Clinical Impact of Single Nucleotide Polymorphism in CD-19 on Treatment Outcome in FMC63-CAR-T Cell Therapy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Study Endpoints
2.3. Monitoring of CAR-T Cell Kinetics
2.4. Statistical Analysis
2.5. CD19 Gene Analysis
3. Results
3.1. Prevalence of the CD19 L174 Allele
3.2. Baseline Clinical Characteristics of the DLBCL Patient Cohort
3.3. Disease Features and CAR-T Cell Treatment
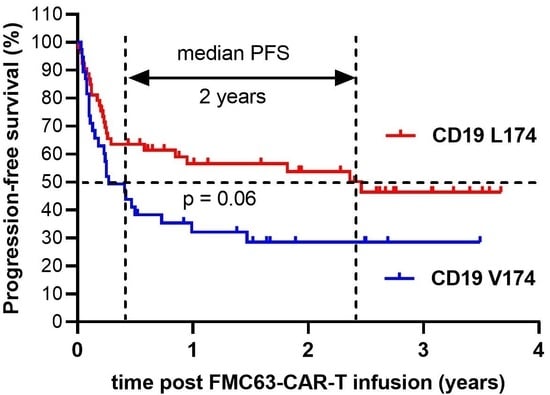
3.4. Treatment Outcome, Univariate Analysis
3.5. Treatment Outcome, Multivariate Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Locke, F.L.; Ghobadi, A.; Jacobson, C.A.; Miklos, D.B.; Lekakis, L.J.; Oluwole, O.O.; Lin, Y.; Braunschweig, I.; Hill, B.T.; Timmerman, J.M.; et al. Long-Term Safety and Activity of Axicabtagene Ciloleucel in Refractory Large B-Cell Lymphoma (ZUMA-1): A Single-Arm, Multicentre, Phase 1–2 Trial. Lancet Oncol. 2019, 20, 31–42. [Google Scholar] [CrossRef]
- Neelapu, S.S.; Locke, F.L.; Bartlett, N.L.; Lekakis, L.J.; Miklos, D.B.; Jacobson, C.A.; Braunschweig, I.; Oluwole, O.O.; Siddiqi, T.; Lin, Y.; et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N. Engl. J. Med. 2017, 377, 2531–2544. [Google Scholar] [CrossRef]
- Schuster, S.J.; Bishop, M.R.; Tam, C.S.; Waller, E.K.; Borchmann, P.; McGuirk, J.P.; Jäger, U.; Jaglowski, S.; Andreadis, C.; Westin, J.R.; et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2019, 380, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Westin, J.R.; Kersten, M.J.; Salles, G.; Abramson, J.S.; Schuster, S.J.; Locke, F.L.; Andreadis, C. Efficacy and Safety of CD19-directed CAR-T Cell Therapies in Patients with Relapsed/Refractory Aggressive B-cell Lymphomas: Observations from the JULIET, ZUMA-1, and TRANSCEND Trials. Am. J. Hematol. 2021, 96, 1295–1312. [Google Scholar] [CrossRef] [PubMed]
- Locke, F.L.; Miklos, D.B.; Jacobson, C.A.; Perales, M.-A.; Kersten, M.-J.; Oluwole, O.O.; Ghobadi, A.; Rapoport, A.P.; McGuirk, J.; Pagel, J.M.; et al. Axicabtagene Ciloleucel as Second-Line Therapy for Large B-Cell Lymphoma. N. Engl. J. Med. 2022, 386, 640–654. [Google Scholar] [CrossRef]
- Bastos-Oreiro, M.; Gutierrez, A.; Reguera, J.L.; Iacoboni, G.; López-Corral, L.; Terol, M.J.; Ortíz-Maldonado, V.; Sanz, J.; Guerra-Dominguez, L.; Bailen, R.; et al. Best Treatment Option for Patients With Refractory Aggressive B-Cell Lymphoma in the CAR-T Cell Era: Real-World Evidence From GELTAMO/GETH Spanish Groups. Front. Immunol. 2022, 13, 855730. [Google Scholar] [CrossRef] [PubMed]
- Chow, V.A.; Gopal, A.K.; Maloney, D.G.; Turtle, C.J.; Smith, S.D.; Ujjani, C.S.; Shadman, M.; Cassaday, R.D.; Till, B.G.; Tseng, Y.D.; et al. Outcomes of Patients with Large B-cell Lymphomas and Progressive Disease Following CD19-specific CAR T-cell Therapy. Am. J. Hematol. 2019, 94, E209–E213. [Google Scholar] [CrossRef] [Green Version]
- Spiegel, J.Y.; Dahiya, S.; Jain, M.D.; Tamaresis, J.S.; Nastoupil, L.; Jacobs, M.T.; Ghobadi, A.; Lin, Y.; Lunning, M.; Lekakis, L.J.; et al. Outcomes of Patients with Large B-Cell Lymphoma Progressing after Axicabtagene Ciloleucel Therapy. Blood 2021, 137, 1832–1835. [Google Scholar] [CrossRef]
- Nydegger, A.; Novak, U.; Kronig, M.-N.; Legros, M.; Zeerleder, S.; Banz, Y.; Bacher, U.; Pabst, T. Transformed Lymphoma Is Associated with a Favorable Response to CAR-T-Cell Treatment in DLBCL Patients. Cancers 2021, 13, 6073. [Google Scholar] [CrossRef]
- Pabst, T.; Joncourt, R.; Shumilov, E.; Heini, A.; Wiedemann, G.; Legros, M.; Seipel, K.; Schild, C.; Jalowiec, K.; Mansouri Taleghani, B.; et al. Analysis of IL-6 Serum Levels and CAR T Cell-Specific Digital PCR in the Context of Cytokine Release Syndrome. Exp. Hematol. 2020, 88, 7–14.e3. [Google Scholar] [CrossRef]
- Wittibschlager, V.; Bacher, U.; Seipel, K.; Porret, N.; Wiedemann, G.; Haslebacher, C.; Hoffmann, M.; Daskalakis, M.; Akhoundova, D.; Pabst, T. CAR T-Cell Persistence Correlates with Improved Outcome in Patients with B-Cell Lymphoma. Int. J. Mol. Sci. 2023, 24, 5688. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.N.; Fry, T.J. Mechanisms of Resistance to CAR T Cell Therapy. Nat. Rev. Clin. Oncol. 2019, 16, 372–385. [Google Scholar] [CrossRef] [PubMed]
- Uckun, F.M.; Jaszcz, W.; Ambrus, J.L.; Fauci, A.S.; Gajl-Peczalska, K.; Song, C.W.; Wick, M.R.; Myers, D.E.; Waddick, K.; Ledbetter, J.A. Detailed Studies on Expression and Function of CD19 Surface Determinant by Using B43 Monoclonal Antibody and the Clinical Potential of Anti-CD19 Immunotoxins. Blood 1988, 71, 13–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailly, S.; Cartron, G.; Chaganti, S.; Córdoba, R.; Corradini, P.; Düll, J.; Ferrarini, I.; Osborne, W.; Rosenwald, A.; Sancho, J.; et al. Targeting CD19 in Diffuse Large B-cell Lymphoma: An Expert Opinion Paper. Hematol. Oncol. 2022, 40, 505–517. [Google Scholar] [CrossRef]
- Viardot, A.; Sala, E. Investigational Immunotherapy Targeting CD19 for the Treatment of Acute Lymphoblastic Leukemia. Expert Opin. Investig. Drugs 2021, 30, 773–784. [Google Scholar] [CrossRef]
- Zhang, Y.; Qian, J.; Gu, C.; Yang, Y. Alternative Splicing and Cancer: A Systematic Review. Signal Transduct. Target. Ther. 2021, 6, 78. [Google Scholar] [CrossRef]
- Maude, S.L.; Frey, N.; Shaw, P.A.; Aplenc, R.; Barrett, D.M.; Bunin, N.J.; Chew, A.; Gonzalez, V.E.; Zheng, Z.; Lacey, S.F.; et al. Chimeric Antigen Receptor T Cells for Sustained Remissions in Leukemia. N. Engl. J. Med. 2014, 371, 1507–1517. [Google Scholar] [CrossRef] [Green Version]
- Maude, S.L.; Laetsch, T.W.; Buechner, J.; Rives, S.; Boyer, M.; Bittencourt, H.; Bader, P.; Verneris, M.R.; Stefanski, H.E.; Myers, G.D.; et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N. Engl. J. Med. 2018, 378, 439–448. [Google Scholar] [CrossRef]
- Cortés-López, M. High-Throughput Mutagenesis Identifies Mutations and RNA-Binding Proteins Controlling CD19 Splicing and CART-19 Therapy Resistance. Nat. Commun. 2022, 13, 5570. [Google Scholar] [CrossRef]
- Fischer, J.; Paret, C.; El Malki, K.; Alt, F.; Wingerter, A.; Neu, M.A.; Kron, B.; Russo, A.; Lehmann, N.; Roth, L.; et al. CD19 Isoforms Enabling Resistance to CART-19 Immunotherapy Are Expressed in B-ALL Patients at Initial Diagnosis. J. Immunother. 2017, 40, 187–195. [Google Scholar] [CrossRef] [Green Version]
- Orlando, E.J.; Han, X.; Tribouley, C.; Wood, P.A.; Leary, R.J.; Riester, M.; Levine, J.E.; Qayed, M.; Grupp, S.A.; Boyer, M.; et al. Genetic Mechanisms of Target Antigen Loss in CAR19 Therapy of Acute Lymphoblastic Leukemia. Nat. Med. 2018, 24, 1504–1506. [Google Scholar] [CrossRef] [PubMed]
- Sotillo, E.; Barrett, D.M.; Black, K.L.; Bagashev, A.; Oldridge, D.; Wu, G.; Sussman, R.; Lanauze, C.; Ruella, M.; Gazzara, M.R.; et al. Convergence of Acquired Mutations and Alternative Splicing of CD19 Enables Resistance to CART-19 Immunotherapy. Cancer Discov. 2015, 5, 1282–1295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oak, J.; Spiegel, J.Y.; Sahaf, B.; Natkunam, Y.; Long, S.R.; Hossain, N.; Mackall, C.L.; Kong, K.A.; Miklos, D.B. Target Antigen Downregulation and Other Mechanisms of Failure after Axicabtagene Ciloleucel (CAR19) Therapy. Blood 2018, 132, 4656. [Google Scholar] [CrossRef]
- Majzner, R.G.; Mackall, C.L. Tumor Antigen Escape from CAR T-Cell Therapy. Cancer Discov. 2018, 8, 1219–1226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sworder, B.J.; Kurtz, D.M.; Alig, S.K.; Frank, M.J.; Shukla, N.; Garofalo, A.; Macaulay, C.W.; Shahrokh Esfahani, M.; Olsen, M.N.; Hamilton, J.; et al. Determinants of Resistance to Engineered T Cell Therapies Targeting CD19 in Large B Cell Lymphomas. Cancer Cell 2023, 41, 210–225.e5. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, X.; Tian, Y.; Li, F.; Zhao, X.; Liu, J.; Yao, C.; Zhang, Y. Point Mutation in CD19 Facilitates Immune Escape of B Cell Lymphoma from CAR-T Cell Therapy. J. Immunother. Cancer 2020, 8, e001150. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Hill, H.A.; Navsaria, L.J.; Wang, M.L. CAR-T and Other Adoptive Cell Therapies for B Cell Malignancies. J. Natl. Cancer Cent. 2021, 1, 88–96. [Google Scholar] [CrossRef]
- Bleakley, M.; Riddell, S.R. Exploiting T Cells Specific for Human Minor Histocompatibility Antigens for Therapy of Leukemia. Immunol. Cell Biol. 2011, 89, 396–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rentsch, V.; Seipel, K.; Banz, Y.; Wiedemann, G.; Porret, N.; Bacher, U.; Pabst, T. Glofitamab Treatment in Relapsed or Refractory DLBCL after CAR T-Cell Therapy. Cancers 2022, 14, 2516. [Google Scholar] [CrossRef]
- Phan, L.; Jin, Y.; Zhang, H.; Quiang, W.; Shekhtman, E.; Shao, D.; Revoe, D.; Villamarin, R.; Ivanchenko, E.; Kimura, M.; et al. ALFA: Allele Frequency Aggregator. Available online: www.ncbi.nlm.nih.gov/snp/docs/gsr/alfa/ (accessed on 5 May 2023).
- Taliun, D.; Harris, D.N.; Kessler, M.D.; Carlson, J.; Szpiech, Z.A.; Torres, R.; Taliun, S.A.G.; Corvelo, A.; Gogarten, S.M.; Kang, H.M.; et al. Sequencing of 53,831 Diverse Genomes from the NHLBI TOPMed Program. Nature 2021, 590, 290–299. [Google Scholar] [CrossRef]
- Spaapen, R.M.; Lokhorst, H.M.; van den Oudenalder, K.; Otterud, B.E.; Dolstra, H.; Leppert, M.F.; Minnema, M.C.; Bloem, A.C.; Mutis, T. Toward Targeting B Cell Cancers with CD4+ CTLs: Identification of a CD19-Encoded Minor Histocompatibility Antigen Using a Novel Genome-Wide Analysis. J. Exp. Med. 2008, 205, 2863–2872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bachy, E.; Le Gouill, S.; Di Blasi, R.; Sesques, P.; Manson, G.; Cartron, G.; Beauvais, D.; Roulin, L.; Gros, F.X.; Rubio, M.T.; et al. A Real-World Comparison of Tisagenlecleucel and Axicabtagene Ciloleucel CAR T Cells in Relapsed or Refractory Diffuse Large B Cell Lymphoma. Nat. Med. 2022, 28, 2145–2154. [Google Scholar] [CrossRef] [PubMed]
- Kamdar, M.; Solomon, S.R.; Arnason, J.; Johnston, P.B.; Glass, B.; Bachanova, V.; Ibrahimi, S.; Mielke, S.; Mutsaers, P.; Hernandez-Ilizaliturri, F.; et al. Lisocabtagene Maraleucel versus Standard of Care with Salvage Chemotherapy Followed by Autologous Stem Cell Transplantation as Second-Line Treatment in Patients with Relapsed or Refractory Large B-Cell Lymphoma (TRANSFORM): Results from an Interim Analysis of an Open-Label, Randomised, Phase 3 Trial. Lancet 2022, 399, 2294–2308. [Google Scholar] [CrossRef]
- Sarkozy, C.; Sehn, L.H. Management of Relapsed/Refractory DLBCL. Best Pract. Res. Clin. Haematol. 2018, 31, 209–216. [Google Scholar] [CrossRef]
- Klesmith, J.R.; Wu, L.; Lobb, R.R.; Rennert, P.D.; Hackel, B.J. Fine Epitope Mapping of the CD19 Extracellular Domain Promotes Design. Biochemistry 2019, 58, 4869–4881. [Google Scholar] [CrossRef] [PubMed]
- Nalls, M.A.; Blauwendraat, C.; Vallerga, C.L.; Heilbron, K.; Bandres-Ciga, S.; Chang, D.; Tan, M.; Kia, D.A.; Noyce, A.J.; Xue, A.; et al. Identification of Novel Risk Loci, Causal Insights, and Heritable Risk for Parkinson’s Disease: A Meta-Analysis of Genome- Wide Association Studies. Lancet Neurol. 2019, 18, 1091–1102. [Google Scholar] [CrossRef]



| Parameter | All Patients | CD19 V174 | CD19 L174 | p-Value * |
|---|---|---|---|---|
| Patients n (%) | 88 (100) | 37 (42) | 51 (58) | |
| Male to female ratio | 49:39 (1.3) | 26:11 (2.4) | 23:28 (0.8) | 0.029 |
| Age at the time of CAR-T cell therapy, median (range) | 67 (35–82) | 68 (42–82) | 66 (35–79) | 0.36 |
| Initial diagnosis | ||||
| DLBCL, n (%) | 88 (100) | |||
| de novo DLBCL, n (%) | 54 (61) | 24 (65) | 30 (59) | 0.66 |
| transformed DLBCL, n (%) | 34 (39) | 13 (35) | 21 (41) | 0.66 |
| Transformed from: | ||||
| FL, n (%) | 24 (27) | 9 (24) | 15 (15) | >0.99 |
| CLL, n (%) | 5(6) | 1 (3) | 4 (8) | 0.63 |
| MCL, n (%) | 3(3) | 1 (3) | 2 (4) | >0.99 |
| other, n (%) | 2 (2) | 2 (5) | 0 | 0.14 |
| Stage at initial diagnosis | ||||
| I, n (%) | 2 (2) | 1 (3) | 1 (2) | >0.99 |
| II, n (%) | 18 (20) | 7 (19) | 11 (22) | 0.79 |
| III, n (%) | 17 (19) | 8 (22) | 9 (18) | 0.78 |
| IV, n (%) | 49 (55) | 21 (57) | 28 (55) | >0.99 |
| Unknown | 2 (3) | 0 | 2 (4) | |
| Number of treatment lines before CAR-T cell therapy, n (%) | ||||
| 1 | 7 (8) | 0 | 7 (14) | 0.019 |
| 2 | 63 (72) | 28 (76) | 33 (65) | 0.35 |
| 3 | 13 (15) | 6 (16) | 8 (16) | >0.99 |
| >3 | 5 (6) | 2 (5) | 3 (6) | >0.99 |
| Previous radiotherapy | 16 (18) | 5 (14) | 11 (22) | 0.41 |
| Previous SCT | 44 (49) | 21 (57) | 23 (45) | 0.39 |
| Autologous SCT | 43 (48) | 20 (54) | 23 (45) | 0.52 |
| Allogeneic SCT | 1 (1) | 1 (3) | 0 | 0.42 |
| Parameter | All Patients | CD19 V174 | CD19 L174 | p-Value * |
|---|---|---|---|---|
| Patients n (%) | n = 88 (100) | n = 37 (42) | n = 51 (58) | |
| IPI before CAR-T cell therapy | ||||
| 1 | 3 (5) | 2 (5) | 1 (2) | 0.57 |
| 2 | 13 (14) | 6 (16) | 7 (14) | 0.77 |
| 3 | 36 (41) | 12 (32) | 24 (47) | 0.19 |
| 4 | 20 (23) | 11 (30) | 9 (18) | 0.31 |
| 5 | 2 (2) | 2 (5) | 0 (0) | 0.17 |
| Nd | 14 (16) | 4 (11) | 10 (20) | |
| Remission Status at CAR-T infusion | ||||
| CR | 3 (3) | 0 | 3 (6) | 0.26 |
| PR | 22 (25) | 11 (30) | 11 (22) | 0.46 |
| SD | 18 (20) | 5 (14) | 13 (25) | 0.19 |
| PD | 45 (51) | 21 (57) | 24 (47) | 0.27 |
| Bridging chemotherapy | 36 (41) | 19 (51) | 17 (33) | 0.40 |
| Bridging radiotherapy | 11(13) | 7 (19) | 7 (14) | 0.56 |
| Lymphodepleting chemotherapy with Fludarabidine, Cyclophosphamide | 88 (100) | |||
| LDH before CAR-T (U/L), median (range) | 458 (164–3949) | 457 (164–2355) | 472 (189–3949) | 0.49 |
| Median time between lymphapheresis and CAR-T cell infusion, days (range) | 40 (13–170) | 41 (26–170) | 38 (13–112) | 0.027 |
| CAR-T cell product | ||||
| Kymriah© | 56 (64) | 22 (59) | 34 (67) | 0.59 |
| Yescarta© | 26 (30) | 11 (30) | 15 (29) | >0.99 |
| Breyanzi© | 6 (7) | 4 (11) | 2 (4) | 0.24 |
| Cytokine release syndrome | 69 (78) | 29 (78) | 40 (78) | >0.99 |
| Grade 1 | 41 (47) | 17 (46) | 27 (53) | 0.66 |
| Grade2 | 21 (24) | 11 (30) | 10 (20) | 0.32 |
| Grade 3 | 3 (4) | 1 (3) | 2 (4) | >0.99 |
| Grade 4 | 4 (5) | 0 | 1 (2) | >0.99 |
| CAR-T-related encephalopathy syndrome | 31 (35) | 16 (43) | 15 (29) | 0.26 |
| Grade 1 | 9 (10) | 4 (11) | 5 (10) | >0.99 |
| Grade 2 | 5 (7) | 2 (5) | 3 (6) | >0.99 |
| Grade 3 | 13 (13) | 8 (22) | 5 (10) | 0.14 |
| Grade 4 | 4 (5) | 1 (3) | 3 (6) | 0.64 |
| Peak value CAR-T copies per µg cfDNA, median (range) | 4860 (37–218,384) | 4212 (37–139,656) | 5432 (193–218,384) | 0.86 |
| Time to peak value, median (range), days | 9 (2–46) | 10 (2–46) | 9 (5–41) | 0.11 |
| Parameter | CD19 V174 | CD19 L174 | p-Value * |
|---|---|---|---|
| n = 37 (42) | n = 51 (58) | ||
| Best response after CAR-T cell therapy | |||
| CR, n (%) | 11 (30) | 26 (51) | 0.05 |
| PR, n (%) | 13 (35) | 13 (25) | 0.35 |
| SD, n (%) | 5 (14) | 5 (10) | 0.73 |
| PD, n (%) | 8 (22) | 6 (12) | 0.25 |
| Time to best response (months), n (%) | |||
| 1, n (%) | 26 (70) | 31 (61) | 0.38 |
| 3, n (%) | 8 (22) | 15 (29) | 0.47 |
| 6, n (%) | 1 (3) | 1 (2) | >0.99 |
| 12, n (%) | 1 (3) | 4 (8) | 0.39 |
| 18, n (%) | 0 (0) | 0 (0) | >0.99 |
| 24, n (%) | 1 (3) | 1 (2) | >0.99 |
| 30, n (%) | 0 (0) | 1 (2) | >0.99 |
| Overall response (CR or PR) within 6 months, n (%) | 24 (65) | 39 (76) | 0.24 |
| CR after 1 year, n (%) | 13 (35) | 25 (49) | 0.27 |
| CR at last follow-up, n (%) | 13 (35) | 27 (53) | 0.13 |
| Primary refractory disease | 12 (32) | 7 (14) | 0.036 |
| Relapse after achieving CR, n (%) | 7 (19) | 11 (22) | 0.79 |
| Relapse treatment | 13 (35) | 16 (31) | 0.82 |
| Pharmacotherapy | 8 (22) | 11 (22) | |
| Radiotherapy | 5 (14) | 6 (12) | |
| Median time to relapse, months (range) | 6.1 (2.2–17.9) | 3.7 (1.4–28.8) | 0.39 |
| Death | 21 (57) | 22 (43) | 0.28 |
| Median time to death, months (range) | 2.77 (0.5–22.27) | 4.73 (0.1–38.2) | 0.60 |
| Median follow up time (months) | 24.33 | 22.85 | 0.96 |
| Median PFS (months) | 6.1 | 22.10 | 0.059 |
| Median OS (months) | 8.27 | 37 | 0.113 |
| PFS | OS | |||
|---|---|---|---|---|
| Predictors | HR (95% CI) | p-Value * | HR (95% CI) | p-Value * |
| CD19 L174 | 0.63 | 0.1172 | 0.64 | 0.1772 |
| Age > 65 | 1.8 | 0.0699 | 2.53 | 0.0101 |
| Male sex | 1.09 | 0.7864 | 1.29 | 0.4234 |
| Transformed DLBCL | 0.56 | 0.0852 | 0.40 | 0.0139 |
| Yescarta© (vs. Kymriah©) | 0.65 | 0.1801 | 0.57 | 0.1125 |
| CR within 6 months | 0.17 | <0.0001 | 0.13 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seipel, K.; Abbühl, M.; Bacher, U.; Nilius, H.; Daskalakis, M.; Pabst, T. Clinical Impact of Single Nucleotide Polymorphism in CD-19 on Treatment Outcome in FMC63-CAR-T Cell Therapy. Cancers 2023, 15, 3058. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers15113058
Seipel K, Abbühl M, Bacher U, Nilius H, Daskalakis M, Pabst T. Clinical Impact of Single Nucleotide Polymorphism in CD-19 on Treatment Outcome in FMC63-CAR-T Cell Therapy. Cancers. 2023; 15(11):3058. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers15113058
Chicago/Turabian StyleSeipel, Katja, Mariesol Abbühl, Ulrike Bacher, Henning Nilius, Michael Daskalakis, and Thomas Pabst. 2023. "Clinical Impact of Single Nucleotide Polymorphism in CD-19 on Treatment Outcome in FMC63-CAR-T Cell Therapy" Cancers 15, no. 11: 3058. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers15113058