Levulinic Acid Production from Delignified Rice Husk Waste over Manganese Catalysts: Heterogeneous Versus Homogeneous
Abstract
:1. Introduction
2. Results
2.1. Catalyst Characterization
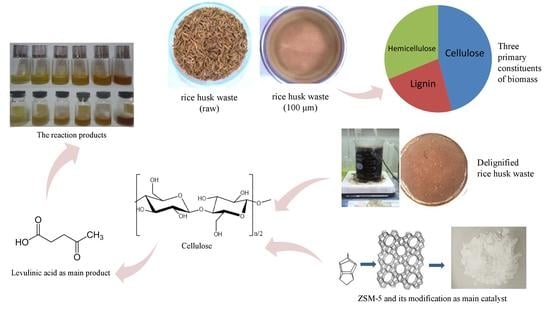
2.2. Cellulose Conversion Process Reaction of Delignified Rice Husk Waste to Levulinic Acid
2.2.1. Pre-Treatment of Rice Husk Waste
2.2.2. Catalytic Test
2.2.3. Side Product Analysis
2.2.4. 5-HMF Intermediate
2.2.5. Formic Acid
3. Discussion
4. Materials and Methods
4.1. Materials and Chemicals
4.2. Preparation of Hierarchical ZSM-5 Zeolite, Hierarchical Mn3O4/ZSM-5 Zeolite, Mn3O4 and Mn(II) ion
4.3. Pre-Treatment of Biomass
4.4. Catalytic Test: Conversion of Delignified Rice Husk
4.5. Product Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Girisuta, B.; Janssen, L.P.B.M.; Heeres, H.J. Green chemicals: A kinetic study on the conversion of glucose to levulinic acid. Chem. Eng. Res. Des. 2006, 84, 339–349. [Google Scholar] [CrossRef]
- Alonso, D.M.; Wettstein, S.G.; Dumesic, J.A. Gamma-valerolactone, a sustainable platform molecule derived from lignocellulosic biomass. Green Chem. 2013, 15, 584–595. [Google Scholar] [CrossRef]
- Kon, K.; Onodera, W.; Shimizu, K.I. Selective hydrogenation of levulinic acid to valeric acid and valeric biofuels by a Pt/HMFI catalyst. Catal. Sci. Technol. 2014, 4, 3227–3234. [Google Scholar] [CrossRef]
- Burton, A. Recent trends in the synthesis of high-silica zeolites. Catal. Rev. Sci. Eng. 2018, 60, 132–175. [Google Scholar] [CrossRef]
- Na, K.; Somorjai, G.A. Hierarchically Nanoporous Zeolites and Their Heterogeneous Catalysis: Current Status and Future Perspectives. Catal. Lett. 2015, 145, 193–213. [Google Scholar] [CrossRef] [Green Version]
- Přech, J.; Pizarro, P.; Serrano, D.P.; Áejka, J. From 3D to 2D zeolite catalytic materials. Chem. Soc. Rev. 2018, 47, 8263–8306. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Zhang, Z.; Yin, C.; Shan, Z.; Xiao, F. Microporous and Mesoporous Materials Hierarchical mesoporous zeolites with controllable mesoporosity templated from cationic polymers. Microporous Mesoporous Mater. 2010, 131, 58–67. [Google Scholar] [CrossRef]
- Inayat, A.; Knoke, I.; Spiecker, E.; Schwieger, W. Assemblies of mesoporous FAU-type zeolite nanosheets. Angew. Chemie Int. Ed. 2012, 51, 1962–1965. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhao, G.; Teng, J.; Wang, Y.; Xie, Z. Morphology control of ZSM-5 zeolites and their application in Cracking reaction of C4 olefin. Inorg. Chem. Front. 2018, 5, 2734–2738. [Google Scholar] [CrossRef]
- Weckhuysen, B.M.; Yu, J. Recent advances in zeolite chemistry and catalysis. Chem. Soc. Rev. 2015, 44, 7022–7024. [Google Scholar] [CrossRef]
- Krisnandi, Y.K.; Putra, B.A.P.; Bahtiar, M.; Zahara; Abdullah, I.; Howe, R.F. Partial Oxidation of Methane to Methanol over Heterogeneous Catalyst Co/ZSM-5. Procedia Chem. 2015, 14, 508–515. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Zhang, C.; Gao, P.; Wang, H.; Li, X.; Zhong, L.; Wei, W.; Sun, Y. A review of the catalytic hydrogenation of carbon dioxide into value-added hydrocarbons. Catal. Sci. Technol. 2017, 7, 4580–4598. [Google Scholar] [CrossRef]
- Chen, H.; Shi, X.; Zhou, F.; Ma, H.; Qiao, K.; Lu, X.; Fu, J.; Huang, H. Catalytic fast pyrolysis of cellulose to aromatics over hierarchical nanocrystalline ZSM-5 zeolites prepared using sucrose as a template. Catal. Commun. 2018, 110, 102–105. [Google Scholar] [CrossRef]
- Antonetti, C.; Galletti, A.M.R.; De Luise, V.; Licursi, D.; Nasso, N. Levulinic Acid Production from waste biomass. J. Natl. Cancer Inst. Monogr. 2014, 7, 1824–1835. [Google Scholar]
- Kumar, P.; Barrett, D.M.; Delwiche, M.J.; Stroeve, P.; Kumar, P.; Barrett, D.M.; Delwiche, M.J.; Stroeve, P. Methods for Pretreatment of Lignocellulosic Biomass for Efficient Hydrolysis and Biofuel Production. Ind. Eng. Chem. Res. 2009, 48, 3713–3729. [Google Scholar] [CrossRef]
- Rahayu, D.U.C.; Nurani, D.A.; Rochmah, L.N.H.; Antra, N.F.; Krisnandi, Y.K. A hierarchical MnOx/ZSM-5 heterogeneous catalyst for the conversion of cellulose from mahogany wood to levulinic acid. IOP Conf. Ser. Mater. Sci. Eng. 2019, 496, 012055. [Google Scholar] [CrossRef]
- Krisnandi, Y.K.; Nurani, D.A.; Agnes, A.; Pertiwi, R.; Antra, N.F.; Anggraini, A.R.; Azaria, A.P.; Howe, R.F. Hierarchical MnOx/ZSM-5 as Heterogeneous Catalysts in Conversion of Delignified Rice Husk to Levulinic Acid. Indones. J. Chem 2019, 19, 115–123. [Google Scholar] [CrossRef]
- Garcés, D.; Faba, L.; Díaz, E.; Ordóñez, S. Aqueous-Phase Transformation of Glucose into Hydroxymethylfurfural and Levulinic Acid by Combining Homogeneous and Heterogeneous Catalysis. ChemSusChem 2019, 12, 924–934. [Google Scholar]
- Treacy, M.M.; Higgins, J.B. Collection of Simulated XRD Powder Patterns for Zeolites; Elsevier: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Wang, L.; Chen, L.; Li, Y.; Ji, H.; Yang, G. Preparation of Mn3O4 nanoparticles at room condition for supercapacitor application. Powder Technol. 2013, 235, 76–81. [Google Scholar] [CrossRef]
- Pang, H.; Abdalla, A.M.; Sahu, R.P.; Duan, Y.; Puri, I.K. Low-temperature synthesis of manganese oxide–carbon nanotube-enhanced microwave-absorbing nanocomposites. J. Mater. Sci. 2018, 53, 16288–16302. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J. Spectrometric Identification of Organic Compounds; John Wiley & Sons: Hoboken, NJ, USA, 2005; ISBN 0-471-39362-2. [Google Scholar]
- Tian, Z.Y.; Mountapmbeme Kouotou, P.; Bahlawane, N.; Tchoua Ngamou, P.H. Synthesis of the catalytically active Mn3O4 spinel and its thermal properties. J. Phys. Chem. C 2013, 117, 6218–6224. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, L.; Yang, X.; Shao, E.; Deng, X.; Liu, N.; Wu, M. Facile synthesis of three-dimensional Mn 3 O 4 hierarchical microstructures and their application in the degradation of methylene blue. J. Mater. Chem. A 2015, 3, 2934–2941. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Liu, J.; Wang, C. A Comparative Study of MFI Zeolite Derived from Different Silica Sources: Synthesis, Characterization and Catalytic Performance. Catalysts 2018, 9, 13. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Wang, F.; Feng, Z.; Liu, Z.; Guan, J. Hierarchical ZSM-5 Zeolite with Enhanced Catalytic Activity for Alkylation of Phenol with Tert-Butanol. Catalysts 2019, 9, 202. [Google Scholar] [CrossRef] [Green Version]
- Al-Thawabeia, R.A.; Hodali, H.A. Use of Zeolite ZSM-5 for Loading and Release of 5-Fluorouracil. J. Chem. 2015, 2015, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Basu, P. Biomass Gasification and Pyrolysis; Elsevier: Oxford, UK, 2010; ISBN 978-0-12-374988-8. [Google Scholar]
- Zhao, X.; Zhang, L.; Liu, D. Biomass recalcitrance. Part II: Fundamentals of different pre-treatments to increase the enzymatic digestibility of lignocellulose. Biofuels Bioprod. Biorefining 2012, 6, 561–579. [Google Scholar] [CrossRef]
- Kumar, A.K.; Sharma, S. Recent updates on different methods of pretreatment of lignocellulosic feedstocks: A review. Bioresour. Bioprocess. 2017, 4, 7. [Google Scholar] [CrossRef] [Green Version]
- Rhadfi, T.; Piquemal, J.Y.; Sicard, L.; Herbst, F.; Briot, E.; Benedetti, M.; Atlamsani, A. Polyol-made Mn3O4 nanocrystals as efficient Fenton-like catalysts. Appl. Catal. A Gen. 2010, 386, 132–139. [Google Scholar] [CrossRef]
- Azadmanesh, J.; Borgstahl, G. A Review of the Catalytic Mechanism of Human Manganese Superoxide Dismutase. Antioxidants 2018, 7, 25. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Li, G.; Yang, F.; Zhang, S. Mn/ZSM-5 participation in the degradation of cellulose under phosphoric acid media. Polym. Degrad. Stab. 2011, 96, 863–869. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Navalon, S.; Alvaro, M.; Garcia, H. Metal nanoparticles as heterogeneous fenton catalysts. ChemSusChem 2012, 5, 46–64. [Google Scholar] [CrossRef] [PubMed]
- Gardner, D.W.; Huo, J.; Hoff, T.C.; Johnson, R.L.; Shanks, B.H.; Tessonnier, J.P. Insights into the Hydrothermal Stability of ZSM-5 under Relevant Biomass Conversion Reaction Conditions. ACS Catal. 2015, 5, 4418–4422. [Google Scholar] [CrossRef]
- Jeong, H.; Park, S.; Ryu, G.; Choi, J.; Kim, J.; Choi, W. Catalytic conversion of hemicellulosic sugars derived from biomass to levulinic acid. Catal. Commun. 2018, 117, 19–25. [Google Scholar] [CrossRef]
- Otromke, M.; White, R.J.; Sauer, J. Hydrothermal base catalyzed depolymerization and conversion of technical lignin—An introductory review. Carbon Resour. Convers. 2019, 2, 59–71. [Google Scholar] [CrossRef]
- Qi, Y.; Song, B.; Qi, Y. The roles of formic acid and levulinic acid on the formation and growth of carbonaceous spheres by hydrothermal carbonization. RSC Adv. 2016, 6, 102428–102435. [Google Scholar] [CrossRef]
- Prodinger, S.; Shi, H.; Eckstein, S.; Hu, J.Z.; Olarte, M.V.; Camaioni, D.M.; Derewinski, M.A.; Lercher, J.A. Stability of Zeolites in Aqueous Phase Reactions. Chem. Mater. 2017, 29, 7255–7262. [Google Scholar] [CrossRef]
- Ravenelle, R.M.; Schübler, F.; Damico, A.; Danilina, N.; Van Bokhoven, J.A.; Lercher, J.A.; Jones, C.W.; Sievers, C. Stability of zeolites in hot liquid water. J. Phys. Chem. C 2010, 114, 19582–19595. [Google Scholar] [CrossRef]
- Poliakoff, M.; Licence, P.; George, M.W. UN sustainable development goals: How can sustainable/green chemistry contribute? By doing things differently. Curr. Opin. Green Sustain. Chem. 2018, 13, 146–149. [Google Scholar] [CrossRef]
- Weingarten, R.; Conner, W.C.; Huber, G.W. Production of levulinic acid from cellulose by hydrothermal decomposition combined with aqueous phase dehydration with a solid acid catalyst. Energy Environ. Sci. 2012, 5, 7559–7574. [Google Scholar] [CrossRef]
- Singh, M.; Pandey, N.; Dwivedi, P.; Kumar, V.; Mishra, B.B. Production of xylose, levulinic acid, and lignin from spent aromatic biomass with a recyclable BrØnsted acid synthesized from d-limonene as renewable feedstock from citrus waste. Bioresour. Technol. 2019, 293, 122105. [Google Scholar] [CrossRef]
- Krisnandi, Y.K.; Nurani, D.A. Akmal Partial Oxidation of Methane Over NiOx / Hierachichal ZSM-5 Catalyst Partial Oxidation of Methane Over NiOx / Hierachichal ZSM-5 Catalyst. IOP Conf. Ser. J. Phys. Conf. Ser. 2018, 1095, 012005. [Google Scholar]
- Minnaar, P.P.; Swan, G.E.; McCrindle, R.I.; De Beer, W.H.J.; Naudé, T.W. A high-performance liquid chromatographic method for the determination of monofluoroacetate. J. Chromatogr. Sci. 2000, 38, 16–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]








| Samples | S BET a (m2/g) | S Micro b (m2/g) | S Meso c (m2/g) | Total Pore Volume d (cm3/g) | Micropore Volume e (cm3/g) | Mesoporous Volume f (cm3/g) | Average Pore Diameter g (nm) | |
|---|---|---|---|---|---|---|---|---|
| Micro- | Meso- | |||||||
| ZSM-5 | 348.6 | 199.1 | 149.5 | 0.2671 | 0.0969 | 0.17025 | 1.8842 | 2.7419 |
| Mn3O4/ZSM-5 | 296.1 | 143.5 | 152.6 | 0.2467 | 0.0704 | 0.17626 | 1.8881 | 2.3979 |
| No. | Chemical Compounds | Yield (wt %) | |
|---|---|---|---|
| Before | After | ||
| Treatment | |||
| 1 | Lignin | 33.53 | 17.24 |
| 2 | α-Cellulose | 42.37 | 25.66 |
| 3 | Hemicellulose | 10.70 | 5.75 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pratama, A.P.; Rahayu, D.U.C.; Krisnandi, Y.K. Levulinic Acid Production from Delignified Rice Husk Waste over Manganese Catalysts: Heterogeneous Versus Homogeneous. Catalysts 2020, 10, 327. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10030327
Pratama AP, Rahayu DUC, Krisnandi YK. Levulinic Acid Production from Delignified Rice Husk Waste over Manganese Catalysts: Heterogeneous Versus Homogeneous. Catalysts. 2020; 10(3):327. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10030327
Chicago/Turabian StylePratama, Arnia Putri, Dyah Utami Cahyaning Rahayu, and Yuni Krisyuningsih Krisnandi. 2020. "Levulinic Acid Production from Delignified Rice Husk Waste over Manganese Catalysts: Heterogeneous Versus Homogeneous" Catalysts 10, no. 3: 327. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10030327