Immune-Checkpoint Inhibitors (ICIs) in Metastatic Colorectal Cancer (mCRC) Patients beyond Microsatellite Instability
Abstract
:Simple Summary
Abstract
1. Introduction
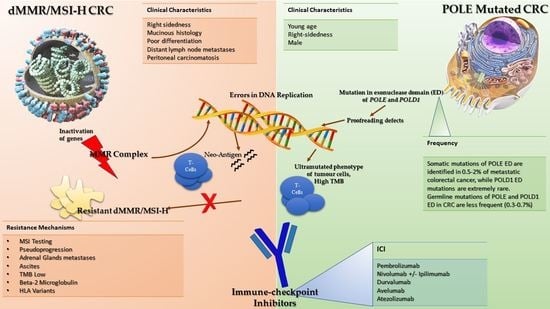
2. Mismatch Repair Complex and Microsatellite Status
3. ICIs in dMMR/MSI-H Metastatic Colorectal Cancer Patients
4. Resistance to ICIs in dMMR/MSI-H mCRC Patients
4.1. MSI Testing and Radiological Assessment
4.2. Site of Metastatic Spread
4.3. Immune-Related Molecular Biomarkers
5. A New Target Population for ICIs in pMMR/MSS CRC: POLE-Mutated Tumors
6. Potential Predictive Biomarkers of ICIs Benefit among dMMR/MSI-H mCRC Patients
6.1. Tumor Mutational Burden (TMB)
6.2. PD-L1 Expression and TILs
6.3. Gut Microbiota
7. Overcoming Resistance to ICIs in dMMR/MSI-H CRC: Future Perspectives
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1–Positive Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rittmeyer, A.; Barlesi, F.; Waterkamp, D.; Park, K.; Ciardiello, F.; von Pawel, J.; Gadgeel, S.M.; Hida, T.; Kowalski, D.M.; Dols, M.C.; et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial. Lancet 2017, 389, 255–265. [Google Scholar] [CrossRef]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef] [Green Version]
- Alemohammad, H.; Najafzadeh, B.; Asadzadeh, Z.; Baghbanzadeh, A.; Ghorbaninezhad, F.; Najafzadeh, A.; Safarpour, H.; Bernardini, R.; Brunetti, O.; Sonnessa, M.; et al. The importance of immune checkpoints in immune monitoring: A future paradigm shift in the treatment of cancer. Biomed. Pharmacother. 2021, 146, 112516. [Google Scholar] [CrossRef]
- Chen, D.S.; Mellman, I. Oncology Meets Immunology: The Cancer-Immunity Cycle. Immunity 2013, 39, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Antoniotti, C.; Rossini, D.; Pietrantonio, F.; Catteau, A.; Salvatore, L.; Lonardi, S.; Boquet, I.; Tamberi, S.; Marmorino, F.; Moretto, R.; et al. Upfront FOLFOXIRI plus bevacizumab with or without atezolizumab in the treatment of patients with metastatic colorectal cancer (AtezoTRIBE): A multicentre, open-label, randomised, controlled, phase 2 trial. Lancet Oncol. 2022, 23, 876–887. [Google Scholar] [CrossRef]
- Napolitano, S.; Martini, G.; Ciardiello, D.; Di Maio, M.; Normanno, N.; Avallone, A.; Martinelli, E.; Maiello, E.; Troiani, T.; Ciardiello, F. CAVE-2 (Cetuximab-AVElumab) mCRC: A Phase II Randomized Clinical Study of the Combination of Avelumab Plus Cetuximab as a Rechallenge Strategy in Pre-Treated RAS/BRAF Wild-Type mCRC Patients. Front. Oncol. 2022, 12, 940523. Available online: https://www.frontiersin.org/articles/10.3389/fonc.2022.940523 (accessed on 10 August 2022). [CrossRef]
- Gruppo Oncologico del Nord-Ovest. Phase II Study of AVELUMAB and CETUXIMAB and Modified FOLFOXIRI as Initial Therapy for RAS Wild-type Unresectable Metastatic Colorectal Cancer Patients. Clinical Trial Registration NCT04513951. Available online: https://clinicaltrials.gov/ct2/show/NCT04513951 (accessed on 9 August 2022).
- André, T.; Shiu, K.-K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab in Microsatellite-Instability–High Advanced Colorectal Cancer. N. Engl. J. Med. 2020, 383, 2207–2218. [Google Scholar] [CrossRef]
- Lenz, H.-J.; Van Cutsem, E.; Limon, M.L.; Wong, K.Y.M.; Hendlisz, A.; Aglietta, M.; García-Alfonso, P.; Neyns, B.; Luppi, G.; Cardin, D.B.; et al. First-Line Nivolumab Plus Low-Dose Ipilimumab for Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer: The Phase II CheckMate 142 Study. J. Clin. Oncol. 2021, 40, 161–170. [Google Scholar] [CrossRef]
- Boland, C.R.; Goel, A. Microsatellite instability in colorectal cancer. Gastroenterology 2010, 138, 2073.e3–2087.e3. [Google Scholar] [CrossRef]
- Dolcetti, R.; Viel, A.; Doglioni, C.; Russo, A.; Guidoboni, M.; Capozzi, E.; Vecchiato, N.; Macrì, E.; Fornasarig, M.; Boiocchi, M. High prevalence of activated intraepithelial cytotoxic T lymphocytes and increased neoplastic cell apoptosis in colorectal carcinomas with microsatellite instability. Am. J. Pathol. 1999, 154, 1805–1813. [Google Scholar] [CrossRef] [Green Version]
- Goldstein, J.; Tran, B.; Ensor, J.; Gibbs, P.; Wong, H.L.; Wong, S.F.; Vilar, E.; Tie, J.; Broaddus, R.; Kopetz, S.; et al. Multicenter retrospective analysis of metastatic colorectal cancer (CRC) with high-level microsatellite instability (MSI-H). Ann. Oncol. 2014, 25, 1032–1038. [Google Scholar] [CrossRef]
- Zhuo, N.; Liu, C.; Zhang, Q.; Li, J.; Zhang, X.; Gong, J.; Lu, M.; Peng, Z.; Zhou, J.; Wang, X.; et al. Characteristics and Prognosis of Acquired Resistance to Immune Checkpoint Inhibitors in Gastrointestinal Cancer. JAMA Netw. Open 2022, 5, e224637. [Google Scholar] [CrossRef]
- Koopman, M.; Kortman, G.A.M.; Mekenkamp, L.; Ligtenberg, M.J.L.; Hoogerbrugge, N.; Antonini, N.F.; Punt, C.J.A.; Van Krieken, J.H.J.M. Deficient mismatch repair system in patients with sporadic advanced colorectal cancer. Br. J. Cancer 2009, 100, 266–273. [Google Scholar] [CrossRef] [Green Version]
- Porkka, N.; Lahtinen, L.; Ahtiainen, M.; Böhm, J.P.; Kuopio, T.; Eldfors, S.; Mecklin, J.-P.; Seppälä, T.; Peltomäki, P. Epidemiological, clinical and molecular characterization of Lynch-like syndrome: A population-based study. Int. J. Cancer 2019, 145, 87–98. [Google Scholar] [CrossRef] [Green Version]
- Wensink, G.E.; Elferink, M.A.G.; May, A.M.; Mol, L.; Hamers, P.A.H.; Bakker, S.D.; Creemers, G.-J.; de Groot, J.W.B.; de Klerk, G.J.; Haberkorn, B.C.M.; et al. Survival of patients with deficient mismatch repair metastatic colorectal cancer in the pre-immunotherapy era. Br. J. Cancer 2021, 124, 399–406. [Google Scholar] [CrossRef]
- Cohen, R.; Meurisse, A.; Pudlarz, T.; Bennouna, J.; Tournigand, C.; De La Fouchardiere, C.; Tougeron, D.; Borg, C.; Mazard, T.; Chibaudel, B.; et al. One-year duration of nivolumab plus ipilimumab in patients (pts) with microsatellite instability-high/mismatch repair-deficient (MSI/dMMR) metastatic colorectal cancer (mCRC): Long-term follow-up of the GERCOR NIPICOL phase II study. J. Clin. Oncol. 2022, 40, 13. [Google Scholar] [CrossRef]
- Diaz, L.A.; Shiu, K.-K.; Kim, T.-W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab versus chemotherapy for microsatellite instability-high or mismatch repair-deficient metastatic colorectal cancer (KEYNOTE-177): Final analysis of a randomised, open-label, phase 3 study. Lancet Oncol. 2022, 23, 659–670. [Google Scholar] [CrossRef]
- Overman, M.J.; Lenz, H.-J.; Andre, T.; Aglietta, M.; Wong, M.K.; Luppi, G.; Van Cutsem, E.; McDermott, R.S.; Hendlisz, A.; Cardin, D.B.; et al. Nivolumab (NIVO) ± ipilimumab (IPI) in patients (pts) with microsatellite instability-high/mismatch repair-deficient (MSI-H/dMMR) metastatic colorectal cancer (mCRC): Five-year follow-up from CheckMate 142. J. Clin. Oncol. 2022, 40, 3510. [Google Scholar] [CrossRef]
- Le, D.T.; Kim, T.W.; Van Cutsem, E.; Geva, R.; Jäger, D.; Hara, H.; Burge, M.; O’Neil, B.; Kavan, P.; Yoshino, T.; et al. Phase II Open-Label Study of Pembrolizumab in Treatment-Refractory, Microsatellite Instability–High/Mismatch Repair–Deficient Metastatic Colorectal Cancer: KEYNOTE-164. J. Clin. Oncol. 2019, 38, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Durham, J.N.; Smith, K.N.; Wang, H.; Bartlett, B.R.; Aulakh, L.K.; Lu, S.; Kemberling, H.; Wilt, C.; Luber, B.S.; et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017, 357, 409–413. [Google Scholar] [CrossRef] [Green Version]
- FDA Approves Nivolumab Plus Low-Dose Ipilimumab for Second-Line Treatment of MSI-H/dMMR Metastatic Colorectal Cancer—The ASCO Post. Available online: https://ascopost.com/News/59044 (accessed on 6 January 2022).
- Bristol-Myers Squibb. A Phase 3 Randomized Clinical Trial of Nivolumab Alone, Nivolumab in Combination With Ipilimumab, or an Investigator’s Choice Chemotherapy in Participants With Microsatellite Instability High (MSI-H) or Mismatch Repair Deficient (dMMR) Metastatic Colorectal Cancer. Clinical Trial Registration NCT04008030. July 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT04008030 (accessed on 4 January 2022).
- Meeting Library | Safety and Efficacy of Anti–PD-1 Antibody Dostarlimab in Patients (Pts) with Mismatch Repair-Deficient (dMMR) Solid Cancers: Results from GARNET Study. Available online: https://meetinglibrary.asco.org/record/194077/abstract (accessed on 9 January 2022).
- Andre, T.; Amonkar, M.; Norquist, J.M.; Shiu, K.-K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.J.A.; Smith, D.; Garcia-Carbonero, R.; et al. Health-related quality of life in patients with microsatellite instability-high or mismatch repair deficient metastatic colorectal cancer treated with first-line pembrolizumab versus chemotherapy (KEYNOTE-177): An open-label, randomised, phase 3 trial. Lancet Oncol. 2021, 22, 665–677. [Google Scholar] [CrossRef]
- FDA. FDA Approves First-Line Immunotherapy for Patients with MSI-H/dMMR Metastatic Colorectal Cancer. 30 June 2020. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-line-immunotherapy-patients-msi-hdmmr-metastatic-colorectal-cancer (accessed on 6 January 2022).
- ESMO. EMA Recommends Extension of Indications for Pembrolizumab. Available online: https://www.esmo.org/oncology-news/ema-recommends-extension-of-indications-for-pembrolizumab4 (accessed on 6 January 2022).
- Tabernero, J.; Grothey, A.; Van Cutsem, E.; Yaeger, R.; Wasan, H.; Yoshino, T.; Desai, J.; Ciardiello, F.; Loupakis, F.; Hong, Y.S.; et al. Encorafenib Plus Cetuximab as a New Standard of Care for Previously Treated BRAF V600E–Mutant Metastatic Colorectal Cancer: Updated Survival Results and Subgroup Analyses from the BEACON Study. J. Clin. Oncol. 2021, 39, 273–284. [Google Scholar] [CrossRef] [PubMed]
- A Study of Encorafenib Plus Cetuximab Taken Together With Pembrolizumab Compared to Pembrolizumab Alone in People With Previously Untreated Metastatic Colorectal Cancer—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT05217446 (accessed on 7 June 2022).
- Russo, M.; Crisafulli, G.; Sogari, A.; Reilly, N.M.; Arena, S.; Lamba, S.; Bartolini, A.; Amodio, V.; Magrì, A.; Novara, L.; et al. Adaptive mutability of colorectal cancers in response to targeted therapies. Science 2019, 366, 1473–1480. [Google Scholar] [CrossRef]
- Morris, V.K.; Parseghian, C.M.; Escano, M.; Johnson, B.; Raghav, K.P.S.; Dasari, A.; Huey, R.; Overman, M.J.; Willis, J.; Lee, M.S.; et al. Phase I/II trial of encorafenib, cetuximab, and nivolumab in patients with microsatellite stable (MSS), BRAFV600E metastatic colorectal cancer. J. Clin. Oncol. 2022, 40, 3598. [Google Scholar] [CrossRef]
- Ludford, K.; Cohen, R.; Svrcek, M.; Foo, W.C.; Colle, R.; Parc, Y.; Thomas, J.V.; Morris, V.K.; Kopetz, S.; Chang, G.J.; et al. Pathological Tumor Response Following Immune Checkpoint Blockade for Deficient Mismatch Repair Advanced Colorectal Cancer. JNCI J. Natl. Cancer Inst. 2020, 113, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Tie, J. Tailoring immunotherapy with liquid biopsy. Nat. Cancer 2020, 1, 857–859. [Google Scholar] [CrossRef]
- Fiz, F.; Viganò, L.; Gennaro, N.; Costa, G.; La Bella, L.; Boichuk, A.; Cavinato, L.; Sollini, M.; Politi, L.S.; Chiti, A.; et al. Radiomics of Liver Metastases: A Systematic Review. Cancers 2020, 12, 2881. [Google Scholar] [CrossRef]
- Sharma, P.; Hu-Lieskovan, S.; Wargo, J.A.; Ribas, A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 2017, 168, 707–723. [Google Scholar] [CrossRef] [PubMed]
- Hodi, F.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.; Rutkowski, P.; Cowey, C.; Lao, C.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Overall survival at 4 years of follow-up in a phase III trial of nivolumab plus ipilimumab combination therapy in advanced melanoma (CheckMate 067). Ann. Oncol. 2018, 29, viii735. [Google Scholar] [CrossRef]
- Cohen, R.; Hain, E.; Buhard, O.; Guilloux, A.; Bardier, A.; Kaci, R.; Bertheau, P.; Renaud, F.; Bibeau, F.; Fléjou, J.-F.; et al. Association of Primary Resistance to Immune Checkpoint Inhibitors in Metastatic Colorectal Cancer With Misdiagnosis of Microsatellite Instability or Mismatch Repair Deficiency Status. JAMA Oncol. 2019, 5, 551–555. [Google Scholar] [CrossRef] [Green Version]
- Luchini, C.; Bibeau, F.; Ligtenberg, M.; Singh, N.; Nottegar, A.; Bosse, T.; Miller, R.; Riaz, N.; Douillard, J.-Y.; Andre, F.; et al. ESMO recommendations on microsatellite instability testing for immunotherapy in cancer, and its relationship with PD-1/PD-L1 expression and tumour mutational burden: A systematic review-based approach. Ann. Oncol. 2019, 30, 1232–1243. [Google Scholar] [CrossRef] [Green Version]
- Seymour, L.; Bogaerts, J.; Perrone, A.; Ford, R.; Schwartz, L.H.; Mandrekar, S.; Lin, N.U.; Litière, S.; Dancey, J.; Chen, A.; et al. iRECIST: Guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017, 18, e143–e152. [Google Scholar] [CrossRef] [Green Version]
- Colle, R.; Radzik, A.; Cohen, R.; Pellat, A.; Lopez-Tabada, D.; Cachanado, M.; Duval, A.; Svrcek, M.; Menu, Y.; André, T. Pseudoprogression in patients treated with immune checkpoint inhibitors for microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer. Eur. J. Cancer 2020, 144, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.; Jonchère, V.; De La Fouchardière, C.; Ratovomanana, T.; Letourneur, Q.; Ayadi, M.; Armenoult, L.; Buisson, A.; Sarabi, M.; Pellat, A.; et al. Adrenal gland as a sanctuary site for immunotherapy in patients with microsatellite instability-high metastatic colorectal cancer. J. Immunother. Cancer 2021, 9, e001903. [Google Scholar] [CrossRef]
- Fucà, G.; Cohen, R.; Lonardi, S.; Shitara, K.; Elez, M.E.; Fakih, M.; Chao, J.; Klempner, S.J.; Emmett, M.; Jayachandran, P.; et al. Ascites and resistance to immune checkpoint inhibition in dMMR/MSI-H metastatic colorectal and gastric cancers. J. Immunother. Cancer 2022, 10, e004001. [Google Scholar] [CrossRef]
- Schrock, A.B.; Ouyang, C.; Sandhu, J.; Sokol, E.; Jin, D.; Ross, J.S.; Miller, V.A.; Lim, D.; Amanam, I.; Chao, J.; et al. Tumor mutational burden is predictive of response to immune checkpoint inhibitors in MSI-high metastatic colorectal cancer. Ann. Oncol. 2019, 30, 1096–1103. [Google Scholar] [CrossRef]
- D’Urso, C.M.; Wang, Z.G.; Cao, Y.; Tatake, R.; Zeff, R.A.; Ferrone, S. Lack of HLA class I antigen expression by cultured melanoma cells FO-1 due to a defect in B2m gene expression. J. Clin. Investig. 1991, 87, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Naranbhai, V.; Viard, M.; Dean, M.; Groha, S.; Braun, D.A.; Labaki, C.; Shukla, S.A.; Yuki, Y.; Shah, P.; Chin, K.; et al. HLA-A*03 and response to immune checkpoint blockade in cancer: An epidemiological biomarker study. Lancet Oncol. 2021, 23, 172–184. [Google Scholar] [CrossRef]
- Heitzer, E.; Tomlinson, I. Replicative DNA polymerase mutations in cancer. Curr. Opin. Genet. Dev. 2014, 24, 107–113. [Google Scholar] [CrossRef] [Green Version]
- Castellucci, E.; He, T.; Goldstein, D.Y.; Halmos, B.; Chuy, J. DNA Polymerase ε Deficiency Leading to an Ultramutator Phenotype: A Novel Clinically Relevant Entity. Oncologist 2017, 22, 497–502. [Google Scholar] [CrossRef] [Green Version]
- Palles, C.; Cazier, J.-B.; Howarth, K.M.; Domingo, E.; Jones, A.M.; Broderick, P.; Kemp, Z.; Spain, S.L.; Guarino, E.; Salguero, I.; et al. Germline mutations affecting the proofreading domains of POLE and POLD1 predispose to colorectal adenomas and carcinomas. Nat. Genet. 2012, 45, 136–144. [Google Scholar] [CrossRef] [Green Version]
- Briggs, S.; Tomlinson, I. Germline and somatic polymerase ϵ and δ mutations define a new class of hypermutated colorectal and endometrial cancers. J. Pathol. 2013, 230, 148–153. [Google Scholar] [CrossRef] [Green Version]
- Mur, P.; García-Mulero, S.; del Valle, J.; Magraner-Pardo, L.; Vidal, A.; Pineda, M.; Cinnirella, G.; Martín-Ramos, E.; Pons, T.; López-Doriga, A.; et al. Role of POLE and POLD1 in familial cancer. Genet. Med. 2020, 22, 2089–2100. [Google Scholar] [CrossRef]
- Domingo, E.; Freeman-Mills, L.; Rayner, E.; Glaire, M.; Briggs, S.; Vermeulen, L.; Fessler, E.; Medema, J.P.; Boot, A.; Morreau, H.; et al. Somatic POLE proofreading domain mutation, immune response, and prognosis in colorectal cancer: A retrospective, pooled biomarker study. Lancet Gastroenterol. Hepatol. 2016, 1, 207–216. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Gong, J.; Tu, T.Y.; Lee, P.P.; Fakih, M. Immune profiling of microsatellite instability-high and polymerase ε (POLE)-mutated metastatic colorectal tumors identifies predictors of response to anti-PD-1 therapy. J. Gastrointest. Oncol. 2018, 9, 404–415. [Google Scholar] [CrossRef]
- Gong, J.; Wang, C.; Lee, P.P.; Chu, P.; Fakih, M. Response to PD-1 Blockade in Microsatellite Stable Metastatic Colorectal Cancer Harboring a POLE Mutation. J. Natl. Compr. Cancer Netw. 2017, 15, 142–147. [Google Scholar] [CrossRef] [Green Version]
- Rousseau, B.; Bieche, I.; Pasmant, E.; Hamzaoui, N.; Leulliot, N.; Michon, L.; de Reynies, A.; Attignon, V.; Foote, M.B.; Masliah-Planchon, J.; et al. PD-1 Blockade in Solid Tumors with Defects in Polymerase Epsilon. Cancer Discov. 2022, 12, 1435–1448. [Google Scholar] [CrossRef]
- ESMO. Nivolumab Shows Promising Activity in Advanced Tumours with Rare Mutations. Available online: https://www.esmo.org/oncology-news/nivolumab-shows-promising-activity-in-advanced-tumours-with-rare-mutations (accessed on 7 January 2022).
- Kim, T.W. A Phase II Study of Durvalumab in Patients With Mismatch Repair Deficient or POLE Mutated Metastatic Colorectal Cancer. Clinical Trial Registration NCT03435107. August 2020. Available online: https://clinicaltrials.gov/ct2/show/NCT03435107 (accessed on 29 December 2021).
- Kim, T.W. A Phase II Study of Avelumab in Patients With Mismatch Repair Deficient or POLE Mutated Metastatic Colorectal Cancer. Clinical Trial Registration NCT03150706. April 2020. Available online: https://clinicaltrials.gov/ct2/show/NCT03150706 (accessed on 5 January 2022).
- Lau, D.; Cunningham, D.; Gillbanks, A.; Crux, R.; Powell, R.; Kalaitzaki, E.; Annels, N.E.; Sclafani, F.; Gerlinger, M.; Chau, I.; et al. POLEM: Avelumab plus fluoropyrimidine-based chemotherapy as adjuvant treatment for stage III dMMR or POLE exonuclease domain mutant colon cancer—A phase III randomized study. J. Clin. Oncol. 2019, 37, TPS3615. [Google Scholar] [CrossRef]
- Subbiah, V.; Solit, D.; Chan, T.; Kurzrock, R. The FDA approval of pembrolizumab for adult and pediatric patients with tumor mutational burden (TMB) ≥10: A decision centered on empowering patients and their physicians. Ann. Oncol. 2020, 31, 1115–1118. [Google Scholar] [CrossRef]
- Rousseau, B.; Foote, M.B.; Maron, S.B.; Diplas, B.H.; Lu, S.; Argilés, G.; Cercek, A.; Diaz, L.A. The Spectrum of Benefit from Checkpoint Blockade in Hypermutated Tumors. N. Engl. J. Med. 2021, 384, 1168–1170. [Google Scholar] [CrossRef]
- Salem, M.E.; Bodor, J.N.; Puccini, A.; Xiu, J.; Goldberg, R.M.; Grothey, A.; Korn, W.M.; Shields, A.F.; Worrilow, W.M.; Kim, E.S.; et al. Relationship between MLH1, PMS2, MSH2 and MSH6 gene-specific alterations and tumor mutational burden in 1057 microsatellite instability-high solid tumors. Int. J. Cancer 2020, 147, 2948–2956. [Google Scholar] [CrossRef]
- Lee, L.H.; Cavalcanti, M.S.; Segal, N.H.; Hechtman, J.F.; Weiser, M.R.; Smith, J.J.; Garcia-Aguilar, J.; Sadot, E.; Ntiamoah, P.; Markowitz, A.J.; et al. Patterns and prognostic relevance of PD-1 and PD-L1 expression in colorectal carcinoma. Mod. Pathol. 2016, 29, 1433–1442. [Google Scholar] [CrossRef] [Green Version]
- Marginean, E.C.; Melosky, B. Is There a Role for Programmed Death Ligand-1 Testing and Immunotherapy in Colorectal Cancer With Microsatellite Instability? Part II—The Challenge of Programmed Death Ligand-1 Testing and Its Role in Microsatellite Instability-High Colorectal Cancer. Arch. Pathol. Lab. Med. 2018, 142, 26–34. [Google Scholar] [CrossRef] [Green Version]
- Loupakis, F.; Depetris, I.; Biason, P.; Intini, R.; Prete, A.A.; Leone, F.; Lombardi, P.; Filippi, R.; Spallanzani, A.; Cascinu, S.; et al. Prediction of Benefit from Checkpoint Inhibitors in Mismatch Repair Deficient Metastatic Colorectal Cancer: Role of Tumor Infiltrating Lymphocytes. Oncologist 2020, 25, 481–487. [Google Scholar] [CrossRef] [Green Version]
- Bose, M.; Mukherjee, P. Role of Microbiome in Modulating Immune Responses in Cancer. Mediat. Inflamm. 2019, 2019, 4107917. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Zhang, X.; Zhang, X.; Zhang, Y.; Zheng, K.; Xiang, Q.; Chen, N.; Chen, Z.; Zhang, N.; Zhu, J.; et al. Antibiotic-Induced Disruption of Gut Microbiota Alters Local Metabolomes and Immune Responses. Front. Cell. Infect. Microbiol. 2019, 9, 99. Available online: https://www.frontiersin.org/articles/10.3389/fcimb.2019.00099 (accessed on 10 August 2022). [CrossRef] [Green Version]
- Cheng, W.Y.; Wu, C.-Y.; Yu, J. The role of gut microbiota in cancer treatment: Friend or foe? Gut 2020, 69, 1867–1876. [Google Scholar] [CrossRef]
- Sivan, A.; Corrales, L.; Hubert, N.; Williams, J.B.; Aquino-Michaels, K.; Earley, Z.M.; Benyamin, F.W.; Lei, Y.M.; Jabri, B.; Alegre, M.-L.; et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 2015, 350, 1084–1089. [Google Scholar] [CrossRef]
- Tahara, T.; Yamamoto, E.; Suzuki, H.; Maruyama, R.; Chung, W.; Garriga, J.; Jelinek, J.; Yamano, H.-O.; Sugai, T.; An, B.; et al. Fusobacterium in Colonic Flora and Molecular Features of Colorectal Carcinoma. Cancer Res. 2014, 74, 1311–1318. [Google Scholar] [CrossRef] [Green Version]
- Mima, K.; Nishihara, R.; Qian, Z.R.; Cao, Y.; Sukawa, Y.; Nowak, J.A.; Yang, J.; Dou, R.; Masugi, Y.; Song, M.; et al. Fusobacterium nucleatumin colorectal carcinoma tissue and patient prognosis. Gut 2016, 65, 1973–1980. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Chen, Y.; Fu, X.; Zhou, X.; Peng, Y.; Shi, L.; Chen, T.; Wu, Y. Invasive Fusobacterium nucleatum may play a role in the carcinogenesis of proximal colon cancer through the serrated neoplasia pathway. Int. J. Cancer 2016, 139, 1318–1326. [Google Scholar] [CrossRef] [Green Version]
- Ito, M.; Kanno, S.; Nosho, K.; Sukawa, Y.; Mitsuhashi, K.; Kurihara, H.; Igarashi, H.; Takahashi, T.; Tachibana, M.; Takahashi, H.; et al. Association of Fusobacterium nucleatum with clinical and molecular features in colorectal serrated pathway. Int. J. Cancer 2015, 137, 1258–1268. [Google Scholar] [CrossRef]
- Park, H.E.; Kim, J.H.; Cho, N.-Y.; Lee, H.S.; Kang, G.H. Intratumoral Fusobacterium nucleatum abundance correlates with macrophage infiltration and CDKN2A methylation in microsatellite-unstable colorectal carcinoma. Virchows Arch. 2017, 471, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Jun, S.-Y.; Lee, I.H.; Kang, B.W.; Park, S.Y.; Kim, H.J.; Park, J.S.; Choi, G.-S.; Yoon, G.; Kim, J.G. CD274, LAG3, and IDO1 expressions in tumor-infiltrating immune cells as prognostic biomarker for patients with MSI-high colon cancer. J. Cancer Res. Clin. Oncol. 2018, 144, 1005–1014. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Jenkins, R.W.; Sullivan, R.J. Mechanisms of Resistance to Immune Checkpoint Blockade. Am. J. Clin. Dermatol. 2018, 20, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Albacker, L.A.; Wu, J.; Smith, P.; Warmuth, M.; Stephens, P.J.; Zhu, P.; Yu, L.; Chmielecki, J. Loss of function JAK1 mutations occur at high frequency in cancers with microsatellite instability and are suggestive of immune evasion. PLoS ONE 2017, 12, e0176181. [Google Scholar] [CrossRef] [PubMed]
- Anichini, A.; Perotti, V.E.; Sgambelluri, F.; Mortarini, R. Immune Escape Mechanisms in Non Small Cell Lung Cancer. Cancers 2020, 12, 3605. [Google Scholar] [CrossRef]
- Huard, B.; Prigent, P.; Tournier, M.; Bruniquel, D.; Triebel, F. CD4/major histocompatibility complex class II interaction analyzed with CD4- and lymphocyte activation gene-3 (LAG-3)-Ig fusion proteins. Eur. J. Immunol. 1995, 25, 2718–2721. [Google Scholar] [CrossRef]
- Koyama, S.; Akbay, E.A.; Li, Y.Y.; Herter-Sprie, G.S.; Buczkowski, K.A.; Richards, W.G.; Gandhi, L.; Redig, A.J.; Rodig, S.J.; Asahina, H.; et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat. Commun. 2016, 7, 10501. [Google Scholar] [CrossRef] [Green Version]
- Hollebecque, A.; Chung, H.C.; de Miguel, M.J.; Italiano, A.; Machiels, J.-P.; Lin, C.-C.; Dhani, N.C.; Peeters, M.; Moreno, V.; Su, W.-C.; et al. Safety and Antitumor Activity of α-PD-L1 Antibody as Monotherapy or in Combination with α-TIM-3 Antibody in Patients with Microsatellite Instability–High/Mismatch Repair–Deficient Tumors. Clin. Cancer Res. 2021, 27, 6393–6404. [Google Scholar] [CrossRef]
- Andre, T.; Sposetti, C.; Gumus, M.; Ahn, J.B.; Wyrwicz, L.; Kwiatkowski, M.; Kim, J.G.; Yalcin, S.; Sendur, M.A.; Odeleye-Ajakaye, A.; et al. Phase 2 study of pembrolizumab-based combination therapy in patients with microsatellite instability-high (MSI-H) or mismatch repair-deficient (dMMR) stage IV colorectal cancer (CRC). J. Clin. Oncol. 2022, 40, TPS3639. [Google Scholar] [CrossRef]
- Kusmartsev, S.; Eruslanov, E.; Kübler, H.; Tseng, T.; Sakai, Y.; Su, Z.; Kaliberov, S.; Heiser, A.; Rosser, C.; Dahm, P.; et al. Oxidative Stress Regulates Expression of VEGFR1 in Myeloid Cells: Link to Tumor-Induced Immune Suppression in Renal Cell Carcinoma. J. Immunol. 2008, 181, 346–353. [Google Scholar] [CrossRef] [Green Version]
- Manning, E.A.; Ullman, J.G.; Leatherman, J.M.; Asquith, J.M.; Hansen, T.R.; Armstrong, T.D.; Hicklin, D.J.; Jaffee, E.M.; Emens, L.A. A Vascular Endothelial Growth Factor Receptor-2 Inhibitor Enhances Antitumor Immunity through an Immune-Based Mechanism. Clin. Cancer Res. 2007, 13, 3951–3959. [Google Scholar] [CrossRef] [Green Version]
- Vincent, J.; Mignot, G.; Chalmin, F.; Ladoire, S.; Bruchard, M.; Chevriaux, A.; Martin, F.; Apetoh, L.; Rébé, C.; Ghiringhelli, F. 5-Fluorouracil Selectively Kills Tumor-Associated Myeloid-Derived Suppressor Cells Resulting in Enhanced T Cell–Dependent Antitumor Immunity. Cancer Res. 2010, 70, 3052–3061. [Google Scholar] [CrossRef] [Green Version]
- Rocha Lima, C.M.S.P.; Yothers, G.; Jacobs, S.A.; Sanoff, H.K.; Cohen, D.J.; Guthrie, A.K.; Henry, N.L.; Ganz, P.A.; Kopetz, S.; Lucas, P.C.; et al. Colorectal cancer metastatic dMMR immuno-therapy (COMMIT) study: A randomized phase III study of atezolizumab (atezo) monotherapy versus mFOLFOX6/bevacizumab/atezo in the first-line treatment of patients (pts) with deficient DNA mismatch repair (dMMR) or microsatellite instability high (MSI-H) metastatic colorectal cancer (mCRC)—NRG-GI004/SWOG-S1610. J. Clin. Oncol. 2022, 40, TPS3647. [Google Scholar] [CrossRef]
- Vall d’Hebron Institute of Oncology. A Phase II Open-label Study with the Anti-PD-L1 Atezolizumab Monoclonal Antibody in Combination With Bevacizumab in Patients With Advanced Chemotherapy Resistant Colorectal Cancer and MSI-like Molecular Signature. Clinical Trial Registration NCT02982694. October 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT02982694 (accessed on 5 January 2022).
| Study Name | Phase | Line of Treatment | N Patients | Agent(s) | Median Follow-Up | Results |
|---|---|---|---|---|---|---|
| GERCOR NIPICOL [19] | II | ≥1st line | N = 57 | Nivolumab plus Ipilimumab # (max 20 cycles) | 34.5 mo | 3-yrs PFS rate: 70% |
| 3-yrs OS rate: 73% | ||||||
| KEYNOTE-177 [10,20] | III | 1st line | N = 307 | Pembrolizumab vs. Chemotherapy plus target agents | 44.5 mo | mPFS: 16.5 vs. 8.2 mo, HR = 0.59, p = 0.0008 |
| mOS: NR vs. 36.7 mo, HR = 0.74; p = 0.036 | ||||||
| [OS superiority not demonstrated] | ||||||
| 3-yrs PFS rate: 42% vs. 11% | ||||||
| ORR: 45% vs. 33% | ||||||
| DCR: 65% vs. 75% | ||||||
| Checkmate-142 [21] | II | 1st line | N = 45 | Nivolumab plus low-dose Ipilimumab ## | 52 mo | 4-yrs PFS rate: 51% |
| 4-yrs OS rate: 72% | ||||||
| ORR: 71% | ||||||
| DCR: 84% | ||||||
| ≥2nd line | N = 119 | Nivolumab plus low-dose Ipilimumab # | 64 mo | 4-yrs PFS rate: 54% | ||
| 4-yrs OS rate: 71% | ||||||
| ORR: 65% | ||||||
| DCR: 81% | ||||||
| ≥2nd line | N = 74 | Nivolumab | 70 mo | 4-yrs PFS rate: 36% | ||
| 4-yrs OS rate: 49% | ||||||
| ORR: 39% | ||||||
| DCR: 69% | ||||||
| KEYNOTE-164 [22] | II | cohort A, ≥3rd line | N = 61 | Pembrolizumab | 31 mo | 12-mo OS rate: 72% |
| ORR: 33% | ||||||
| DCR: 51% | ||||||
| cohort B, ≥2nd line | N= 63 | Pembrolizumab | 24 mo | 12-mo PFS rate: 41% | ||
| 12-mo OS rate: 76% | ||||||
| ORR: 33% | ||||||
| DCR: 57% | ||||||
| KEYNOTE-016 [4] | II | ≥3rd line | N = 11 | Pembrolizumab | 36 weeks | 20-weeks PFS rate: 78% |
| ORR: 40% | ||||||
| DCR: 90% |
| Target | Site of Expression | Biological Role |
|---|---|---|
| CD274 | APC | Reduce T-CD4+ function |
| LAG-3 | T-cells | Activation of Tregs and suppression of CD8+ T-cells and DC |
| IDO1 | T-cells DC Macrophages | Catabolic enzyme involved in the degradation of tryptophan. Increase immunosuppressive cells, such as Treg |
| TIM-3 | T-cells | Inhibition of Th1 cell response |
| TIGIT | T-cells NK cells | Decrease of cytokine production and T-effector functions |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borelli, B.; Antoniotti, C.; Carullo, M.; Germani, M.M.; Conca, V.; Masi, G. Immune-Checkpoint Inhibitors (ICIs) in Metastatic Colorectal Cancer (mCRC) Patients beyond Microsatellite Instability. Cancers 2022, 14, 4974. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers14204974
Borelli B, Antoniotti C, Carullo M, Germani MM, Conca V, Masi G. Immune-Checkpoint Inhibitors (ICIs) in Metastatic Colorectal Cancer (mCRC) Patients beyond Microsatellite Instability. Cancers. 2022; 14(20):4974. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers14204974
Chicago/Turabian StyleBorelli, Beatrice, Carlotta Antoniotti, Martina Carullo, Marco Maria Germani, Veronica Conca, and Gianluca Masi. 2022. "Immune-Checkpoint Inhibitors (ICIs) in Metastatic Colorectal Cancer (mCRC) Patients beyond Microsatellite Instability" Cancers 14, no. 20: 4974. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers14204974