1 | 7.783541 × 10−8 | 4.001226 × 10−6 | 100 | 9 | |
| Vinorelbine | PI-3K cascade:FGFR2 | 7.783541 × 10−8 | 4.001226 × 10−6 | 100 | 9 | |
| Vinorelbine | PI-3K cascade:FGFR3 | 7.783541 × 10−8 | 4.001226 × 10−6 | 100 | 9 | |
| Vinorelbine | PI-3K cascade:FGFR4 | 7.783541 × 10−8 | 4.001226 × 10−6 | 100 | 9 | |
| Vinorelbine | PI3K events in ERBB2 signaling | 7.783541 × 10−8 | 4.001226 × 10−6 | 100 | 9 | |
| Vinorelbine | PI3K events in ERBB4 signaling | 7.783541 × 10−8 | 4.001226 × 10−6 | 100 | 9 | |
| Vinorelbine | PIP3 activates AKT signaling | 7.783541 × 10−8 | 4.001226 × 10−6 | 100 | 9 | |
| Vinorelbine | GAB1 signalosome | 1.096689 × 10−7 | 5.466829 × 10−6 | 104 | 9 | |
| Vinorelbine | Fc epsilon receptor (FCERI) signaling | 1.417907 × 10−7 | 6.860169 × 10−6 | 223 | 12 | |
| Vinorelbine | Immune System | 1.922234 × 10−7 | 8.825209 × 10−6 | 1232 | 27 | X |
| Vinorelbine | Signaling by Interleukins | 1.931353 × 10−7 | 8.825209 × 10−6 | 111 | 9 | X |
| Vinorelbine | PI3K/AKT Signaling in Cancer | 3.077967 × 10−7 | 0.000014 | 85 | 8 | |
| Vinorelbine | Innate Immune System | 8.845427 × 10−7 | 0.000038 | 689 | 19 | |
| Vinorelbine | Adaptive Immune System | 2.458655 × 10−6 | 0.000104 | 665 | 18 | |
| Vinorelbine | Cytokine Signaling in Immune system | 4.429201 × 10−6 | 0.000182 | 308 | 12 | |
| Vinorelbine | CD28 costimulation | 4.691035 × 10−6 | 0.000187 | 32 | 5 | |
| Vinorelbine | Regulation of mRNA stability by proteins that bind AU-rich elements | 4.785766 × 10−6 | 0.000187 | 86 | 7 | |
| Vinorelbine | Regulation of KIT signaling | 6.232763 × 10−6 | 0.000238 | 16 | 4 | |
| Vinorelbine | Insulin receptor signalling cascade | 8.078116 × 10−6 | 0.000302 | 93 | 7 | |
| Vinorelbine | Regulation of signaling by CBL | 0.000010 | 0.000379 | 18 | 4 | |
| Vinorelbine | CD28 dependent PI3K/Akt signaling | 0.000020 | 0.000711 | 21 | 4 | |
| Vinorelbine | HuR stabilizes mRNA | 0.000026 | 0.000922 | 8 | 3 | |
| Vinorelbine | Constitutive Signaling by AKT1 E17K in Cancer | 0.000035 | 0.001189 | 24 | 4 | |
| Vinorelbine | Signaling by Insulin receptor | 0.000036 | 0.001223 | 117 | 7 | |
| Vinorelbine | GPVI-mediated activation cascade | 0.000040 | 0.001291 | 49 | 5 | |
| Vinorelbine | Interleukin-2 signaling | 0.000040 | 0.001291 | 49 | 5 | |
| Vinorelbine | SHC-related events | 0.000041 | 0.001299 | 25 | 4 | |
| Vinorelbine | VEGFR2 mediated vascular permeability | 0.000048 | 0.001497 | 26 | 4 | |
| Vinorelbine | Signal Transduction | 0.000055 | 0.001666 | 2260 | 33 | |
| Vinorelbine | Integrin alphaIIb beta3 signaling | 0.000056 | 0.001683 | 27 | 4 | |
| Vinorelbine | Interleukin receptor SHC signaling | 0.000065 | 0.001917 | 28 | 4 | |
| Vinorelbine | IRS-related events | 0.000070 | 0.002024 | 89 | 6 | |
| Vinorelbine | Interleukin-6 signaling | 0.000076 | 0.002165 | 11 | 3 | |
| Vinorelbine | IGF1R signaling cascade | 0.000090 | 0.002460 | 93 | 6 | |
| Vinorelbine | Signaling by Type 1 Insulin-like Growth Factor 1 Receptor (IGF1R) | 0.000090 | 0.002460 | 93 | 6 | |
| Vinorelbine | Disease | 0.000106 | 0.002860 | 714 | 16 | |
| Vinorelbine | SHC1 events in ERBB2 signaling | 0.000112 | 0.002969 | 32 | 4 | |
| Vinorelbine | Signalling to RAS | 0.000143 | 0.003724 | 34 | 4 | |
| Vinorelbine | Signal attenuation | 0.000166 | 0.004255 | 14 | 3 | |
| Vinorelbine | Signalling to STAT3 | 0.000187 | 0.004720 | 3 | 2 | |
| Vinorelbine | TP53 Regulates Metabolic Genes | 0.000195 | 0.004720 | 68 | 5 | |
| Vinorelbine | Transcriptional Regulation by TP53 | 0.000195 | 0.004720 | 68 | 5 | |
| Vinorelbine | VEGFA-VEGFR2 Pathway | 0.000195 | 0.004720 | 107 | 6 | |
| Vinorelbine | Platelet Aggregation (Plug Formation) | 0.000200 | 0.004756 | 37 | 4 | |
| Vinorelbine | Constitutive Signaling by EGFRvIII | 0.000206 | 0.004768 | 15 | 3 | |
| Vinorelbine | Signaling by EGFRvIII in Cancer | 0.000206 | 0.004768 | 15 | 3 | |
| Vinorelbine | Costimulation by the CD28 family | 0.000272 | 0.006221 | 73 | 5 | |
| Vinorelbine | Signaling by VEGF | 0.000289 | 0.006514 | 115 | 6 | |
| Vinorelbine | Platelet activation, signaling and aggregation | 0.000340 | 0.007564 | 221 | 8 | |
| Vinorelbine | SHC activation | 0.000372 | 0.008165 | 4 | 2 | |
| Vinorelbine | Signalling to ERKs | 0.000393 | 0.008513 | 44 | 4 | |
| Vinorelbine | Constitutive Signaling by Ligand-Responsive EGFR Cancer Variants | 0.000428 | 0.008920 | 19 | 3 | |
| Vinorelbine | Signaling by EGFR in Cancer | 0.000428 | 0.008920 | 19 | 3 | |
| Vinorelbine | Signaling by Ligand-Responsive EGFR Variants in Cancer | 0.000428 | 0.008920 | 19 | 3 | |
| Vinorelbine | G beta:gamma signalling through PI3Kgamma | 0.000550 | 0.011319 | 48 | 4 | |
| Vinorelbine | IRS-mediated signalling | 0.000583 | 0.011844 | 86 | 5 | |
| Vinorelbine | SHC1 events in EGFR signaling | 0.000669 | 0.013264 | 22 | 3 | |
| Vinorelbine | SHC-mediated signalling | 0.000669 | 0.013264 | 22 | 3 | |
| Vinorelbine | G-protein beta:gamma signalling | 0.000694 | 0.013600 | 51 | 4 | |
| Vinorelbine | IRS-related events triggered by IGF1R | 0.000718 | 0.013900 | 90 | 5 | |
| Vinorelbine | Growth hormone receptor signaling | 0.000870 | 0.016635 | 24 | 3 | |
| Vinorelbine | Activation of BH3-only proteins | 0.000983 | 0.018369 | 25 | 3 | |
| Vinorelbine | SHC-related events triggered by IGF1R | 0.000983 | 0.018369 | 25 | 3 | |
| Vinorelbine | Antigen activates B Cell Receptor (BCR) leading to generation of second messengers | 0.001059 | 0.019578 | 57 | 4 | |
| Vinorelbine | Signaling by Leptin | 0.001105 | 0.020001 | 26 | 3 | |
| Vinorelbine | CLEC7A (Dectin-1) signaling | 0.001106 | 0.020001 | 99 | 5 | |
| Vinorelbine | Constitutive Signaling by Aberrant PI3K in Cancer | 0.001367 | 0.024339 | 61 | 4 | |
| Vinorelbine | SHC1 events in ERBB4 signaling | 0.001376 | 0.024339 | 28 | 3 | |
| Vinorelbine | Apoptosis | 0.001649 | 0.028564 | 160 | 6 | |
| Vinorelbine | GRB2 events in ERBB2 signaling | 0.001686 | 0.028564 | 30 | 3 | |
| Vinorelbine | Hemostasis | 0.001698 | 0.028564 | 497 | 11 | |
| Vinorelbine | Negative regulation of the PI3K/AKT network | 0.001702 | 0.028564 | 8 | 2 | |
| Vinorelbine | Release of eIF4E | 0.001702 | 0.028564 | 8 | 2 | |
| Vinorelbine | Programmed Cell Death | 0.001813 | 0.030122 | 163 | 6 | |
| Vinorelbine | TGF-beta receptor signaling activates SMADs | 0.002037 | 0.033502 | 32 | 3 | |
| Vinorelbine | AKT phosphorylates targets in the nucleus | 0.002177 | 0.035451 | 9 | 2 | |
| Vinorelbine | Glutathione conjugation | 0.002228 | 0.035926 | 33 | 3 | |
| Vinorelbine | PI3K Cascade | 0.002276 | 0.036351 | 70 | 4 | |
| Vinorelbine | EPHA-mediated growth cone collapse | 0.002429 | 0.038426 | 34 | 3 | |
| Vinorelbine | Signaling by TGF-beta Receptor Complex | 0.002524 | 0.039538 | 72 | 4 | |
| Vinorelbine | C-type lectin receptors (CLRs) | 0.002888 | 0.044825 | 123 | 5 | |
| Vinorelbine | S6K1-mediated signalling | 0.003291 | 0.050602 | 11 | 2 | |
| Vinorelbine | Intrinsic Pathway for Apoptosis | 0.003348 | 0.050997 | 38 | 3 | |
| Vinorelbine | Chk1/Chk2(Cds1) mediated inactivation of Cyclin B:Cdk1 complex | 0.003929 | 0.059302 | 12 | 2 | |
| Vinorelbine | p75 NTR receptor-mediated signalling | 0.004223 | 0.063160 | 83 | 4 | |
| Vinorelbine | deactivation of the beta-catenin transactivating complex | 0.004454 | 0.066013 | 42 | 3 | |
| Vinorelbine | Downregulation of ERBB2:ERBB3 signaling | 0.004620 | 0.066667 | 13 | 2 | |
| Vinorelbine | mTORC1-mediated signalling | 0.004620 | 0.066667 | 13 | 2 | |
| Vinorelbine | Regulation of Rheb GTPase activity by AMPK | 0.004620 | 0.066667 | 13 | 2 | |
| Vinorelbine | TCF dependent signaling in response to WNT | 0.004891 | 0.069957 | 199 | 6 | |
| Vinorelbine | FCERI mediated MAPK activation | 0.004996 | 0.070851 | 87 | 4 | |
| Vinorelbine | Prolactin receptor signaling | 0.006156 | 0.086551 | 15 | 2 | |
| Vinorelbine | EPH-Ephrin signaling | 0.006567 | 0.091554 | 94 | 4 | |
| Vinorelbine | G2/M DNA damage checkpoint | 0.006999 | 0.095156 | 16 | 2 | |
| Vinorelbine | Rap1 signalling | 0.006999 | 0.095156 | 16 | 2 | |
| Vinorelbine | Spry regulation of FGF signaling | 0.006999 | 0.095156 | 16 | 2 | |
| Vinorelbine | Developmental Biology | 0.007292 | 0.098323 | 517 | 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sachdev, P.; Ronen, R.; Dutkowski, J.; Littlefield, B.A. Systematic Analysis of Genetic and Pathway Determinants of Eribulin Sensitivity across 100 Human Cancer Cell Lines from the Cancer Cell Line Encyclopedia (CCLE). Cancers 2022, 14, 4532. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers14184532
Sachdev P, Ronen R, Dutkowski J, Littlefield BA. Systematic Analysis of Genetic and Pathway Determinants of Eribulin Sensitivity across 100 Human Cancer Cell Lines from the Cancer Cell Line Encyclopedia (CCLE). Cancers. 2022; 14(18):4532. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers14184532
Chicago/Turabian StyleSachdev, Pallavi, Roy Ronen, Janusz Dutkowski, and Bruce A. Littlefield. 2022. "Systematic Analysis of Genetic and Pathway Determinants of Eribulin Sensitivity across 100 Human Cancer Cell Lines from the Cancer Cell Line Encyclopedia (CCLE)" Cancers 14, no. 18: 4532. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers14184532