Platinum Group Metal-Free Catalysts for Oxygen Reduction Reaction: Applications in Microbial Fuel Cells
Abstract
:1. Introduction
2. Main Types of Bioelectrochemical Systems
2.1. Enzymatic Fuel Cells
2.2. Microbial Fuel Cells (MFCs)
3. Oxygen Reduction Reaction at the Cathode Side of MFCs: Electrode Kinetics and Electrocatalysis
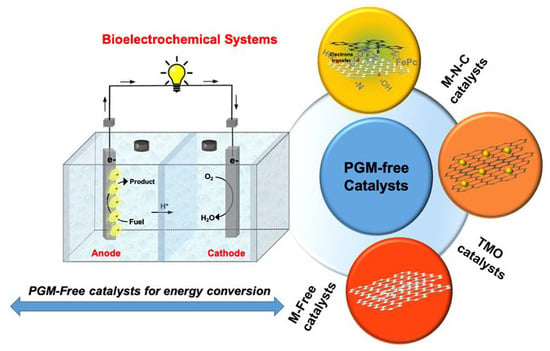
4. Oxygen Reducing Catalysts Based on Platinum Group Metal-Free Materials
4.1. Transition Metal-Nitrogen-Carbon (M-N-C) Catalysts
4.1.1. Pyrolyzed Catalysts
4.1.2. Molecular Catalysts
4.2. Transition Metal Oxides (TMOs)
4.3. Metal-Free Catalysts
5. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Jung, S.; Lee, J.; Park, Y.K.; Kwon, E.E. Bioelectrochemical systems for a circular bioeconomy. Bioresour. Technol. 2020, 300, 122748. [Google Scholar] [CrossRef]
- Ivase, T.J.P.; Nyakuma, B.B.; Oladokun, O.; Abu, P.T.; Hassan, M.N. Review of the principal mechanisms, prospects, and challenges of bioelectrochemical systems. Environ. Prog. Sustain. Energy 2020, 39, e13298. [Google Scholar] [CrossRef]
- Zhang, X.; Li, X.; Zhao, X.; Li, Y. Factors affecting the efficiency of a bioelectrochemical system: A review. RSC Adv. 2019, 9, 19748–19761. [Google Scholar] [CrossRef]
- Rinaldi, A.; Mecheri, B.; Garavaglia, V.; Licoccia, S.; Di Nardo, P.; Traversa, E. Engineering materials and biology to boost performance of microbial fuel cells: A critical review. Energy Environ. Sci. 2008, 1, 417–429. [Google Scholar] [CrossRef]
- Katz, E. Biofuel Cells with Switchable Power Output. Electroanalysis 2010, 22, 744–756. [Google Scholar] [CrossRef]
- Yuan, Y.; Yuan, T.; Wang, D.; Tang, J.; Zhou, S. Sewage sludge biochar as an efficient catalyst for oxygen reduction reaction in a microbial fuel cell. Biosour. Technol. 2013, 144, 115–120. [Google Scholar] [CrossRef]
- Vries, S.C.; van de Ven, G.W.J.; van Ittersum, M.K.; Giller, K.E. Resource use efficiency and environmental performance of nine major biofuel crops, processed by first-generation conversion techniques. Biomass Bioenergy 2010, 34, 588–601. [Google Scholar] [CrossRef]
- Cecconet, D.; Callegari, A.; Capodaglio, A.G. Bioelectrochemical systems for removal of selected metals and perchlorate from groundwater: A review. Energies 2018, 11, 2643. [Google Scholar] [CrossRef] [Green Version]
- Fang, C.; Achal, V. The potential of microbial fuel cells for remediation of heavy metals from soil and water—Review of application. Microorganisms 2019, 7, 697. [Google Scholar] [CrossRef] [Green Version]
- Włodarczyk, P.P.; Włodarczyk, B. Microbial fuel cell with Ni-Co cathode powered with yeast wastewater. Energies 2018, 11, 3194. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, D.V.P.; Jacobs, D.; Gregory, K.; Huang, J.; Hu, Y.; Vidic, R.; Yun, M. Changes in carbon electrode morphology affect microbial fuel cell performance with Shewanella oneidensis MR-1. Energies 2015, 8, 1817–1829. [Google Scholar] [CrossRef] [Green Version]
- Włodarczyk, P.P.; Włodarczyk, B. Preparation and analysis of ni–co catalyst use for electricity production and COD reduction in microbial fuel cells. Catalysts 2019, 9, 1042. [Google Scholar] [CrossRef] [Green Version]
- Kabutey, F.T.; Zhao, Q.; Wei, L.; Ding, J.; Antwi, P.; Quashie, F.K.; Wang, W. An overview of plant microbial fuel cells (PMFCs): Configurations and applications. Renew. Sustain. Energy Rev. 2019, 110, 402–414. [Google Scholar] [CrossRef]
- Christwardana, M.; Frattini, D.; Accardo, G.; Yoon, S.P.; Kwon, Y. Effects of methylene blue and methyl red mediators on performance of yeast based microbial fuel cells adopting polyethylenimine coated carbon felt as anode. J. Power Sources 2018, 396, 1–11. [Google Scholar] [CrossRef]
- Jannelli, N.; Anna Nastro, R.; Cigolotti, V.; Minutillo, M.; Falcucci, G. Low pH, high salinity: Too much for microbial fuel cells? Appl. Energy 2017, 192, 543–550. [Google Scholar] [CrossRef] [Green Version]
- Addi, H.; Mateo-Ramírez, F.; Ortiz-Martínez, V.M.; Salar-García, M.J.; Hernández-Fernández, F.J.; de los Ríos, A.P.; Godínez, C.; Lotfi, E.M.; Mahi, M.E.; Blanco, L.J.L. Treatment of mineral oil refinery wastewater in microbial fuel cells using ionic liquid based separators. Appl. Sci. 2018, 8, 438. [Google Scholar] [CrossRef] [Green Version]
- Santoro, C.; Arbizzani, C.; Erable, B.; Ieropoulos, I. Microbial fuel cells: From fundamentals to applications. A review. J. Power Sources 2017, 356, 225–244. [Google Scholar] [CrossRef]
- Do, M.H.; Ngo, H.H.; Guo, W.S.; Liu, Y.; Chang, S.W.; Nguyen, D.D.; Nghiem, L.D.; Ni, B.J. Challenges in the application of microbial fuel cells to wastewater treatment and energy production: A mini review. Sci. Total Environ. 2018, 639, 910–920. [Google Scholar] [CrossRef]
- Santoro, S.; Kodali, M.; Herrera, S.; Serov, A.; Ieropoulos, I.; Atanassov, P. Power generation in microbial fuel cells using platinum group metal-free cathode catalyst: Effect of the catalyst loading on performance and costs. J. Power Sources 2018, 378, 169–175. [Google Scholar] [CrossRef]
- Oliveira, M.A.C.; Mecheri, B.; D’Epifanio, A.; Placidi, E.; Arciprete, F.; Valentini, F.; Perandini, A.; Valentini, V.; Licoccia, S. Graphene oxide nanoplatforms to enhance catalytic performance of iron phthalocyanine for oxygen reduction reaction in bioelectrochemical systems. J. Power Sources 2017, 356, 381–388. [Google Scholar] [CrossRef]
- Santoro, C.; Serov, A.; Stariha, L.; Kodali, M.; Gordon, J.; Babanova, S.; Bretschger, O.; Artyushkova, K.; Atanassov, P. Iron based catalysts from novel low-cost organic precursors for enhanced oxygen reduction reaction in neutral media microbial fuel cells. Energy Environ. Sci. 2016, 9, 2346–2353. [Google Scholar] [CrossRef] [Green Version]
- Rojas-Carbonell, S.; Santoro, C.; Serov, A.; Atanassov, P. Transition metal-nitrogen-carbon catalysts for oxygen reduction reaction in neutral electrolyte. Electrochem. Commun. 2017, 75, 38–42. [Google Scholar] [CrossRef]
- Bajracharya, S.; Sharma, M.; Mohanakrishna, G.; Benneton, X.D.; Strik, D.P.B.T.B.; Sarma, P.M.; Pant, D. An overview on emerging bioelectrochemical systems (BESs): Technology for sustainable electricity, waste remediation, resource recovery, chemical production and beyond. Renew. Energy 2016, 98, 153–170. [Google Scholar] [CrossRef]
- Xiao, X.; Xia, H.Q.; Wu, R.; Bai, L.; Yan, L.; Magner, E.; Cosnier, S.; Lojou, E.; Zhu, Z.; Liu, A. Tackling the challenges of enzymatic (bio) fuel cells. Chem. Rev. 2019, 119, 9509–9958. [Google Scholar] [CrossRef] [PubMed]
- Osman, M.H.; Shah, A.A.; Walsh, F.C. Biosensors and Bioelectronics Recent progress and continuing challenges in bio-fuel cells. Part I: Enzymatic cells. Biosens. Bioelectron. 2011, 26, 3087–3102. [Google Scholar] [CrossRef] [PubMed]
- Willner, I.; Katz, E. Integration of layered redox proteins and conductive supports for bioelectronic applications. Angew. Chem. Int. Ed. 2000, 39, 1180–1218. [Google Scholar] [CrossRef]
- Mecheri, B.; De Porcellinis, D.; Campana, P.T.; Rainer, A.; Trombetta, M.; Marletta, A.; Oliveira, N.O., Jr.; Licoccia, S. Tuning structural changes in glucose oxidase for enzyme fuel cell applications. ACS Appl. Mater. Interfaces 2015, 7, 28311–28318. [Google Scholar] [CrossRef]
- Mano, N.; De Poulpiquet, A. O2 reduction in enzymatic biofuel cells. Chem. Rev. 2018, 118, 2392–2468. [Google Scholar] [CrossRef] [Green Version]
- Shleev, S.; Tkac, J.; Christenson, A.; Ruzgas, T.; Yaropolov, A.I.; Whittaker, J.W.; Gorton, L. Direct electron transfer between copper-containing proteins and electrodes. Biosens. Bioelectron. 2005, 20, 2517–2554. [Google Scholar] [CrossRef]
- Kamitaka, Y.; Tsujimura, S.; Kataoka, S.; Sakurai, T.; Ikeda, T.; Kano, K. Effects of axial ligand mutation of the type I copper site in bilirubin oxidase on direct electron transfer-type bioelectrocatalytic reduction of dioxygen. J. Electroanal. Chem. 2007, 601, 119–124. [Google Scholar] [CrossRef]
- Miura, Y.; Tsujimura, S.; Kurose, S.; Kamitaka, Y.; Kataoka, K.; Sakurai, T.; Kano, K. Direct electrochemistry of CueO and its mutants at residues to and near type I Cu for oxygen-reducing biocathode. Fuel Cells 2009, 9, 70–78. [Google Scholar] [CrossRef]
- Gooding, J.J.; Wibowo, R.; Liu, J.; Yang, W.; Losic, D.; Orbons, S.; Mearns, F.J.; Shapter, J.G.; Hibbert, D.B. Protein electrochemistry using aligned carbon nanotube arrays. J. Am. Chem. Soc. 2003, 125, 9006–9007. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Li, Q.; Su, L.; Yan, Y.; Zhang, J.; Mao, L. Direct electrochemistry of multi-copper oxidases at carbon nanotubes noncovalently functionalized with cellulose derivatives. Electroanalysis 2006, 18, 587–594. [Google Scholar] [CrossRef]
- Cass, A.E.G.; Davis, G.; Francis, G.D.; Hill, H.A.O.; Aston, W.J.; Higgins, I.J.; Plotkin, E.V.; Scott, L.D.L.; Turner, A.P.F. Ferrocene-mediated enzyme electrode for amperometric determination of glucose. Anal. Chem. 1984, 56, 667–671. [Google Scholar] [CrossRef]
- Tsujimura, S.; Tatsumi, H.; Ogawa, J.; Shimizu, S.; Kano, K.; Ikeda, T. Bioelectrocatalytic reduction of dioxygen to water at neutral pH using bilirubin oxidase as an enzyme and 2,2-azinobis (3-ethylbenzothiazolin-6-sulfonate) as an electron transfer mediator. J. Electroanal. Chem. 2001, 496, 69–75. [Google Scholar] [CrossRef]
- Patolsky, F.; Lichtenstein, A.; Willner, I. Electronic transduction of DNA sensing processes on surfaces: Amplification of DNA detection and analysis of single-base mismatches by tagged liposomes. J. Am. Chem. Soc. 2001, 123, 5194–5205. [Google Scholar] [CrossRef] [PubMed]
- Ohnuki, H.; Wako, T.; Mecheri, B.; Wu, H.; Tsuya, D.; Endo, H. Self-powered hydrogen peroxide sensor and its application as a biosensor. Jpn. J. Appl. Phys. 2019, 58, SBBG16. [Google Scholar] [CrossRef]
- Ohnuki, H.; Saiki, T.; Kusakari, A.; Endo, H.; Ichihara, M.; Izumi, M. Incorporation of glucose oxidase into langmuir-blodgett films based on prussian blue applied to amperometric glucose biosensor. Langmuir 2007, 23, 4675–4681. [Google Scholar] [CrossRef]
- Mano, N.; Mao, F.; Heller, A. On the parameters affecting the characteristics of the ‘wired’ glucose oxidase anode. J. Electroanal. Chem. 2005, 574, 347–357. [Google Scholar] [CrossRef]
- Heller, A. Electron-conducting redox hydrogels: Design, characteristics and synthesis. Curr. Opin. Chem. Biol. 2006, 10, 664–672. [Google Scholar] [CrossRef]
- Wang, H.; Ohnuki, H.; Endo, H.; Izumi, M. Impedimetric and amperometric bifunctional glucose biosensor based on hybrid organic-inorganic thin films. Bioelectrochemistry 2005, 101, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.M.; Akers, N.L.; Hill, A.D.; Johnson, Z.C.; Minteer, S.D. Improving the environment for immobilized dehydrogenase enzymes by modifying nafion with tetraalkylammonium bromides. Biomacromolecules 2004, 5, 1241–1247. [Google Scholar] [CrossRef] [PubMed]
- Beneyton, T.; El Harrak, A.; Griffiths, A.D.; Hellwig, P.; Taly, V. Immobilization of CotA, an extremophilic laccase from Bacillus subtilis, on glassy carbon electrodes for biofuel cell applications. Electrochem. Commun. 2011, 13, 24–27. [Google Scholar] [CrossRef]
- Nimje, V.R.; Chen, C.C.; Chen, H.R.; Chen, C.Y.; Tseng, M.J.; Cheng, K.C.; Shih, R.C.; Chang, Y.F. A single-chamber microbial fuel cell without an air cathode. Int. J. Mol. Sci. 2012, 13, 3933–3948. [Google Scholar] [CrossRef] [Green Version]
- Cheng, S.; Liu, H.; Logan, B.E. Increased performance of single-chamber microbial fuel cells using an improved cathode structure. Electrochem. Commun. 2006, 8, 489–494. [Google Scholar] [CrossRef]
- Logan, B.E.; Cheng, S.; Watson, V.; Estadt, G. Graphite fiber brush anodes for increased power production in air-cathode microbial fuel cells. Environ. Sci. Technol. 2007, 9, 3341–3346. [Google Scholar] [CrossRef]
- Rozendal, R.A.; Hamelers, H.V.M.; Rabaey, K.; Keller, J.; Buisman, C.J.N. Towards practical implementation of bioelectrochemical wastewater treatment. Trends Biotechnol. 2008, 26, 450–459. [Google Scholar] [CrossRef]
- Rismani-Yazdi, S.M.H.; Carver, A.D.; Christy, O.H. Cathodic limitations in microbial fuel cells: An overview. J. Power Sources 2008, 180, 683–694. [Google Scholar] [CrossRef]
- Wang, Z.; Mahadevan, G.D.; Wu, Y.; Zhao, F. Progress of air-breathing cathode in microbial fuel cells. J. Power Sources 2017, 356, 245–255. [Google Scholar] [CrossRef]
- Li, Y.; Li, Q.; Wang, H.; Zhang, L.; Wilkinson, D.P.; Zhang, J. Recent progresses in oxygen reduction reaction electrocatalysts for electrochemical energy applications. Electrochem. Energy Rev. 2019, 2, 518–538. [Google Scholar] [CrossRef] [Green Version]
- Choi, C.H.; Kwon, H.C.; Yook, S.; Shin, H.; Kim, H.; Choi, M. Hydrogen peroxide synthesis via enhanced two-electron oxygen reduction pathway on carbon-coated Pt surface. J. Phys. Chem. C 2014, 118, 30063–30070. [Google Scholar] [CrossRef]
- Ma, R.; Lin, G.; Zhou, Y.; Liu, Q.; Zhang, T.; Shan, G.; Yang, M.; Wang, J. A review of oxygen reduction mechanisms for metal-free carbon-based electrocatalysts. npj Comput. Mater. 2019, 5, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Zhou, R.; Zheng, Y.; Jaroniec, M.; Qiao, S.-Z. Determination of the electron transfer number for the oxygen reduction reaction: From theory to experiment. ACS Catal. 2016, 6, 4720–4728. [Google Scholar] [CrossRef]
- Yang, T.; Wang, M.; Ju, X.; Zhao, J.; Fu, C. The efficient oxygen reduction catalysts based on the non-noble metal and conducting polymers. Int. J. Electrochem. Sci. 2017, 12, 12125–12139. [Google Scholar] [CrossRef]
- Ramaswamy, N.; Mukerjee, S. Fundamental mechanistic understanding of electrocatalysis of oxygen reduction on Pt and non-Pt surfaces: Acid versus Alkaline Media. Adv. Phys. Chem. 2012, 491604, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.-H.; Li, P.-C. Electron transfer number control of the oxygen reduction reaction on nitrogen-doped reduced graphene oxides for the air electrodes of zinc-air batteries and organic degradation. Mater. Chem. Phys. 2016, 183, 551–560. [Google Scholar] [CrossRef]
- Brocato, S.; Serov, A.; Atanassov, P. PH dependence of catalytic activity for ORR of the non-PGM catalyst derived from heat-treated Fe-phenanthroline. Electrochim. Acta 2013, 87, 361–365. [Google Scholar] [CrossRef]
- Xie, J.; Li, S.; Zhang, X.; Zhang, J.; Wang, R.; Zhang, H.; Pan, B.; Xie, Y. Atomically thin molybdenum nitride nanosheets with exposed active surface sites for efficient hydrogen evolution. Chem. Sci. 2014, 5, 4615–4620. [Google Scholar] [CrossRef]
- Chen, W.; Fan, Z.; Gu, L.; Bao, X.; Wang, C. Enhanced capacitance of manganese oxide via confinement inside carbon nanotubes. Chem. Commun. 2010, 46, 3905–3907. [Google Scholar] [CrossRef]
- Madkikar, P.; Menga, D.; Harzer, G.S.; Mittermeier, T.; Siebel, A.; Wagner, F.E.; Merz, M.; Schuppler, S.; Nagel, P.; Muñoz-García, A.B.; et al. Nanometric Fe-substituted ZrO2 on carbon black as PGM-free ORR catalyst for PEMFCs. J. Electrochem. Soc. 2019, 166, F3032–F3043. [Google Scholar] [CrossRef]
- Mecheri, B.; Ficca, V.C.A.; Oliveira, M.A.C.; Placidi, E.; Arciprete, F.; D’Epifanio, A.; Licoccia, S. Facile synthesis of graphene-phthalocyanine composites as oxygen reduction electrocatalysts in microbial fuel cells. Appl. Catal. B-Environ. 2018, 237, 699–707. [Google Scholar] [CrossRef]
- Oliveira, M.A.C.; Mecheri, B.; Zurlo, F.; D’Epifanio, A.; Licoccia, S. Optimization of PGM-free cathodes for oxygen reduction in microbial fuel cells. Electrochim. Acta 2020, 334, 135650. [Google Scholar] [CrossRef]
- Jiang, K.; Back, S.; Akey, A.J.; Xia, C.; Hu, Y.; Liang, W.; Schaak, D.; Stavitski, E.; Nørskov, J.K.; Siahrostami, S.; et al. Highly selective oxygen reduction to hydrogen peroxide on transition metal single atom coordination. Nat. Commun. 2019, 10, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.; Wang, L.; Zhao, K.; Shi, S.; Shao, S.; Zhang, L.; Sun, X.; Zhao, Y.; Zhang, J. Atomically dispersed metal catalysts for the oxygen reduction reaction: Synthesis, characterization, reaction mechanisms and electrochemical energy applications. Energy Environ. Sci. 2019, 12, 2890–2923. [Google Scholar] [CrossRef]
- Setzler, B.P.; Zhuang, Z.; Wittkop, J.A.; Yan, Y. Activity targets for nanostructured platinum-group-metal-free catalysts in hydroxide exchange membrane fuel cells. Nat. Nanotechnol. 2016, 11, 1020–1025. [Google Scholar] [CrossRef]
- Lefèvre, M.; Proietti, E.; Jaouen, F.; Dodelet, J.P. Iron-based catalysts with improved oxygen reduction activity in polymer electrolyte fuel cells. Science 2009, 324, 71–74. [Google Scholar] [CrossRef]
- Jaouen, F.; Proietti, E.; Lefèvre, M.; Chenitz, R.; Dodelet, J.P.; Wu, G.; Chung, H.T.; Johnston, C.M.; Zelenay, P. Recent advances in non-precious metal catalysis for oxygen-reduction reaction in polymer electrolyte fuel cells. Energy Environ. Sci. 2011, 4, 114–130. [Google Scholar] [CrossRef]
- Artyushkova, K.; Serov, A.; Rojas-Carbonell, S.; Atanassov, P. Chemistry of multitudinous active sites for oxygen reduction reaction in transition metal-nitrogen-carbon electrocatalysts. J. Phys. Chem. C 2015, 119, 25917–25928. [Google Scholar] [CrossRef]
- Mohamed, H.O.; Sayed, E.T.; Obaid, M.; Choi, Y.J.; Park, S.G.; Al-Qaradawi, S.; Chae, K.J. Transition metal nanoparticles doped carbon paper as a cost-effective anode in a microbial fuel cell powered by pure and mixed biocatalyst cultures. Int. J. Hydrog. Energy 2018, 43, 21560–21571. [Google Scholar] [CrossRef]
- Azargohar, R.; Dalai, A.K. Biochar is a precursor of activated carbon. Appl. Biochem. Biotech. 2006, 131, 762–773. [Google Scholar] [CrossRef]
- Mineva, T.; Matanovic, I.; Atanassov, P.; Sougrati, M.-T.; Stievano, L.; Clémancey, M.; Kochem, A.; Latour, J.-M.; Jaouen, F. Understanding active sites in pyrolyzed Fe-N-C catalysts for fuel cell cathodes by bridging density functional theory calculations and 57Fe mössbauer spectroscopy. ACS Catal. 2019, 9, 9359–9371. [Google Scholar] [CrossRef]
- Xu, X.; Xia, Z.; Zhang, X.; Sun, R.; Sun, X.; Li, H.; Wu, C.; Wang, J.; Wang, S.; Sun, G. Atomically dispersed Fe-N-C derived from dual metal-organic frameworks as efficient oxygen reduction electrocatalysts in direct methanol fuel cells. Appl. Catal. B Environ. 2019, 259, 118042. [Google Scholar] [CrossRef]
- Yin, X.; Utetiwabo, W.; Sun, S.; Lian, Y.; Chen, R.; Yang, W. Incorporation of CeF3 on single-atom dispersed Fe/N/C with oxophilic interface as highly durable electrocatalyst for proton exchange membrane fuel cell. J. Catal. 2019, 374, 43–50. [Google Scholar] [CrossRef]
- Ratso, S.; Sougrati, M.T.; Käärik, M.; Merisalu, M.; Rähn, M.; Kisand, V.; Kikas, A.; Paiste, P.; Leis, J.; Sammelselg, V.; et al. Effect of ball-milling on the oxygen reduction reaction activity of iron and nitrogen co-doped carbide-derived carbon catalysts in acid media. ACS Appl. Energy Mater. 2019, 2, 7952–7962. [Google Scholar] [CrossRef]
- Nabae, Y.; Moriya, S.; Matsubayashi, K.; Lyth, S.M.; Malon, M.; Wu, L.; Islam, N.M.; Koshigoe, Y.; Kuroki, S.; Kakimoto, M.A.; et al. The role of Fe species in the pyrolysis of Fe phthalocyanine and phenolic resin for preparation of carbon-based cathode catalysts. Carbon 2010, 48, 2613–2624. [Google Scholar] [CrossRef]
- Miller, H.A.; Bellini, M.; Oberhauser, W.; Deng, X.; Chen, H.; He, Q.; Passaponti, M.; Innocenti, M.; Yang, Y.; Sun, F.; et al. Heat treated carbon supported iron (II) phthalocyanine oxygen reduction catalysts: Elucidation of the structure-activity relationship using X-ray absorption spectroscopy. Phys. Chem. Chem. Phys. 2016, 18, 33142–33151. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chung, H.T.; Cullen, D.A.; Wagner, S.; Kramm, U.I.; More, K.L.; Zelenay, P.; Wu, G. High-performance fuel cell cathodes exclusively containing atomically dispersed iron active sites. Energy Environ. Sci. 2019, 12, 2548–2558. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, J.; Wang, F.; Dai, L. Efficient oxygen reduction reaction (ORR) catalysts based on single iron atoms dispersed on a hierarchically structured porous carbon framework. Angew. Chem. Int. Ed. 2018, 57, 1–7. [Google Scholar]
- Ao, X.; Zhang, W.; Li, Z.; Lv, L.; Ruan, Y.; Wu, H.-H.; Chiang, W.-H.; Wang, C.; Liue, M.; Zeng, X.C. Unraveling the high-activity nature of Fe-N-C electrocatalysts for oxygen reduction reaction: The extraordinary synergy between Fe-N4 and Fe4N. J. Mater. Chem. A 2012, 7, 11792–11801. [Google Scholar] [CrossRef]
- Chung, D.Y.; Kim, M.J.; Kang, N.; Yoo, J.M.; Shin, H.; Kim, O.-H.; Sung, Y.-E. Low-temperature and gram-scale synthesis of two-dimensional Fe-N-C carbon sheets for robust electrochemical oxygen reduction reaction. Chem. Mater. 2017, 29, 2890–2898. [Google Scholar] [CrossRef]
- Chen, M.; He, Y.; Spendelow, J.S.; Wu, G. Atomically dispersed metal catalysts for oxygen reduction. ACS Energy Lett. 2019, 4, 1619–1633. [Google Scholar] [CrossRef]
- Huang, X.; Yang, Z.; Dong, B.; Wang, Y.; Tang, T.; Hou, Y. In-situ Fe2N@N-doped porous carbon hybrids as superior catalysts for oxygen reduction reaction. Nanoscale 2013, 9, 8102–8106. [Google Scholar] [CrossRef] [PubMed]
- Monteverde Videla, A.H.A.; Osmieri, L.; Specchia, S. Non-noble metal (NNM) catalysts for fuel cells: Tuning the activity by a rational step by step single variable evolution. In Electrochemistry of N4 Macrocyclic Metal Complexes; Bedioui, F., Zagal, J.H., Eds.; Springer International Publishing AG: Basel, Switzerland, 2016; pp. 69–101. [Google Scholar]
- Monteverde Videla, A.H.A.; Osmieri, L.; Armandi, M.; Specchia, S. Varying the morphology of Fe-N-C electrocatalysts by templating Iron Phthalocyanine precursor with different porous SiO2 to promote the Oxygen Reduction Reaction. Electrochim. Acta 2014, 177, 43–50. [Google Scholar] [CrossRef]
- Zagal, J.H.; Fethi, B. (Eds.) Electrochemistry of N4 Macrocyclic Metal Complexes; Springer: Berlin/Heidelberg, Germany, 2016; Volume 1: Energy. [Google Scholar]
- Zhang, Z.; Dou, M.; Ji, J.; Wang, F. Phthalocyanine tethered iron phthalocyanine on graphitized carbon black as superior electrocatalyst for oxygen reduction reaction. Nano Energy 2017, 34, 338–343. [Google Scholar] [CrossRef]
- Cui, L.; Lv, G.; Dou, Z.; He, X. Fabrication of iron phthalocyanine/graphene micro/nanocomposite by solvothermally assisted π–π assembling method and its application for oxygen reduction reaction. Electrochim. Acta 2013, 106, 272–278. [Google Scholar] [CrossRef]
- Taniguchi, T.; Tateishi, H.; Miyamoto, S.; Hatakeyama, K.; Ogata, C.; Funatsu, A.; Hayami, A.; Makinose, Y.; Matsushita, N.; Koinuma, M.; et al. A self-assembly route to an iron phthalocyanine/reduced graphene oxide hybrid electrocatalyst affording an ultrafast oxygen reduction reaction. Part. Part. Syst. Charact. 2013, 30, 1063–1070. [Google Scholar] [CrossRef]
- Jiang, Y.; Lu, Y.; Lv, X.; Han, D.; Zhang, Q.; Niu, L.; Chen, W. Enhanced catalytic performance of Pt free iron phthalocyanine by graphene support for efficient oxygen reduction reaction. ACS Catal. 2013, 3, 1263–1271. [Google Scholar] [CrossRef]
- Zhang, C.; Hao, Z.; Yin, H.; Liu, H.; Hou, L. Iron phthalocyanine and nitrogen-doped graphene composite as a novel non-precious catalyst for the oxygen reduction reaction. Nanoscale 2013, 4, 7326–7329. [Google Scholar] [CrossRef]
- Oliveira, M.A.C.; Ficca, V.C.A.; Gokhale, R.; Santoro, C.; Mecheri, B.; D’Epifanio, A.; Licoccia, S.; Atanassov, P. Iron(II) phthalocyanine (FePc) over carbon support for oxygen reduction reaction electrocatalysts operating in alkaline electrolyte. J. Solid State Electrochem. 2020, 1–12. [Google Scholar] [CrossRef]
- Osmieri, L.; Monteverde Videla, A.H.A.; Armandi, M.; Specchia, S. Influence of different transition metals on the properties of Me-N-C (Me = Fe, Co, Cu, Zn) catalysts synthesized using SBA-15 as tubular nano-silica reactor for oxygen reduction reaction. Int. J. Hydrog. Energy 2016, 41, 22570–22588. [Google Scholar] [CrossRef]
- Santoro, C.; Rojas-Carbonell, S.; Awais, R.; Gokhale, R.; Kodali, M.; Serov, A.; Artyushkova, K.; Atanassov, P. Influence of platinum group metal-free catalyst synthesis on microbial fuel cell performance. J. Power Sources 2018, 375, 11–20. [Google Scholar] [CrossRef]
- Chen, Y.; Artyushkova, K.; Rojas-Carbonell, S.; Serov, A.; Matanovic, I.; Santoro, C.; Atanassov, P. Inhibition of surface chemical moieties by tris(hydroxymethyl)aminomethane: A key to understanding oxygen reduction on iron–nitrogen–carbon catalysts. ACS Appl. Energy Mater. 2018, 1, 1942–1949. [Google Scholar] [CrossRef]
- Mecheri, B.; Gokhale, R.; Santoro, C.; Oliveira, M.A.C.; D’Epifanio, A.; Licoccia, S.; Serov, A.; Artyushkova, K.; Atanassov, P. Oxygen reduction reaction electrocatalysts derived from iron salt and benzimidazole and aminobenzimidazole precursors and their application in microbial fuel cell cathode. ACS Appl. Energy Mater. 2018, 1, 5755–5765. [Google Scholar] [CrossRef] [PubMed]
- Birry, L.; Mehta, P.; Jaouen, F.; Dodelet, J.-P.; Guiot, S.R.; Tartakovsky, B. Application of iron-based cathode catalysts in a microbial fuel cell. Electrochim. Acta 2011, 56, 1505–1511. [Google Scholar] [CrossRef] [Green Version]
- Kodali, M.; Santoro, C.; Serov, A.; Kabir, S.; Artyushkova, K.; Matanovic, I.; Atanassov, P. Air breathing cathodes for microbial fuel cell using Mn-, Fe-, Co- and Ni-containing platinum group metal-free catalysts. Electrochim. Acta 2017, 231, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, Q.; Xu, T.; Wei, K.; Yin, M.; Liang, P.; Huang, X.; Zhang, X. One-step ball milling-prepared nano Fe2O3 and nitrogen-doped graphene with high oxygen reduction activity and its application in microbial fuel cells. Front. Environ. Sci. Eng. 2020, 14, 30. [Google Scholar] [CrossRef]
- Yang, W.; Wang, X.; Rossi, R.; Logan, B.E. Low-cost Fe-N-C catalyst derived from Fe (III)–chitosan hydrogel to enhance power production in microbial fuel cells. Chem. Eng. J. 2019, 380, 122522. [Google Scholar] [CrossRef]
- Mahalingam, S.; Ayyaru, S.; Ahn, Y.-H. Enhanced cathode performance of Fe2O3, boron nitride-doped rGO nanosheets for microbial fuel cell applications. Sustain. Energy Fuels 2020, 4, 1454–1468. [Google Scholar] [CrossRef]
- Tang, H.; Cai, S.; Xie, S.; Wang, Z.; Tong, Y.; Pan, M.; Lu, X. Metal-organic-framework-derived dual metal- and nitrogen-doped carbon as efficient and robust oxygen reduction reaction catalysts for microbial fuel cells. Adv. Sci. 2020, 3, 1500265. [Google Scholar] [CrossRef]
- Greenmana, G.J.; Santoro, C.; Serov, A.; Melhuish, C.; Atanassov, P.; Ieropoulos, I.A. Improved power and long- term performance of microbial fuel cell with Fe-N-C catalyst in air-breathing cathode. Energy 2018, 144, 1073–1079. [Google Scholar]
- Erable, B.; Oliot, M.; Lacroix, R.; Bergel, A.; Serov, A.; Kodali, M.; Santoro, C.; Atanassov, P. Iron-nicarbazin derived platinum group metal-free electrocatalyst in scalable-size air-breathing cathodes for microbial fuel cells. Electrochim. Acta 2018, 277, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fan, Y.S. Pyrolysis of iron phthalocyanine on activated carbon as highly efficient non noble metal oxygen reduction catalyst in microbial fuel cells. Chem. Eng. J. 2019, 361, 416–427. [Google Scholar] [CrossRef]
- Jasinski, R. A new fuel cell cathode catalyst. Nature 1964, 201, 1212–1213. [Google Scholar] [CrossRef]
- Zion, N.; Friedman, A.; Levy, N.; Elbaz, L. Bioinspired electrocatalysis of oxygen reduction reaction in fuel cells using molecular catalysts. Adv. Mater. 2018, 30, 1800406. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Liu, C.; Wang, Z.; Zhang, Z.; Zhang, M.; Chang, X.; Zhang, W.; Cao, R. Reactivity and Mechanism Studies of Hydrogen Evolution Catalyzed by Copper Corroles. ACS Catal. 2016, 6, 6429–6437. [Google Scholar] [CrossRef]
- Raggio, M.; Mecheri, B.; Nardis, S.; D’Epifanio, A.; Licoccia, S.; Paolesse, R. Metallo-corroles supported on carbon nanostructures as oxygen reduction electrocatalysts in neutral media. Eur. J. Inorg. Chem. 2019, 44, 4760–4765. [Google Scholar] [CrossRef]
- Shpilman, J.S.; Friedman, A.; Zion, N.; Levy, N.; Major, D.T.; Elbaz, L. Combined experimental and theoretical study of cobalt corroles as catalysts for oxygen reduction reaction. J. Phys. Chem. C 2019, 123, 30129–30136. [Google Scholar] [CrossRef]
- Levy, N.; Shpilman, J.S.; Honig, H.C.; Major, D.T.; Elbaz, L. A surprising substituent effect in corroles on the electrochemical activation of oxygen reduction. Chem. Commun. 2017, 53, 12942–12945. [Google Scholar] [CrossRef]
- Levy, N.; Mahammed, A.; Kosa, M.; Major, D.T.; Gross, Z.; Elbaz, L. Metallocorroles as nonprecious-metal catalysts for oxygen reduction. Angew. Chem. Int. Ed. 2015, 54, 14080–14084. [Google Scholar] [CrossRef] [PubMed]
- Zagal, J.H.; Kruusenberg, I.; Tammeveski, K.; Recio, J.; Muñoz, K.; Venegas, R. Oxygen reduction on carbon-supported metallophthalocyanines and metalloporphyrins. Elsevier Encycl. Interfacial Chem. Electrochem. 2018, 812–819. [Google Scholar] [CrossRef]
- Dong, G.; Huang, M.; Guan, L. Iron phthalocyanine coated on single-walled carbon nanotubes composite for the oxygen reduction reaction in alkaline media. Phys. Chem. Chem. Phys. 2012, 14, 2557–2559. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Long, Y.-T. Superior Catalytic Activity of Electrochemically Reduced Graphene Oxide Supported Iron Phthalocyanines toward Oxygen Reduction Reaction. ACS Appl. Mater. Interfaces 2015, 7, 24063–24068. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Toshimitsu, F.; Yang, Z.; Fujigaya, T.; Nakashima, N. Pristine carbon nanotube/iron phthalocyanine hybrids with a well-defined nanostructure show excellent efficiency and durability for oxygen reduction reaction. J. Mater. Chem. A 2013, 5, 1184–1191. [Google Scholar] [CrossRef]
- Van Veen, J.A.R.; Colijn, H.A.; van Baar, J.F. On the effect of a heat treatment on the structure of carbon-supported metalloporphyrins and phthalocyanines. Electrochim. Acta 1998, 33, 801–804. [Google Scholar] [CrossRef]
- Nguyen, M.-T.; Mecheri, B.; Iannaci, A.; D’Epifanio, A.; Licoccia, S. Iron/polyindole-based electrocatalysts to enhance oxygen reduction in microbial fuel cells. Electrochim. Acta 2016, 190, 388–395. [Google Scholar] [CrossRef]
- Iannaci, A.; Mecheri, B.; D’Epifanio, A.; Elorri, M.J.L.; Liccocia, S. Iron–nitrogen-functionalized carbon as efficient oxygen reduction reaction electrocatalyst in microbial fuel cells. Int. J. Hydrog. Energy 2016, 41, 19637–19644. [Google Scholar] [CrossRef]
- Santoro, C.; Gokhale, A.; Mecheri, B.; D’Epifanio, A.; Licoccia, S.; Serov, A.; Artyushkova, K.; Atanassov, P. Design of iron (II) phthalocyanine-derived oxygen reduction electrocatalysts for high-power-density microbial fuel cells. ChemSusChem 2017, 10, 3243–3251. [Google Scholar] [CrossRef] [Green Version]
- Osgood, H.; Devaguptapu, S.V.; Xu, H.; Cho, J.; Wu, G. Transition metal (Fe, Co, Ni, and Mn) oxides for oxygen reduction and evolution bifunctional catalysts in alkaline media. Nano Today 2016, 11, 601–625. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Wei, Z. Transition-metal-oxide-based catalysts for the oxygen reduction reaction. J. Mater. Chem. A 2018, 6, 8194–8209. [Google Scholar] [CrossRef]
- Goswami, C.; Kashyap Hazarika, K.; Bharali, P. Transition metal oxide nanocatalysts for oxygen reduction reaction. Mater. Sci. Energy Technol. 2018, 1, 117–128. [Google Scholar] [CrossRef]
- Sun, M.; Liu, H.; Liu, Y.; Qu, J.; Li, J. Graphene-based transition metal oxide nanocomposites for the oxygen reduction reaction. Nanoscale 2015, 7, 1250–1269. [Google Scholar] [CrossRef] [PubMed]
- Seonghee, K.; Kato, S.; Ishizaki, T.; Li, O.L.; Kang, J. Transition metal (Fe, Co, Ni) nanoparticles on selective amino-N-doped carbon as high-performance oxygen reduction reaction electrocatalyst. Nanomaterials 2019, 9, 742. [Google Scholar]
- Su, Y.; Zhu, Y.; Yang, X.; Shen, J.; Lu, J.; Zhang, X.; Chen, J.; Li, C. A highly efficient catalyst toward oxygen reduction reaction in neutral media for microbial fuel cells. Ind. Eng. Chem. Res. 2013, 52, 6076–6082. [Google Scholar] [CrossRef]
- Li, X.; Hu, B.; Suib, S.; Lei, Y.; Li, B. Manganese dioxide as a new cathode catalyst in microbial fuel cells. J. Power Sources 2010, 195, 2586–2591. [Google Scholar] [CrossRef]
- Gautam, R.K.; Bhattacharjee, H.; Mohan, S.V.; Verma, A. Nitrogen doped graphene supported α-MnO2 nanorods for efficient ORR in a microbial fuel cell. RSC Adv. 2016, 6, 110091–110101. [Google Scholar] [CrossRef]
- Farahani, F.S.; Mecheri, B.; Majidi, M.R.; Oliveira, M.A.C.; D’Epifanio, A.; Zurlo, F.; Placidi, E.; Arciprete, F.; Licoccia, S. MnOx-based electrocatalysts for enhanced oxygen reduction in microbial fuel cell air cathodes. J. Power Sources 2018, 390, 45–53. [Google Scholar] [CrossRef]
- Farahani, F.S.; Mecheri, B.; Majidi, M.R.; Placidi, E.; D’Epifanio, A. Carbon-supported Fe/Mn-based perovskite-type oxides boost oxygen reduction in bioelectrochemical systems. Carbon 2019, 145, 716–724. [Google Scholar] [CrossRef]
- Huang, Q.; Zhou, P.; Yang, H.; Zhu, L.; Wu, H. In situ generation of inverse spinel CoFe2O4 nanoparticles onto nitrogen-doped activated carbon for an effective cathode electrocatalyst of microbial fuel cells. Chem. Eng. J. 2017, 325, 466–473. [Google Scholar] [CrossRef]
- Burkitt, R.; Whiffen, T.R.; Yu, E.H. Iron phthalocyanine and MnOx composite catalysts for microbial fuel cell applications. Appl. Catal. B Environ. 2016, 181, 279–288. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Deng, L.; Hu, H.; Qiao, Y.; Yuan, H.; Chen, D.; Chang, M.; Wei, H. Pomelo peel-derived, N-doped biochar microspheres as an efficient and durable metal-free ORR catalyst in microbial fuel cells. Sustain. Energy Fuels 2020, 4, 1642–1653. [Google Scholar] [CrossRef]
- Mao, K.; Zhang, W.; Dai, J.; Zeng, X.C. Carbon fragments as highly active metal-free catalysts for the oxygen reduction reaction: A mechanistic study. Nanoscale 2019, 11, 19422–19428. [Google Scholar] [CrossRef] [PubMed]
- Zhong, K.; Li, M.; Yang, Y.; Zhang, H.; Zhang, B.; Tang, J.; Yan, J.; Su, M.; Yang, Z. Nitrogen-doped biochar derived from watermelon rind as oxygen reduction catalyst in air cathode microbial fuel cells. Appl. Energy 2019, 242, 516–525. [Google Scholar]
- Liu, X.; Zhang, Y.; Li, Z.; Feng, R.; Zhang, Y. Characterization of corncob-derived biochar and pyrolysis kinetics in comparison with corn stalk and sawdust. Bioresour. Technol. 2014, 170, 76–82. [Google Scholar] [CrossRef]
- Yuan, H.; Deng, L.; Qi, Y.; Kobayashi, N.; Tang, J. Nonactivated and activated biochar derived from bananas as alternative cathode catalyst in microbial fuel cells. Sci. World J. 2014, 2014, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agrafioti, E.; Bouras, G.; Kalderis, D.; Diamadopoulos, E. Biochar production by sewage sludge pyrolysis. J. Anal. Appl. Pyrolysis 2018, 101, 72–78. [Google Scholar] [CrossRef]
- Goldfarb, J.L.; Buessing, L.; Gunn, E.; Lever, M.; Billias, A.; Casoliba, E.; Schievano, A.; Adani, F. Novel integrated biorefinery for olive mill waste management: Utilization of secondary waste for water treatment. ACS Sustain. Chem. Eng. 2017, 5, 876–884. [Google Scholar] [CrossRef]
- Goldfarb, J.L.; Dou, G.; Salari, M.; Grinstaff, M.W. Biomass-based fuels and activated carbon electrode materials: An integrated approach to green energy systems. ACS Sustain. Chem. Eng. 2017, 5, 3046–3054. [Google Scholar] [CrossRef]
- Hagemann, N.; Spokas, K.; Schmidt, H.-P.; Kägi, R.; Böhler, M.A.; Bucheli, T.D. Activated carbon, biochar and charcoal: Linkages and synergies across pyrogenic carbon’s ABCs. Water 2018, 10, 182. [Google Scholar] [CrossRef] [Green Version]
- Dhelipan, A.; Arunchander, A.; Sahu, A.K.; Kalpana, D. Activated carbon from orange peels as supercapacitor electrode and catalyst support for oxygen reduction reaction in proton exchange membrane fuel cell. J. Saudi Chem. Soc. 2017, 21, 487–494. [Google Scholar] [CrossRef]
- Lv, Y.; Gan, L.; Liu, M.; Xiong, W.; Xua, Z.; Zhu, D.; Wright, D.S. A self-template synthesis of hierarchical porous carbon foams based on banana peel for supercapacitor electrodes. J. Power Sources 2012, 209, 152–157. [Google Scholar] [CrossRef]
- Deng, L.-F.; Dong, G.; Cai, X.-X. Biochar derived from the inner membrane of passion fruit as cathode catalyst of microbial fuel cells in neutral solution. J. Fuel Chem. Technol. 2018, 46, 120–128. [Google Scholar]
- Li, M.; Zhang, H.; Xiao, T.; Wang, S.; Zhang, B.; Chen, D.; Su, M.; Tang, J. Low-cost biochar derived from corncob as oxygen reduction catalyst in air cathode microbial fuel cells. Electrochim. Acta 2018, 283, 780–788. [Google Scholar] [CrossRef]
- Chang, H.-C.; Gustave, W.; Yuan, Z.-F.; Xiao, Y.; Chen, Z. One-step fabrication of binder-free air cathode for microbial fuel cells by using balsa wood biochar. Environ. Technol. Innov. 2020, 18, 100615. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, Y.; Zhou, W.; Li, L.; Huang, S.; Chen, S. Biomass-derived heteroatoms-doped mesoporous carbon for efficient oxygen reduction in microbial fuel cells. Biosens. Bioelectron. 2017, 98, 350–356. [Google Scholar] [CrossRef]
- Miran, W.; Nawaz, M.; Jang, J.; Lee, D.S. Conversion of orange peel waste biomass to bioelectricity using a mediator-less microbial fuel cell. Sci. Total Environ. 2016, 547, 197–205. [Google Scholar] [CrossRef]
- Sciarria, T.P.; Tenca, A.; D’Epifanio, A.; Mecheri, B.; Merlino, G.; Barbato, M.; Borin, S.; Licoccia, S.; Garavaglia, V.; Adani, F. Using olive mill wastewater to improve performance in producing electricity from domestic wastewater by using single-chamber microbial fuel cell. Bioresour. Technol. 2013, 147, 246–253. [Google Scholar] [CrossRef] [Green Version]
- Sciarria, T.P.; Costa de Oliveira, M.A.; Mecheri, B.; D’Epifanio, A.; Goldfarb, J.L.; Adani, F. Metal-free activated biochar as an oxygen reduction reaction catalyst in single chamber microbial fuel cells. J. Power Sources 2020, 462, 228183. [Google Scholar] [CrossRef]
- Xue, H.; He, T.; Chabu, J.M.; Liu, J.; Wu, H.; Zheng, J.; Tan, M.; Ma, J.; Shen, R.; Deng, L.; et al. Iron single clusters anchored on N-doped porous carbon as superior trace-metal catalysts toward oxygen reduction. Adv. Mater. Interfaces 2018, 5, 1701345. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, J.; Huang, T. The key roles of trace iron for nitrogen, sulfur dual-doped carbon nanospheres as high efficient oxygen reduction catalyst. J. Mater. Sci. 2018, 53, 1404–1413. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Konkena, B.; Jayaramulu, K.; Schuhmann, W.; Maji, T.K. Synthesis of nano-porous carbon and nitrogen doped carbon dots from an anionic MOF: A trace cobalt metal residue in carbon dots promotes electrocatalytic ORR activity. J. Mater. Chem. A 2017, 5, 13573–13580. [Google Scholar] [CrossRef]
- Ye, R.; Dong, J.; Wang, L.; Mendoza-Cruz, R.; Li, Y.; An, P.-F.; Yacamán, M.J.; Yakobson, B.I.; Chen, D.; Tour, J.M. Manganese deception on graphene and implications in catalysis. Carbon 2018, 132, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Masa, J.; Xia, W.; Muhler, M.; Schuhmann, W. On the role of metals in nitrogen-doped carbon electrocatalysts for oxygen reduction. Angew. Chem. Int. Ed. 2015, 54, 10102–10120. [Google Scholar] [CrossRef] [PubMed]
| 1 | [96] |





| pH | Pathway | Reactions | E0 vs. RHE1 |
|---|---|---|---|
| <7 | Direct four-electron | O2 + 4H+ + 4e− → 2 H2O | 1.230 |
| <7 | two-electron | O2 + 2H+ + 2e− → H2O2 | 0.695 |
| <7 | - | H2O2 + 2H+ + 2e− → 2 H2O | 1.776 |
| >7 | Direct four-electron | O2 + 2 H2O + 4e− → 4 OH− | 1.230 |
| >7 | two-electron | O2 + H2O + 2e− → H2O− + OH− | 0.695 |
| >7 | - | H2O− + H2O + 2e− ⇨ OH− | 1.776 |
| Carbon Source | Iron Source | Nitrogen Source | Pyrolysis T | PD | Ref. |
|---|---|---|---|---|---|
| Benzimidazole, Aminobenzimidazole | Fe(NO3)3 | Benzimidazole, Aminobenzimidazole | 900 °C | 1620 mWm−2 | [95] |
| Ketjen Black | ClFeTMPP, FePc | NH3(g) | 700 °C | 590 mWm−2 | [96] |
| Aminoantipyrine | Fe(NO3)3 | Aminoantipyrine | 950 °C | 2510 mWm−2 | [97] |
| Graphene | Fe2O3 | Pyrrole | 600 °C | 1380 mWm−2 | [98] |
| Activated carbon | FeCl3 | Chitosan | 800 °C | 2400 mWm−2 | [99] |
| Graphene oxide | Fe2O3 | BNNS1s | 550 °C | 1673 mWm−2 | [100] |
| 2-methylimidazole | FeCl2 | 2-methylimidazole | 800 °C | 4335 mWm−2 | [101] |
| Aminoantipyrine | Fe(NO3)3 | Aminoantipyrine | 950 °C | 1300 mWm−2 | [102] |
| Nicarbazin | Fe(NO3)3 | NH3(g), Nicarbazin | 900 °C | 1850 mWm−2 | [103] |
| Activated carbon | Fe(II)Pc | Fe(II)Pc | 900 °C | 1092 mWm−2 | [104] |
| Phenolic resin | Fe(II)Pc | Fe(II)Pc | 600 °C | 330 mWm−2 | [75] |
| Activated carbon | Fe(II)Pc | Fe(II)Pc | 400–1000 °C | 120 mWm−2 | [76] |
| Ricobendazole, niclosamide | Fe(NO3)3 | Ricobendazole, niclosamide | 975 °C | 2510 mWm−2 | [21] |
| Biomass | Pyrolysis T | Power Density | Ref. |
| Sewage sludge | 900 °C | 500 mWm−2 | [6] |
| Banana | 900 °C | 528 mWm−2 | [136] |
| Passion fruit | 900 °C | 1153 mWm−2 | [143] |
| Corncob | 650 °C | 459 mWm−2 | [144] |
| Balsa wood | 800 °C | 200 mWm−2 | [145] |
| Egg | 900 °C | 737 mWm−2 | [146] |
| Orange peel | 50 °C | 359 mWm−2 | [147] |
| Pt/C | - | 704 mWm−2 | [146] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa de Oliveira, M.A.; D’Epifanio, A.; Ohnuki, H.; Mecheri, B. Platinum Group Metal-Free Catalysts for Oxygen Reduction Reaction: Applications in Microbial Fuel Cells. Catalysts 2020, 10, 475. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10050475
Costa de Oliveira MA, D’Epifanio A, Ohnuki H, Mecheri B. Platinum Group Metal-Free Catalysts for Oxygen Reduction Reaction: Applications in Microbial Fuel Cells. Catalysts. 2020; 10(5):475. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10050475
Chicago/Turabian StyleCosta de Oliveira, Maida Aysla, Alessandra D’Epifanio, Hitoshi Ohnuki, and Barbara Mecheri. 2020. "Platinum Group Metal-Free Catalysts for Oxygen Reduction Reaction: Applications in Microbial Fuel Cells" Catalysts 10, no. 5: 475. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10050475