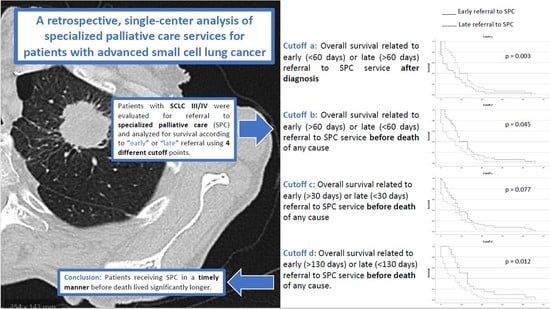
A Retrospective, Single-Center Analysis of Specialized Palliative Care Services for Patients with Advanced Small-Cell Lung Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Design
2.2. Setting
2.2.1. Patients
2.2.2. Definition of SPC Services at Our University-Based Referral Center
2.2.3. Definition of Referral Policy at Our Hospital to the SCP-Team
2.2.4. Definition of Transition Points for Referral to SPC
2.2.5. Data Collection
2.3. Statistical Methods
3. Results
3.1. Patient Characteristics
3.2. Evaluation of SPC Services for Patients with Locally Advanced or Metastatic SCLC
3.3. OS Related to the Prevalence of SPC Services for Advanced or Metastatic SCLC Patients (Stage III/IV)
3.4. Referral to SPC Services in Stage III/IV SCLC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASCO | American Society of Clinical Oncology |
| ECOG | Eastern Cooperative Oncology Group |
| ICD | International Statistical Classification of Diseases and Related Health Problems |
| NSCLC | Non–small cell lung cancer |
| NCCN | National Comprehensive Cancer Network |
| QoL | Quality of life |
| PC | Palliative Care |
| PD-L1 | Programmed death-ligand 1 |
| SCLC | Small-cell lung cancer |
| SPC | Specialized palliative care |
| UICC | Union Internationale Contre le Cancer |
| WHO | World Health Organization |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Travis, W.D.; Brambilla, E.; Nicholson, A.G.; Yatabe, Y.; Austin, J.H.; Beasley, M.B.; Chirieac, L.R.; Dacic, S.; Duhig, E.; Flieder, D.B. The 2015 World Health Organization classification of lung tumors: Impact of genetic, clinical and radiologic advances since the 2004 classification. J. Thorac. Oncol. 2015, 10, 1243–1260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Billingy, N.E.; Tromp, V.N.; van den Hurk, C.J.; Becker-Commissaris, A.; Walraven, I. Health-Related Quality of Life and Survival in Metastasized Non-Small Cell Lung Cancer Patients with and without a Targetable Driver Mutation. Cancers 2021, 13, 4282. [Google Scholar] [CrossRef]
- Temel, J.S.; Petrillo, L.A.; Greer, J.A. Patient-centered palliative care for patients with advanced lung cancer. J. Clin. Oncol. 2022, 40, 626–634. [Google Scholar] [CrossRef]
- Hui, D.; Kim, S.H.; Roquemore, J.; Dev, R.; Chisholm, G.; Bruera, E. Impact of timing and setting of palliative care referral on quality of end-of-life care in cancer patients. Cancer 2014, 120, 1743–1749. [Google Scholar] [CrossRef] [Green Version]
- WHO Definition of Palliative Care. Available online: https://www.who.int/health-topics/palliative-care (accessed on 2 September 2022).
- Ferrell, B.R.; Temel, J.S.; Temin, S.; Alesi, E.R.; Balboni, T.A.; Basch, E.M.; Firn, J.I.; Paice, J.A.; Peppercorn, J.M.; Phillips, T.; et al. Integration of Palliative Care into Standard Oncology Care: American Society of Clinical Oncology Clinical Practice Guideline Update. J. Clin. Oncol. 2017, 35, 96–112. [Google Scholar] [CrossRef] [Green Version]
- Smith, T.J.; Temin, S.; Alesi, E.R.; Abernethy, A.P.; Balboni, T.A.; Basch, E.M.; Ferrell, B.R.; Loscalzo, M.; Meier, D.E.; Paice, J.A. American Society of Clinical Oncology provisional clinical opinion: The integration of palliative care into standard oncology care. J. Clin. Oncol. 2012, 30, 880–887. [Google Scholar] [CrossRef]
- Hui, D.; Heung, Y.; Bruera, E. Timely Palliative Care: Personalizing the Process of Referral. Cancers 2022, 14, 1047. [Google Scholar] [CrossRef]
- Bruera, E.H.I.; Ripamonti, C.; von Gunten, C.F. Textbook of Palliative Medicine; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Hui, D.; Kim, S.H.; Kwon, J.H.; Tanco, K.C.; Zhang, T.; Kang, J.H.; Rhondali, W.; Chisholm, G.; Bruera, E. Access to palliative care among patients treated at a comprehensive cancer center. Oncologist 2012, 17, 1574–1580. [Google Scholar] [CrossRef] [Green Version]
- Bruera, E.; Hui, D. Conceptual models for integrating palliative care at cancer centers. J. Palliat. Med. 2012, 15, 1261–1269. [Google Scholar] [CrossRef]
- Hui, D.; Hoge, G.; Bruera, E. Models of supportive care in oncology. Curr. Opin. Oncol. 2021, 33, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Mo, L.; Urbauer, D.L.; Bruera, E.; Hui, D. Recommendations for supportive care and best supportive care in NCCN clinical practice guidelines for treatment of cancer: Differences between solid tumor and hematologic malignancy guidelines. Support. Care Cancer 2021, 29, 7385–7392. [Google Scholar] [CrossRef] [PubMed]
- Quill, T.E.; Abernethy, A.P. Generalist plus specialist palliative care—Creating a more sustainable model. N. Engl. J. Med. 2013, 368, 1173–1175. [Google Scholar] [CrossRef] [Green Version]
- Kaasa, S.; Loge, J.H.; Aapro, M.; Albreht, T.; Anderson, R.; Bruera, E.; Brunelli, C.; Caraceni, A.; Cervantes, A.; Currow, D.C.; et al. Integration of oncology and palliative care: A Lancet Oncology Commission. Lancet Oncol. 2018, 19, e588–e653. [Google Scholar] [CrossRef] [Green Version]
- Bakitas, M.; Lyons, K.D.; Hegel, M.T.; Balan, S.; Brokaw, F.C.; Seville, J.; Hull, J.G.; Li, Z.; Tosteson, T.D.; Byock, I.R. Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: The Project ENABLE II randomized controlled trial. JAMA 2009, 302, 741–749. [Google Scholar] [CrossRef] [Green Version]
- Bakitas, M.A.; Tosteson, T.D.; Li, Z.; Lyons, K.D.; Hull, J.G.; Li, Z.; Dionne-Odom, J.N.; Frost, J.; Dragnev, K.H.; Hegel, M.T. Early versus delayed initiation of concurrent palliative oncology care: Patient outcomes in the ENABLE III randomized controlled trial. J. Clin. Oncol. 2015, 33, 1438. [Google Scholar] [CrossRef]
- Maltoni, M.; Scarpi, E.; Dall’Agata, M.; Schiavon, S.; Biasini, C.; Codecà, C.; Broglia, C.M.; Sansoni, E.; Bortolussi, R.; Garetto, F. Systematic versus on-demand early palliative care: A randomised clinical trial assessing quality of care and treatment aggressiveness near the end of life. Eur. J. Cancer 2016, 69, 110–118. [Google Scholar] [CrossRef]
- Temel, J.S.; Greer, J.A.; Admane, S.; Gallagher, E.R.; Jackson, V.A.; Lynch, T.J.; Lennes, I.T.; Dahlin, C.M.; Pirl, W.F. Longitudinal perceptions of prognosis and goals of therapy in patients with metastatic non-small-cell lung cancer: Results of a randomized study of early palliative care. J. Clin. Oncol. 2011, 29, 2319–2326. [Google Scholar] [CrossRef] [Green Version]
- Temel, J.S.; Greer, J.A.; El-Jawahri, A.; Pirl, W.F.; Park, E.R.; Jackson, V.A.; Back, A.L.; Kamdar, M.; Jacobsen, J.; Chittenden, E.H. Effects of early integrated palliative care in patients with lung and GI cancer: A randomized clinical trial. J. Clin. Oncol. 2017, 35, 834. [Google Scholar] [CrossRef]
- Zimmermann, C.; Swami, N.; Krzyzanowska, M.; Hannon, B.; Leighl, N.; Oza, A.; Moore, M.; Rydall, A.; Rodin, G.; Tannock, I. Early palliative care for patients with advanced cancer: A cluster-randomised controlled trial. Lancet 2014, 383, 1721–1730. [Google Scholar] [CrossRef]
- Davis, M.P.; Temel, J.S.; Balboni, T.; Glare, P. A review of the trials which examine early integration of outpatient and home palliative care for patients with serious illnesses. Ann. Palliat. Med. 2015, 4, 99–121. [Google Scholar]
- Zimmermann, C.; Riechelmann, R.; Krzyzanowska, M.; Rodin, G.; Tannock, I. Effectiveness of specialized palliative care: A systematic review. JAMA 2008, 299, 1698–1709. [Google Scholar] [CrossRef] [PubMed]
- Temel, J.S.; Greer, J.A.; Muzikansky, A.; Gallagher, E.R.; Admane, S.; Jackson, V.A.; Dahlin, C.M.; Blinderman, C.D.; Jacobsen, J.; Pirl, W.F. Early palliative care for patients with metastatic non–small-cell lung cancer. N. Engl. J. Med. 2010, 363, 733–742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collins, A.; Sundararajan, V.; Burchell, J.; Millar, J.; McLachlan, S.-A.; Krishnasamy, M.; Le, B.H.; Mileshkin, L.; Hudson, P.; Philip, J. Transition points for the routine integration of palliative care in patients with advanced cancer. J. Pain Symptom Manag. 2018, 56, 185–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Earle, C.C.; Neville, B.A.; Landrum, M.B.; Ayanian, J.Z.; Block, S.D.; Weeks, J.C. Trends in the aggressiveness of cancer care near the end of life. J. Clin. Oncol. 2004, 22, 315–321. [Google Scholar] [CrossRef]
- Ziegler, L.E.; Craigs, C.L.; West, R.M.; Carder, P.; Hurlow, A.; Millares-Martin, P.; Hall, G.; Bennett, M.I. Is palliative care support associated with better quality end-of-life care indicators for patients with advanced cancer? A retrospective cohort study. BMJ Open 2018, 8, e018284. [Google Scholar] [CrossRef]
- Gaertner, J.; Siemens, W.; Meerpohl, J.J.; Antes, G.; Meffert, C.; Xander, C.; Stock, S.; Mueller, D.; Schwarzer, G.; Becker, G. Effect of specialist palliative care services on quality of life in adults with advanced incurable illness in hospital, hospice, or community settings: Systematic review and meta-analysis. BMJ 2017, 357, j2925. [Google Scholar] [CrossRef] [Green Version]
- Kaplan, E.L.; Meier, P. Nonparametric estimation from incomplete observations. J. Am. Stat. Assoc. 1958, 53, 457–481. [Google Scholar] [CrossRef]
- Toh, C.-K.; Gao, F.; Lim, W.-T.; Leong, S.-S.; Fong, K.-W.; Yap, S.-P.; Hsu, A.A.; Eng, P.; Koong, H.-N.; Thirugnanam, A. Differences between small-cell lung cancer and non-small-cell lung cancer among tobacco smokers. Lung Cancer 2007, 56, 161–166. [Google Scholar] [CrossRef]
- Scarpi, E.; Dall’Agata, M.; Zagonel, V.; Gamucci, T.; Bertè, R.; Sansoni, E.; Amaducci, E.; Broglia, C.M.; Alquati, S.; Garetto, F. Systematic vs. on-demand early palliative care in gastric cancer patients: A randomized clinical trial assessing patient and healthcare service outcomes. Support. Care Cancer 2019, 27, 2425–2434. [Google Scholar] [CrossRef] [Green Version]
- Vanbutsele, G.; Pardon, K.; Van Belle, S.; Surmont, V.; De Laat, M.; Colman, R.; Eecloo, K.; Cocquyt, V.; Geboes, K.; Deliens, L. Effect of early and systematic integration of palliative care in patients with advanced cancer: A randomised controlled trial. Lancet Oncol. 2018, 19, 394–404. [Google Scholar] [CrossRef]
- Temel, J.S.; Sloan, J.; Zemla, T.; Greer, J.A.; Jackson, V.A.; El-Jawahri, A.; Kamdar, M.; Kamal, A.; Blinderman, C.D.; Strand, J. Multisite, randomized trial of early integrated palliative and oncology care in patients with advanced lung and gastrointestinal cancer: Alliance A221303. J. Palliat. Med. 2020, 23, 922–929. [Google Scholar] [CrossRef] [PubMed]
- Franciosi, V.; Maglietta, G.; Degli Esposti, C.; Caruso, G.; Cavanna, L.; Bertè, R.; Bacchini, G.; Bocchi, L.; Piva, E.; Monfredo, M. Early palliative care and quality of life of advanced cancer patients-a multicenter randomized clinical trial. Ann. Palliat. Med. 2019, 8, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Rodin, R.; Swami, N.; Pope, A.; Hui, D.; Hannon, B.; Le, L.W.; Zimmermann, C. Impact of early palliative care according to baseline symptom severity: Secondary analysis of a cluster-randomized controlled trial in patients with advanced cancer. Cancer Med. 2022, 11, 1869–1878. [Google Scholar] [CrossRef] [PubMed]
- Schenker, Y.; Arnold, R. Toward palliative care for all patients with advanced cancer. JAMA Oncol. 2017, 3, 1459–1460. [Google Scholar] [CrossRef]
- Hui, D.; Bruera, E. Models of palliative care delivery for patients with cancer. J. Clin. Oncol. 2020, 38, 852. [Google Scholar] [CrossRef]
- Davis, M.P.; Bruera, E.; Morganstern, D. Early integration of palliative and supportive care in the cancer continuum: Challenges and opportunities. Am. Soc. Clin. Oncol. Educ. Book 2013, 33, 144–150. [Google Scholar] [CrossRef]
- Lamont, E.B.; Christakis, N.A. Physician factors in the timing of cancer patient referral to hospice palliative care. Cancer 2002, 94, 2733–2737. [Google Scholar] [CrossRef]
- Cherny, N.I.; Catane, R. Attitudes of medical oncologists toward palliative care for patients with advanced and incurable cancer: Report on a survey by the European Society of Medical Oncology Taskforce on Palliative and Supportive Care. Cancer 2003, 98, 2502–2510. [Google Scholar]
- Earle, C.C.; Landrum, M.B.; Souza, J.M.; Neville, B.A.; Weeks, J.C.; Ayanian, J.Z. Aggressiveness of cancer care near the end of life: Is it a quality-of-care issue? J. Clin. Oncol. 2008, 26, 3860. [Google Scholar] [CrossRef] [Green Version]
- Hui, D.; Hannon, B.L.; Zimmermann, C.; Bruera, E. Improving patient and caregiver outcomes in oncology: Team-based, timely, and targeted palliative care. CA A Cancer J. Clin. 2018, 68, 356–376. [Google Scholar] [CrossRef]
- Glare, P.; Plakovic, K.; Schloms, A.; Egan, B.; Epstein, A.S.; Kelsen, D.; Saltz, L. Study using the NCCN guidelines for palliative care to screen patients for palliative care needs and referral to palliative care specialists. J. Natl. Compr. Cancer Netw. 2013, 11, 1087–1096. [Google Scholar] [CrossRef] [PubMed]
- Greer, J.A.; Pirl, W.F.; Jackson, V.A.; Muzikansky, A.; Lennes, I.T.; Heist, R.S.; Gallagher, E.R.; Temel, J.S. Effect of early palliative care on chemotherapy use and end-of-life care in patients with metastatic non-small-cell lung cancer. J. Clin. Oncol. 2012, 30, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Meyers, F.J.; Linder, J.; Beckett, L.; Christensen, S.; Blais, J.; Gandara, D.R. Simultaneous care: A model approach to the perceived conflict between investigational therapy and palliative care. J. Pain Symptom Manag. 2004, 28, 548–556. [Google Scholar] [CrossRef]
- Chosich, B.; Burgess, M.; Earnest, A.; Franco, M.; Runacres, F.; William, L.; Poon, P.; Yoong, J. Cancer patients’ perceptions of palliative care. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2020, 28, 1207–1214. [Google Scholar] [CrossRef] [PubMed]
- Hui, D.; Kim, Y.J.; Park, J.C.; Zhang, Y.; Strasser, F.; Cherny, N.; Kaasa, S.; Davis, M.P.; Bruera, E. Integration of oncology and palliative care: A systematic review. Oncologist 2015, 20, 77–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamal, A.H.; Peppercorn, J.M. The generalizability paradox within palliative care clinical trials. Ann. Palliat. Med. 2013, 2, 101–104. [Google Scholar] [PubMed]
- Kaasa, S.; Loge, J.H. Early integration of palliative care-new evidence and old questions. Lancet Oncol. 2018, 19, 280–281. [Google Scholar] [CrossRef]
- Philip, J.; Collins, A.; Le, B.; Sundararajan, V.; Brand, C.; Hanson, S.; Emery, J.; Hudson, P.; Mileshkin, L.; Ganiatsas, S. A randomised phase II trial to examine feasibility of standardised, early palliative (STEP) care for patients with advanced cancer and their families [ACTRN12617000534381]: A research protocol. Pilot Feasibility Stud. 2019, 5, 44. [Google Scholar] [CrossRef]



| Number of Patients (%) | 152 (100) |
|---|---|
| Age (years), median (range) | 68 (37–88) |
| Male, n (%) | 90 (59.2) |
| Female, n (%) | 62 (40.8) |
| UICC stage at diagnosis: | |
| 4 (2.6) |
| 5 (3.3) |
| 47 (31.0) |
| 96 (63.1) |
| Metastatic disease at time of diagnosis: | 96 (63.1) |
| 32 (21.1) |
| 46 (30.3) |
| 18 (11.8) |
| 12 (7.9) |
| 46 (30.3) |
| 18 (11.8) |
| 33 (21.7) |
| History of smoking, n (%) | 141 (92.8) |
| Targetable mutation, n (%) | 0 |
| PD-L1 ≥ 1%, n (%) | 34 (22.4) |
| ECOG at time of diagnosis, n (%) (n = 125): | |
| 38 (25.0) |
| 69 (45.4) |
| 12 (7.9) |
| 4 (2.6) |
| 2 (1.3) |
| Therapeutic approaches (first line), n (%): | |
| 8 (5.3) |
| 11 (7.2) |
| 112 (73.7) |
| 21 (13.8) |
| Deaths from any cause, n (%) | 106 (69.7) |
| Survival time (months), median (range) | 8 (0–61) |
| Referral to SPC Services (n = 75) | No Referral to SPC Services (n = 68) | p-Value | |
|---|---|---|---|
| Age (years), median (range) | 67 (46–85) | 68 (37–80) | n.s. † |
| Male, n (%) | 45 (60) | 40 (41.2) | n.s. * |
| Female, n (%) | 30 (40) | 28 (58.8) | n.s. * |
| UICC stage at diagnosis: | <0.001 * | ||
| 12 (16) | 36 (52.9) | |
| 63 (84) | 32 (47.1) | |
| History of smoking, n (%) | 72 (96) | 62 (91.2) | n.s. * |
| PD-L1 ≥ 1%, n (%) | 15 (20) | 19 (27.9) | n.s. * |
| ECOG ≥ 2 at time of diagnosis, n (%) (n = 116) | 14 (22.2) | 3 (5.7) | 0.010 # |
| Metastatic disease at time of diagnosis: | |||
| 20 (26.7) | 12 (17.6) | n.s. * |
| 30 (40) | 16 (23.5) | 0.048 * |
| 8 (10.7) | 10 (14.7) | n.s. |
| 9 (12) | 3 (4.4) | n.s. |
| 28 (37.3) | 18 (26.5) | n.s. |
| 13 (17.3) | 5 (7.4) | n.s. |
| 23 (30.7) | 10 (14.7) | 0.019 # |
| Therapeutic approaches (first line): | n.s. * | ||
| 2 (2.7) | 1 (1.5) | |
| 0 | 2 (2.9) | |
| 2 (2.7) | 5 (7.4) | |
| 62 (82.7) | 48 (70.6) | |
| 9 (12) | 12 (17.6) | |
| Referral indications: | |||
| 21 (28) | ||
| 9 (12) | ||
| 15 (20) | ||
| 4 (5.3) | ||
| 2 (2.7) | ||
| 4 (5.3) | ||
| 20 (26.7) | ||
| Duration of SPC services (days), median (range) | 75 (1–1876) | - | - |
| Time from diagnosis till start of SPC (days), median (range) | 47 (0–1315) | - | - |
| Pat referred to SPC services ≤ 60 days after diagnosis, n (%) (cutoff a) | 42 (56) | - | - |
| Pat referred to SPC services ≥ 60 days before death, n (%) (cutoff b) | 33 (44) | - | - |
| Pat referred to SPC services ≥ 30 days before death, n (%) (cutoff c) | 40 (53.3) | ||
| Pat referred to SPC services ≥ 130 days before death, n (%) (cutoff d) | 25 (33.3) | ||
| Deaths from any cause, n (%) | 65 (86.7) | 38 (56.7) | <0.001 * |
| Survival time (months), median (range) | 8 (0–61) | 17 (0–32) | 0.014 ‡ |
| Overall Survival | ||
|---|---|---|
| Univariate | Multivariate | |
| Variable | HR (95% CI); p-Value | HR (95% CI); p-Value |
| Age | 0.08 (−0.73–0.23); 0.312 | 0.05 (−0.12–0.22); 0.552 |
| Gender | 0.05 (−0.10–0.20); 0.544 | 0.06 (−0.11–0.23); 0.472 |
| ECOG ≥ 2 | −0.09 (−0.32–0.14); 0.446 | −0.19 (−0.43–0.05); 0.124 |
| First-line anticancer therapy | −0.15 (−0.61–0.06); 0.169 | −0.03 (−0.25–0.18); 0.768 |
| Referral to SPC services | 0.28 (0.14–0.42); <0.001 | 0.37 (0.19–0.55); <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wachter, C.; Hackner, K.; Groissenberger, I.; Jutz, F.; Tschurlovich, L.; Le, N.-S.; Kreye, G. A Retrospective, Single-Center Analysis of Specialized Palliative Care Services for Patients with Advanced Small-Cell Lung Cancer. Cancers 2022, 14, 4988. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers14204988
Wachter C, Hackner K, Groissenberger I, Jutz F, Tschurlovich L, Le N-S, Kreye G. A Retrospective, Single-Center Analysis of Specialized Palliative Care Services for Patients with Advanced Small-Cell Lung Cancer. Cancers. 2022; 14(20):4988. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers14204988
Chicago/Turabian StyleWachter, Claudia, Klaus Hackner, Iris Groissenberger, Franziska Jutz, Lisa Tschurlovich, Nguyen-Son Le, and Gudrun Kreye. 2022. "A Retrospective, Single-Center Analysis of Specialized Palliative Care Services for Patients with Advanced Small-Cell Lung Cancer" Cancers 14, no. 20: 4988. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers14204988