1. Introduction
Lung cancer has been identified as one of the most common malignant tumors. In recent years, the incidence of lung cancer has gradually increased. Among different malignancies, lung cancer has the fastest-growing incidence and mortality, becoming the biggest threat to people’s health and life [
1,
2]. Lung cancer is divided into two types: non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC). NSCLC accounts for about 80–85% of lung cancers, including squamous cell carcinoma, adenocarcinoma, and large cell carcinoma [
3]. Compared with SCLC, NSCLC cells grow and divide slowly and metastasize relatively late [
4]. The early clinical symptoms of lung cancer are not obvious; thus, lung cancer is usually diagnosed at an advanced stage, and optimal treatment and surgical opportunities are lost. Thus, improving the early detection rate of lung cancer is needed to immediately adopt positive treatment measures to reduce the harm of the disease.
Tumor markers reflect the presence of the tumor, and changes in the presence and level of markers indicate the nature of the tumor. Detecting tumor markers facilitates the early diagnosis and operation of tumor development. In recent years, more serum tumor markers have been identified for the early diagnosis of lung cancer. Due to the low sensitivity and specificity of single serum tumor markers, detecting multiple tumor markers has been used to improve the sensitivity and specificity of clinical diagnosis in lung cancer patients. Therefore, the application of single tumor markers has gradually progressed to the diagnostic use of multiple markers, thus improving the positive rate of diagnosis and monitoring the development of lung cancer [
5,
6]. Clinically significant serum tumor markers for lung cancer include carcinoembryonic antigen (CEA), cytokeratin-19 fragment (CYFRA 21-1), neuron-specific enolase (NSE), squamous cell carcinoma antigen (SCC-Ag), pro-gastrin-releasing peptide (ProGRP), total prostate-specific antigen (TPSA) and carbohydrate antigen 199 (CA199) [
7,
8,
9,
10]. Numerous studies have reported the application of these tumor markers in the diagnosis of lung cancer. Clinical studies have also focused on the use of these markers for monitoring treatment efficacy and prognosis; furthermore, progress has been made in the application of these markers [
11,
12,
13,
14,
15]. High levels of tumor markers at baseline are correlated with worse survival in stage III-IV NSCLC patients [
13]. Tumor markers such as CYFRA21-1, SCC-Ag, NSE, and CEA in the serum of lung cancer patients are significantly increased, and the degree of elevation are significantly correlated with tumor invasion, clinical stage and lymph node metastasis [
16].
Serum tumor markers have been used for the early diagnosis of lung cancer and the clinical practice of tumor efficacy monitoring for more than 10 years. However, confirmative studies with large clinical sample size on the consistency of various tumor markers for determining the pathology and tumor progression of lung cancer remain lacking. Thus, the purpose of this study was to explore the effectiveness of serum markers in determining tumor metastasis and stage in lung cancer patients from two clinical centers with a large sample size.
2. Materials and Methods
2.1. Patients and Control Subjects
In total, 4690 lung cancer patients admitted to Tianjin Union Medical Center from September 2016 to September 2019 and 2700 lung cancer patients admitted to Tianjin Medical University Cancer Institute and Hospital from January 2018 to September 2019 were screened as study subjects. All patients were screened using case data, relevant laboratory examination data, imaging data, and pathological examination data. Patients were determined to be all Chinese from north China and northeast China. The inclusion criteria were complete information including age, sex, smoking history, and other basic data of the patients. Patients were excluded from the study if they had other tumors, inflammation in the lungs or areas other than the lungs, or a history of chronic gastritis or ulcer in the digestive system. A total of 3272 patients with NSCLC were included in this study.
Fasting blood samples were taken for determination of lung cancer-related serum tumor markers before surgery, chemotherapy, radiotherapy, or other special treatments at the first admission. The pathological diagnosis was based on lung cancer surgery, lung puncture biopsy or tracheoscopy. Pathological diagnoses of lung cancer included squamous cell carcinoma, adenocarcinoma, adenosquamous cell carcinoma, etc. Data entry for all patients included smoking index, intrapulmonary, lymphatic and distant metastasis, and tumor stage (according to the International Association for the Study of Lung Cancer, IASLC 2015 TNM stage for lung cancer).
2.2. Sample Collection and Measurement
All the patients had an empty stomach the morning after the first admission without any treatment. Venous blood (5 mL) was collected from each patient to detect lung cancer-related tumor markers. The whole blood was separated into serum and cellular fractions within 2 h by centrifugation at 4000 rpm for 10 min. Serum samples were obtained after separation, and serum concentrations of tumor markers were determined. CEA, SCC-Ag and CA199 were determined by chemiluminescent microparticle immunoassay (CMIA) using an Abbott ARCHITECT I2000SR automatic chemical microparticle immune analyzer and its supporting reagents. CYFRA 21-1, NSE, ProGRP, and TPSA were determined using a Roche Elecsys 2010 automatic electrochemiluminescence immune analyzer and its supporting reagents.
2.3. Statistical Analysis
All tumor markers had non-normal distribution, and markers were represented by the median (P25–P75). Nonparametric tests were used to compare the concentrations of tumor markers and the smoking index between the different groups. Chi-square tests were also used to determine the distribution differences of basic information (age, sex and smoking index) and tumor markers among different groups. The Bonferroni method was used for paired comparisons. Binary logistic analysis was used to analyze the influencing factors for lung tumor metastasis, lymphatic metastasis and distant metastasis, while ordinal logistic analysis was used in examining the influencing factors for tumor stage. The two logistic analyses were divided into two steps: (1) univariate factor analysis and logistic analysis for each potential influencing factor was conducted; (2) influencing factors of
p < 0.2 [
17] in univariate analysis were included in a multivariate logistic analysis. Finally, the prediction probabilities of tumor markers with
p < 0.05 were reassessed by logistic analysis using multivariate analysis, and the receiver operating characteristic (ROC) curves were used for joint predictions. SPSS 24.0 (IBM, Chicago, IL, USA) was used for all analyses. For two-sided tests, a
p value less than 0.05 was considered significant.
4. Discussion
Tumor markers have been widely used in the clinical diagnosis and treatment of malignant tumors as they serve as important indicators of disease outcome monitoring. At present, varied tumor markers, such as CEA, SCC-Ag, CYFRA 21-1, NSE, are applied in diagnosing lung cancer, which can also be used to monitor metastasis and recurrence of NSCLC [
18]. Although tumor markers are widely used in clinical practice, the clinical analysis and validation of these markers using a large sample size remain lacking. In this study, a large sample of lung cancer patients from two medical centers was selected to verify the accuracy of tumor markers from multiple perspectives, including predicting tumor metastasis and clinical stage. Our results support the use of these markers in clinical practice.
CEA is widely found in adult cancer tissues and has been used in the auxiliary diagnosis, efficacy observation, prognostic judgment, and recurrence prediction of cancer [
19,
20]. CEA elevation is common in multisystem tumors, including lung cancer [
21]. Due to the non-specificity of this indicator, CEA is often used in combination with other tumor markers in clinical practice [
22,
23]. SCC-Ag participates in the regulation of protein decomposition during malignant transformation, and it is the preferred tumor marker for cervical squamous cell carcinoma [
24,
25]. Additionally, this marker is observed to increase in lung squamous cell cancers [
26]. CYFRA 21-1 is highly expressed in lung squamous cell carcinoma compared with adenocarcinoma and SCLC [
27]. The use of increased serum levels of CYFRA 21-1 for predicting postoperative recurrence in lung cancer patients shows good sensitivity and specificity. CYFRA 21-1 is also a highly sensitive and specific biomarker for the prediction of post-chemotherapy progression [
28].
A high concentration of serum NSE is a specific marker of neuroendocrine tumors [
29,
30]. SCLC regulates the secretion of a variety of related enzymes, active peptides, and hormones [
31]. Thus, NSE is a preferred marker for SCLC. NSE is only significantly changed in middle and advanced SCLC. NSE has been found to be related to changes in tumor growth and can be combined with clinical observations and monitoring to predict metastasis and recurrence for NSCLC [
32]. ProGRP is a marker of small cell lung cancer. Serum CA199 can be used for pancreatic cancer. Auxiliary diagnostic indicators for malignant tumors such as gallbladder cancer are mainly used as indicators for disease monitoring and predicting recurrence.
In this study, we have analyzed the differences in tumor markers among patients with different metastases and tumor stages. The results showed that the levels of SCC-Ag, ProGRP and CA199 in patients with lymphatic metastasis, and the levels of CEA, CYFRA 21-1 and NSE in patients with intrapulmonary, lymphatic, and distant metastasis, were significantly higher than those patients with non-metastasis. This data indicates that the increased tumor markers significantly correlate with NSCLC metastasis [
13]. Lung cancer markers have been also associated with the clinical stage of lung cancer. Tumor markers related to NSCLC, such as CEA, SCC-Ag and CYFRA 21-1, show a clear relationship with tumor stage [
12,
33]. The results of this study showed that there were statistical differences in the numeric levels and proportion of six tumor markers, including SCC-Ag, CEA, CYFRA 21-1, NSE, CA199 and TPSA, among patients with different tumor stages of NSCLC.
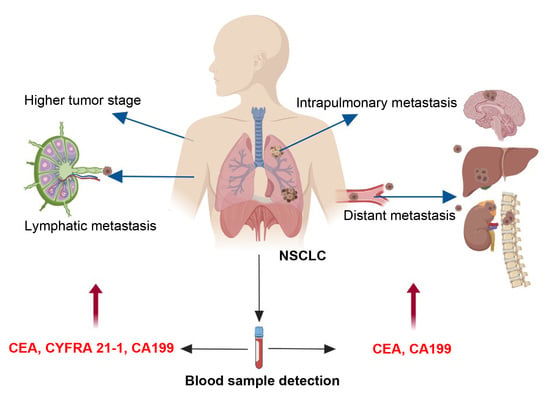
The results of risk factors showed that the patients, with increased levels of CEA, CYFRA 21-1, NSE and CA199, tended to have higher tumor stages. The risk factors for intrapulmonary metastasis were smoking index > 600, and increased levels of CEA and CA199. The risk factors for lymphatic metastasis were higher levels of CEA, CYFRA 21-1 and CA199. The risk factors for distant metastasis were elevated CEA and CA199 levels.
Combined detection of certain serum tumor markers in lung cancer patients can significantly improve diagnostic sensitivity and the roles of monitoring tumor progression [
34,
35]. At last, joint predictions of combined lung cancer-related tumor markers for tumor metastasis have been performed by ROC curve analyses. The result showed that the combined elevations in CEA and CA199 were also useful for the diagnoses of lymphatic metastasis and distant metastasis, respectively. These results are in accordance with previous reports [
36].