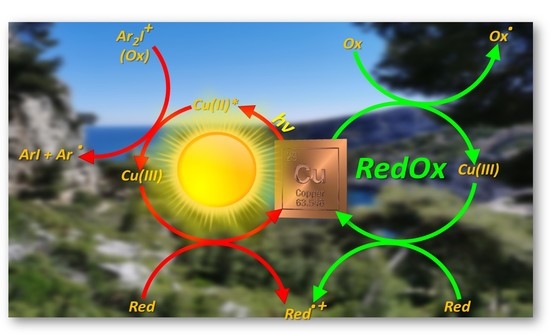
Recent Advances on Copper Complexes as Visible Light Photoinitiators and (Photo) Redox Initiators of Polymerization
Abstract
:1. Introduction
2. Copper Complexes as Visible Light Photoinitiators of Polymerization
2.1. Copper Complexes as Photoredox Catalysts
2.2. Copper Complexes as Photoinitiators for Polymerization Processes in Shadowed Area
2.3. Copper Complexes as Photoinitiators for Dark Polymerization Processes
2.4. Green Approach towards the Synthesis of Copper-Based Photoinitiators of Polymerization
2.5. Green Approach towards the Synthesis of Copper-Based Redox and Photoredox Initiators of Polymerization
2.6. Complexes with Elongated Excited State Lifetimes: The Basis of Chemical Engineering
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jasinski, F.; Zetterlund, P.B.; Braun, A.M.; Chemtob, A. Photopolymerization in dispersed systems. Prog. Polym. Sci. 2018, 84, 47–88. [Google Scholar] [CrossRef] [Green Version]
- Ng, G.; Yeow, J.; Xu, J.; Boyer, C. Application of oxygen tolerant PET-RAFT to polymerization-induced self-assembly. Polym. Chem. 2017, 8, 2841–2851. [Google Scholar] [CrossRef]
- Pan, X.; Tasdelen, M.A.; Laun, J.; Junkers, T.; Yagci, Y.; Matyjaszewski, K. Photomediated controlled radical polymerization. Prog. Polym. Sci. 2016, 62, 73–125. [Google Scholar] [CrossRef] [Green Version]
- Abdallah, M.; Hijazi, A.; Dumur, F.; Lalevée, J. Coumarins as powerful photosensitizers for the cationic polymerization of epoxy-silicones under near-UV and visible light and applications for 3D printing technology. Molecules 2020, 25, 2063. [Google Scholar] [CrossRef]
- Noè, C.; Malburet, S.; Bouvet-Marchand, A.; Graillot, A.; Loubat, C.; Sangermano, M. Cationic photopolymerization of bio-renewable epoxidized monomers. Prog. Org. Coat. 2019, 133, 131–138. [Google Scholar] [CrossRef]
- Lalevée, J.; Mokbel, H.; Fouassier, J.-P. Recent developments of versatile photoinitiating systems for cationic ring opening polymerization operating at any wavelengths and under low light intensity sources. Molecules 2015, 20, 7201–7221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Corrigan, N.; Bagheri, A.; Jin, J.; Boyer, C. A versatile 3D and 4D printing system through photocontrolled raft polymerization. Angew. Chem. 2019, 131, 18122–18131. [Google Scholar] [CrossRef]
- Lee, A.Y.; An, J.; Chua, C.K. Two-way 4D-printing: A review on the reversibility of 3d-printed shape memory materials. Engineering 2017, 3, 663–674. [Google Scholar] [CrossRef]
- Bagheri, A.; Jin, J. Photopolymerization in 3D Printing. ACS Appl. Polym. Mat. 2019, 1, 593–611. [Google Scholar] [CrossRef] [Green Version]
- Aduba, D.C., Jr.; Margaretta, E.D.; Marnot, A.E.C.; Heifferon, K.V.; Surbey, W.R.; Chartrain, N.A.; Whittington, A.R.; Long, T.E.; Williams, C.B. Vat photopolymerization 3D printing of acid-cleavable PEG-methacrylate networks for biomaterial applications. Mat. Today Commun. 2019, 19, 204–211. [Google Scholar] [CrossRef]
- Mendes-Felipe, C.; Oliveira, J.; Etxebarria, I.; Vilas-Vilela, J.L.; Lanceros-Mendez, S. State-of-the-Art and Future Challenges of UV Curable Polymer-Based Smart Materials for Printing Technologies. Adv. Mat. Technol. 2019, 4, 1800618. [Google Scholar] [CrossRef] [Green Version]
- Yagci, Y.; Jockusch, S.; Turro, N.J. Photoinitiated Polymerization: Advances, Challenges, and Opportunities. Macromolecules 2010, 43, 6245–6260. [Google Scholar] [CrossRef]
- Shao, J.; Huang, Y.; Fan, Q. Visible light initiating systems for photopolymerization: Status, development and challenges. Polym. Chem. 2014, 5, 4195–4210. [Google Scholar] [CrossRef]
- Dietlin, C.; Schweizer, S.; Xiao, P.; Zhang, J.; Morlet-Savary, F.; Graff, B.; Fouassier, J.P.; Lalevée, J. Photopolymerization upon LEDs: New photoinitiating systems and strategies. Polym. Chem. 2015, 6, 3895–3912. [Google Scholar] [CrossRef]
- Ibrahim-Ouali, M.; Dumur, F. Recent advances on metal-based near-infrared and infrared emitting OLEDs. Molecules 2019, 24, 1412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dumur, F. Zinc complexes in OLEDs: An overview. Synth. Met. 2014, 195, 241–251. [Google Scholar] [CrossRef]
- Dumur, F. Recent advances in organic light-emitting devices comprising copper complexes: A realistic approach for low-cost and highly emissive devices? Org. Electron. 2015, 21, 27–39. [Google Scholar] [CrossRef]
- Yoshino, F.; Yoshida, A. Effects of blue-light irradiation during dental treatment. Jap. Dent. Sci. Rev. 2018, 54, 160–168. [Google Scholar] [CrossRef]
- Khairunisah Ghazali, S.; Syaimima Syaiful Azim, F.; Adrus, N.; Jamaluddin, J. The Effectiveness of UV-LED Photopolymerisation over Conventional UV-Mercury for Polyurethane Acrylate Coating. J. Photopolym. Sci. Technol. 2019, 32, 705–710. [Google Scholar] [CrossRef]
- Xiao, P.; Zhang, J.; Dumur, F.; Tehfe, M.-A.; Morlet-Savary, F.; Graff, B.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Photoinitiating systems: Recent progress in visible light induced cationic and radical photopolymerization reactions under soft conditions. Prog. Polym. Sci. 2015, 41, 32–66. [Google Scholar] [CrossRef]
- Bonardi, A.H.; Dumur, F.; Grant, T.M.; Noirbent, G.; Gigmes, D.; Lessard, B.H.; Fouassier, J.-P.; Lalevée, J. High performance near infrared (NIR) photoinitiating systems operating under low light intensity and in presence of oxygen. Macromolecules 2018, 51, 1314–1324. [Google Scholar] [CrossRef]
- Garra, P.; Dietlin, C.; Morlet-Savary, F.; Dumur, F.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Photopolymerization processes of thick films and in shadow areas: A review for the access to composites. Polym. Chem. 2017, 8, 7088–7101. [Google Scholar] [CrossRef]
- Dumur, F. Recent advances on carbazole-based photoinitiators of polymerization. Eur. Polym. J. 2020, 125, 109503. [Google Scholar] [CrossRef]
- Dumur, F. Recent advances on pyrene-based photoinitiators of polymerization. Eur. Polym. J. 2020, 126, 109564. [Google Scholar] [CrossRef]
- Noirbent, G.; Dumur, F. Recent advances on naphthalic anhydrides and 1,8-naphthalimide-based photoinitiators of polymerization. Eur. Polym. J. 2020, 132, 109702. [Google Scholar] [CrossRef]
- Pigot, C.; Noirbent, G.; Brunel, D.; Dumur, F. Recent advances on push-pull organic dyes as visible light photoinitiators of polymerization. Eur. Polym. J. 2020, 133, 109797. [Google Scholar] [CrossRef]
- Tehfe, M.-A.; Dumur, F.; Graff, B.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Blue-to-red light sensitive push-pull structured photoinitiators: Indanedione derivatives for radical and cationic photopolymerization reactions. Macromolecules 2013, 46, 3332–3341. [Google Scholar] [CrossRef]
- Tehfe, M.-A.; Dumur, F.; Graff, B.; Morlet-Savary, F.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. New push-pull dyes derived from Michler’s ketone for polymerization reactions upon visible lights. Macromolecules 2013, 46, 3761–3770. [Google Scholar] [CrossRef]
- Tehfe, M.-A.; Dumur, F.; Graff, B.; Morlet-Savary, F.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Push-pull (thio)barbituric acid derivatives in dye photosensitized radical and cationic polymerization reactions under 457/473 nm Laser beams or blue LEDs. Polym. Chem. 2013, 4, 3866–3875. [Google Scholar] [CrossRef]
- Mokbel, H.; Telitel, S.; Dumur, F.; Vidal, L.; Versace, D.-L.; Tehfe, M.-A.; Graff, B.; Toufaily, J.; Fouassier, J.-P.; Gigmes, D.; et al. Photoinitiating systems of polymerization and in-situ incorporation of metal nanoparticles in polymer matrixes upon visible lights: Push-pull malonate and malonitrile based dyes. Polym. Chem. 2013, 4, 5679–5687. [Google Scholar] [CrossRef]
- Xiao, P.; Frigoli, M.; Dumur, F.; Graff, B.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Julolidine or fluorenone based push-pull dyes for polymerization upon soft polychromatic visible light or green light. Macromolecules 2014, 47, 106–112. [Google Scholar] [CrossRef]
- Sun, K.; Pigot, C.; Chen, H.; Nechab, M.; Gigmes, D.; Morlet-Savary, F.; Graff, B.; Liu, S.; Xiao, P.; Dumur, F.; et al. Free radical photopolymerization and 3D printing using newly developed dyes: Indane-1,3-dione and 1H-cyclopentanaphthalene-1,3-dione derivatives as photoinitiators in three-component systems. Catalysts 2020, 10, 463. [Google Scholar] [CrossRef] [Green Version]
- Xiao, P.; Dumur, F.; Graff, B.; Vidal, L.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Structural effects in the indanedione skeleton for the design of low intensity 300-500 nm light sensitive initiators. Macromolecules 2014, 47, 26–34. [Google Scholar] [CrossRef]
- Xiao, P.; Dumur, F.; Graff, B.; Fouassier, J.-P.; Gigmes, D.; Lalevée, J. Cationic and thiol-ene photopolymerization upon red lights using anthraquinone derivatives as photoinitiators. Macromolecules 2013, 46, 6744–6750. [Google Scholar] [CrossRef]
- Dumur, F.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Organic Electronics: An El Dorado in the quest of new photocatalysts as photoinitiators of polymerization. Acc. Chem. Res. 2016, 49, 1980–1989. [Google Scholar] [CrossRef]
- Xiao, P.; Dumur, F.; Tehfe, M.-A.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Red-light-induced cationic photopolymerization: Perylene derivatives as efficient photoinitiators. Macromol. Rapid. Commun. 2013, 34, 1452–1458. [Google Scholar] [CrossRef]
- Lalevée, J.; Peter, M.; Dumur, F.; Gigmes, D.; Blanchard, N.; Tehfe, M.-A.; Morlet-Savary, F.; Fouassier, J.-P. Subtle ligand effects in oxidative photocatalysis with iridium complexes: Application to photopolymerization. Chem. Eur. J. 2011, 17, 15027–15031. [Google Scholar] [CrossRef]
- Lalevée, J.; Tehfe, M.-A.; Dumur, F.; Gigmes, D.; Blanchard, N.; Morlet-Savary, F.; Fouassier, J.-P. Iridium photocatalysts in free radical photopolymerization under visible lights. ACS Macro Lett. 2012, 1, 286–290. [Google Scholar] [CrossRef]
- Lalevée, J.; Dumur, F.; Mayer, C.R.; Gigmes, D.; Nasr, G.; Tehfe, M.-A.; Telitel, S.; Morlet-Savary, F.; Graff, B.; Fouassier, J.-P. Photopolymerization of N-vinylcarbazole using visible-light harvesting iridium complexes as photoinitiators. Macromolecules 2012, 45, 4134–4141. [Google Scholar] [CrossRef]
- Telitel, S.; Dumur, F.; Telitel, S.; Soppera, O.; Lepeltier, M.; Guillaneuf, Y.; Poly, J.; Morlet-Savary, F.; Fioux, P.; Fouassier, J.-P.; et al. Photoredox catalysis using a new iridium complex as an efficient toolbox for radical, cationic and controlled polymerizations under soft blue to green lights. Polym. Chem. 2015, 6, 613–624. [Google Scholar] [CrossRef]
- Telitel, S.; Dumur, F.; Lepeltier, M.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Photoredox process induced polymerization reactions: Iridium complexes for panchromatic photoinitiating systems. C. R. Chimie 2016, 19, 71–78. [Google Scholar] [CrossRef]
- Tehfe, M.-A.; Lepeltier, M.; Dumur, F.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Structural effects in the iridium complex series: Photoredox catalysis and photoinitiation of polymerization reactions under visible lights. Macromol. Chem. Phys. 2017, 218, 1700192. [Google Scholar] [CrossRef]
- Tehfe, M.-A.; Lalevée, J.; Dumur, F.; Telitel, S.; Gigmes, D.; Contal, E.; Bertin, D.; Fouassier, J.-P. Zinc-based metal complexes as new photocatalysts in polymerization initiating systems. Eur. Polym. J. 2013, 49, 1040–1049. [Google Scholar] [CrossRef]
- Versace, D.-L.; Bourgon, J.; Leroy, E.; Dumur, F.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Zinc complex based photoinitiating systems for acrylate polymerization under air; in situ formation of Zn-based fillers and composites. Polym. Chem. 2014, 5, 6569–6576. [Google Scholar] [CrossRef]
- Al Mousawi, A.; Poriel, C.; Dumur, F.; Toufaily, J.; Hamieh, T.; Fouassier, J.-P.; Lalevée, J. Zinc tetraphenylporphyrin as high performance visible-light photoinitiator of cationic photosensitive resins for LED projector 3D printing applications. Macromolecules 2017, 50, 746–753. [Google Scholar] [CrossRef]
- Zhang, J.; Campolo, D.; Dumur, F.; Xiao, P.; Fouassier, J.-P.; Gigmes, D.; Lalevée, J. Iron complexes as photoinitiators for radical and cationic polymerization through photoredox catalysis processes. J. Polym. Sci. A Polym. Chem. 2015, 53, 42–49. [Google Scholar] [CrossRef]
- Xiao, P.; Zhang, J.; Campolo, D.; Dumur, F.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Copper and iron complexes as visible-light-sensitive photoinitiators of polymerization. J. Polym. Sci. A Polym. Chem. 2015, 53, 2673–2684. [Google Scholar] [CrossRef]
- Telitel, S.; Dumur, F.; Campolo, D.; Poly, J.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Iron complexes as potential photocatalysts for controlled radical photopolymerizations: A tool for modifications and patterning of surfaces. J. Polym. Sci. A Polym. Chem. 2016, 54, 702–713. [Google Scholar] [CrossRef]
- Zhang, J.; Campolo, D.; Dumur, F.; Xiao, P.; Fouassier, J.-P.; Gigmes, D.; Lalevée, J. Visible-light-sensitive photoredox catalysis by iron complexes: Applications in cationic and radical polymerization reactions. J. Polym. Sci. A Polym. Chem. 2016, 54, 2247–2253. [Google Scholar] [CrossRef]
- Zhang, J.; Campolo, D.; Dumur, F.; Xiao, P.; Fouassier, J.-P.; Gigmes, D.; Lalevée, J. Novel iron complexes in visible-light-sensitive photoredox catalysis: Effect of ligands on their photoinitiation efficiencies. ChemCatChem 2016, 8, 2227–2233. [Google Scholar] [CrossRef]
- Di Bernardo, P.; Zanonato, P.L.; Tamburini, S.; Tomasin, P.; Vigato, P.A. Complexation behaviour and stability of Schiff bases in aqueous solution. The case of an acyclic diimino(amino) diphenol and its reduced triamine derivative. Dalton Trans. 2006, 39, 4711–4721. [Google Scholar] [CrossRef] [PubMed]
- Garra, P.; Brunel, D.; Noirbent, G.; Graff, B.; Morlet-Savary, F.; Dietlin, C.; Sidorkin, V.F.; Dumur, F.; Duché, D.; Gigmes, D.; et al. Ferrocene-based (photo)redox polymerization under long wavelengths. Polym. Chem. 2019, 10, 1431–1441. [Google Scholar] [CrossRef]
- Brunel, D.; Noirbent, G.; Dumur, F. Ferrocene: An unrivaled electroactive building block for the design of push-pull dyes with near-infrared and infrared absorptions. Dyes Pigm. 2019, 170, 107611. [Google Scholar] [CrossRef]
- Cuttell, D.G.; Kuang, S.-M.; Fanwick, P.E.; McMillin, D.R.; Walton, R.A. Simple Cu(I) Complexes with Unprecedented Excited-State Lifetimes. J. Am. Chem. Soc. 2002, 124, 6–7. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhou, Q.; Cheng, Y.; Wang, L.; Ma, D.; Jing, X.; Wang, F. Highly efficient electroluminescence from green-light-emitting electrochemical cells based on CuI complexes. Adv. Funct. Mat. 2006, 16, 1203–1208. [Google Scholar] [CrossRef]
- Zhang, Q.; Komino, T.; Huang, S.; Matsunami, S.; Goushi, K.; Adachi, C. Triplet exciton confinement in green organic light-emitting diodes containing luminescent charge-transfer Cu(I) complexes. Adv. Funct. Mat. 2012, 22, 2327–2336. [Google Scholar] [CrossRef]
- Armaroli, N.; Accorsi, G.; Holler, M.; Moudam, O.; Nierengarten, J.F.; Zhou, Z.; Wegh, R.T.; Welter, R. Highly luminescent CuI complexes for light-emitting electrochemical cells. Adv. Mat. 2006, 18, 1313–1316. [Google Scholar] [CrossRef]
- Hernandez-Perez, A.C.; Vlassova, A.; Collins, S.K. Toward a visible light mediated photocyclization: Cu-based sensitizers for the synthesis of [5]helicene. Org. Lett. 2012, 14, 2988–2991. [Google Scholar] [CrossRef]
- Pirtsch, M.; Paria, S.; Matsuno, T.; Isobe, H.; Reiser, O. [Cu(dap)2Cl] as an efficient visible-light-driven photoredox catalyst in carbon–carbon bond-forming reactions. Chem. Eur. J. 2012, 18, 7336–7340. [Google Scholar] [CrossRef]
- Gong, T.; Adzima, B.J.; Baker, N.H.; Bowman, C.N. Photopolymerization reactions using the photoinitiated copper (I)-catalyzed azide-alkyne cycloaddition (CuAAC) reaction. Adv. Mat. 2013, 25, 2024–2028. [Google Scholar] [CrossRef]
- Alzahrani, A.A.; Erbse, A.H.; Bowman, C.N. Evaluation and development of novel photoinitiator complexes for photoinitiating the copper-catalyzed azide-alkyne cycloaddition reaction. Polym. Chem. 2014, 5, 1874–1882. [Google Scholar] [CrossRef]
- Xiao, P.; Dumur, F.; Zhang, J.; Fouassier, J.-P.; Gigmes, D.; Lalevée, J. Copper complexes in radical photoinitiating systems: Applications to free radical and cationic polymerization under visible lights. Macromolecules 2014, 47, 3837–3844. [Google Scholar] [CrossRef]
- Poli, R.; Allan, L.E.N.; Shaver, M.P. Iron-mediated reversible deactivation controlled radical polymerization. Prog. Polym. Sci. 2014, 39, 1827–1845. [Google Scholar] [CrossRef]
- Wang, J.S.; Matyjaszewski, K. Controlled/“living” radical polymerization atom transfer radical polymerization in the presence of transition-metal complexes. J. Am. Chem. Soc. 1995, 117, 5614–5615. [Google Scholar] [CrossRef]
- Kamigaito, M.; Ando, T.; Sawamoto, M. Metal-catalyzed living radical polymerization. Chem. Rev. 2001, 101, 3689–3746. [Google Scholar] [CrossRef]
- Cunningham, A.F.; Desobry, V. Radiation Curing in Polymer Science and Technology; Fouassier, J.-P., Rabek, J.-F., Eds.; Elsevier: Barking, UK, 1993; Volume 2, pp. 323–374. [Google Scholar]
- Garra, P.; Dumur, F.; Nechab, M.; Morlet-Savary, F.; Dietlin, C.; Graff, B.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Stable copper acetylacetonate-based oxidizing agents in redox (NIR photoactivated) polymerization: An opportunity for the one pot grafting from approach and an example on a 3D printed object. Polym. Chem. 2018, 9, 2173–2182. [Google Scholar] [CrossRef]
- Garra, P.; Carré, M.; Dumur, F.; Morlet-Savary, F.; Dietlin, C.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Copper-based (photo)redox initiating systems as highly efficient systems for interpenetrating polymer network preparation. Macromolecules 2018, 51, 679–688. [Google Scholar] [CrossRef]
- Garra, P.; Dumur, F.; Morlet-Savary, F.; Dietlin, C.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Mechanosynthesis of a copper complex for redox initiating systems with a unique near infrared light activation. J. Polym. Sci. A Polym. Chem. 2017, 55, 3646–3655. [Google Scholar] [CrossRef]
- Garra, P.; Morlet-Savary, F.; Graff, B.; Dumur, F.; Monnier, V.; Dietlin, C.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Metal acetylacetonate-bidentate ligand interaction (MABLI) as highly efficient free radical generating systems for polymer synthesis. Polym. Chem. 2018, 9, 1371–1378. [Google Scholar] [CrossRef]
- Garra, P.; Dumur, F.; Gigmes, D.; Nechab, M.; Morlet-Savary, F.; Dietlin, C.; Gree, S.; Fouassier, J.P.; Lalevée, J. Metal acetylacetonate-bidentate ligand interaction (MABLI) (photo)activated polymerization: Toward high performance amine-free, peroxide-free redox radical (photo)initiating systems. Macromolecules 2018, 51, 2706–2715. [Google Scholar] [CrossRef]
- Dumur, F.; Bertin, D.; Gigmes, D. Iridium (III) complexes as promising emitters for solid-state light-emitting electrochemical cells (LECs). Int. J. Nanotechnol. 2012, 9, 377–395. [Google Scholar] [CrossRef]
- Dumur, F.; Contal, E.; Wantz, G.; Gigmes, D. Photoluminescence of zinc complexes: Easily tunable optical properties by the bridge between the imido groups of Schiff-base ligands. Eur. J. Inorg. Chem. 2014, 4186–4198. [Google Scholar] [CrossRef]
- Villotte, S.; Gigmes, D.; Dumur, F.; Lalevée, J. Design of iodonium salts for UV or near-UV LEDs for photoacid generator and polymerization purposes. Molecules 2020, 25, 149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakai, Y.; Sagara, Y.; Nomura, H.; Nakamura, N.; Suzuki, Y.; Miyazaki, H.; Adachi, C. Zinc complexes exhibiting highly efficient thermally activated delayed fluorescence and their application to organic light-emitting diodes. Chem. Commun. 2015, 51, 3181–3184. [Google Scholar] [CrossRef] [PubMed]
- Uoyama, H.; Goushi, K.; Shizu, K.; Nomura, H.; Adachi, C. Highly efficient organic light-emitting diodes from delayed fluorescence. Nature 2012, 492, 234–238. [Google Scholar] [CrossRef]
- Atkins, C.E.; Park, S.E.; Blaszak, J.A.; McMillin, D.R. A two-level approach to deconvoluting absorbance data involving multiple species. Applications to copper systems. Inorg. Chem. 1984, 23, 569–572. [Google Scholar] [CrossRef]
- Lennert, A.; Guldi, D.M. Homoleptic and Heteroleptic Copper Complexes as Redox Couples in Dye-Sensitized Solar Cells. ChemPhotoChem 2019, 3, 636–644. [Google Scholar] [CrossRef]
- Kuang, S.-M.; Cuttell, D.G.; McMillin, D.R.; Fanwick, P.E.; Walton, R.A. Synthesis and structural characterization of Cu(I) and Ni(II) complexes that contain the bis[2-(diphenylphosphino) phenyl]ether ligand novel emission properties for the Cu(I) species. Inorg. Chem. 2002, 41, 3313–3322. [Google Scholar] [CrossRef]
- Xiao, P.; Dumur, F.; Zhang, J.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Copper complexes: The effect of ligands on their photoinitiation efficiencies in radical polymerization reactions under visible light. Polym. Chem. 2014, 5, 6350–6357. [Google Scholar] [CrossRef]
- Yang, Q.; Balverde, S.; Dumur, F.; Lalevée, J.; Poly, J. Synergetic effect of the epoxide functional groups in the photocatalyzed atom transfer radical copolymerization of glycidyl methacrylate. Polym. Chem. 2016, 7, 6084–6093. [Google Scholar] [CrossRef]
- Al Mousawi, A.; Kermagoret, A.; Versace, D.-L.; Toufaily, J.; Hamieh, T.; Graff, B.; Dumur, F.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Copper photoredox catalysts for polymerization upon near UV or visible light: Structure/reactivity/efficiency relationships and use in LED projector 3D printing resins. Polym. Chem. 2017, 8, 568–580. [Google Scholar] [CrossRef]
- Chen, X.-L.; Yu, R.; Zhang, Q.-K.; Zhou, L.-J.; Wu, X.-Y.; Zhang, Q.; Lu, C.-Z. Rational design of strongly blue-emitting cuprous complexes with thermally activated delayed fluorescence and application in solution-processed OLEDs. Chem. Mat. 2013, 25, 3910–3920. [Google Scholar] [CrossRef]
- Czerwieniec, R.; Leitl, M.J.; Homeier, H.H.H.; Yersin, H. Cu(I) complexes—Thermally activated delayed fluorescence. Photophysical approach and material design. Coord. Chem. Rev. 2016, 325, 2–28. [Google Scholar] [CrossRef]
- Rehm, D.; Weller, A. Kinetics of fluorescence quenching by electron and H-Atom transfer. Isr. J. Chem. 1970, 8, 259–271. [Google Scholar] [CrossRef]
- Mousawi, A.A.; Dietlin, C.; Graff, B.; Morlet-Savary, F.; Toufaily, J.; Hamieh, T.; Fouassier, J.-P.; Chachaj-Brekiesz, A.; Ortyl, J.; Lalevée, J. Meta-terphenyl derivative/iodonium salt/9H-carbazole-9-ethanol photoinitiating systems for free radical promoted cationic polymerization upon visible lights. Macromol. Chem. Phys. 2016, 217, 1955–1965. [Google Scholar] [CrossRef]
- Fouassier, J.-P.; Lalevée, J. Photoinitiators for Polymer Synthesis: Scope, Reactivity, and Efficiency; John Wiley & Sons: Weinheim, Germany, 2013. [Google Scholar]
- Fouassier, J.-P. Photoinitiation, Photopolymerization, and Photocuring: Fundamentals and Applications; Hanser: Munich, Germany, 1995. [Google Scholar]
- Crivello, J.V.; Dietliker, K.; Bradley, G. Photoinitiators for Free Radical Cationic & Anionic Photopolymerisation; John Wiley & Sons: Chichester, UK, 1999. [Google Scholar]
- Chen, L.; Yu, Q.; Wang, Y.; Li, H. BisGMA/TEGDMA dental composite containing high aspect-ratio hydroxyapatite nanofibers. Dent. Mat. 2011, 27, 1187–1195. [Google Scholar] [CrossRef] [Green Version]
- Amirouche-Korichi, A.; Mouzali, M.; Watts, D.C. Shrinkage strain—Rates study of dental composites based on (BisGMA/TEGDMA) monomers. Arab. J. Chem. 2017, 10, S190–S195. [Google Scholar] [CrossRef]
- Mello de Paiva Campos, L.; Cidreira Boaro, L.; Perez Ferreira, H.; Gomes dos Santos, L.K.; Ribeiro dos Santos, T.; Fernandes Parra, D. Evaluation of polymerization shrinkage in dental restorative experimental composites based: BisGMA/TEGDMA, filled with MMT. J. Appl. Polym. Sci. 2016, 133, 43543. [Google Scholar]
- Sautrot-Ba, P.; Al Mousawi, A.; Lalevée, J.; Mazeran, P.-E.; Park, S.J.; Kang, I.-K.; Laurent-Brocq, M.; Langlois, V.; Versace, D.-L. Copper complex: A key role in the synthesis of biocidal polymer coatings. Chem. Afr. 2019, 2, 241–251. [Google Scholar]
- Catilaz-Simonin, L.; Fouassier, J.-P. Investigation of a system capable of photoinitiating radical polymerizations in thick pigmented media. J. Appl. Polym. Sci. 2001, 79, 1911–1923. [Google Scholar] [CrossRef]
- Grotzinger, C.; Burget, D.; Fouassier, J.-P. Polymerization activity in film and photochemical behaviour of a four components photoinitiating system. Trends Photochem. Photobiol. 2001, 7, 71–84. [Google Scholar]
- Aguirre-Soto, A.; Lim, C.-H.; Hwang, A.T.; Musgrave, C.B.; Stansbury, J.W. Visible-light organic photocatalysis for latent radical-initiated polymerization via 2e−/1H+ transfers: Initiation with parallels to photosynthesis. J. Am. Chem. Soc. 2014, 136, 7418–7427. [Google Scholar] [CrossRef] [PubMed]
- Auerbach, S.S.; Bristol, D.W.; Peckham, J.C.; Travlos, G.S.; Hébert, C.D.; Chhabra, R.S. Toxicity and carcinogenicity studies of methylene blue trihydrate in F344N rats and B6C3F1 mice. Food Chem. Toxicol. 2010, 48, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Albert, M.; Lessin, M.S.; Gilchrist, B.F. Methylene blue: Dangerous dye for neonates. J. Pediatr. Surg. 2003, 38, 1244–1245. [Google Scholar] [CrossRef]
- Ng, B.K.W.; Cameron, A.J.D. The role of methylene blue in serotonin syndrome: A systematic review. Psychosomatics 2010, 51, 194–200. [Google Scholar] [CrossRef]
- Bynum, S.; Tullier, M.; Morejon-Garcia, C.; Guidry, J.; Runnoe, E.; Pojman, J.A. The effect of acrylate functionality on frontal polymerization velocity and temperature. J. Polym. Sci. A Polym. Chem. 2019, 57, 982–988. [Google Scholar] [CrossRef]
- Riazi, H.; Shamsabadi, A.A.; Grady, M.C.; Rappe, A.M.; Soroush, M. Experimental and theoretical study of the self-initiation reaction of methyl acrylate in free-radical polymerization. Ind. Eng. Chem. Res. 2018, 57, 532–539. [Google Scholar] [CrossRef]
- Mokbel, H.; Anderson, D.; Plenderleith, R.; Dietlin, C.; Morlet-Savary, F.; Dumur, F.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Copper photoredox catalyst “G1”: A new high performance photoinitiator for near-UV and visible LEDs. J. Polym. Chem. 2017, 8, 5580–5592. [Google Scholar] [CrossRef]
- Mokbel, H.; Anderson, D.; Plenderleith, R.; Dietlin, C.; Morlet-Savary, F.; Dumur, F.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Simultaneous initiation of radical and cationic polymerization reactions using the “G1” copper complex as photoredox catalyst: Applications of free radical/cationic hybrid photopolymerization in the composites and 3D printing fields. Prog. Org. Coat. 2019, 132, 50–61. [Google Scholar] [CrossRef]
- Garra, P.; Dumur, F.; Mokbel, H.; Monnier, V.; Morlet-Savary, F.; Dietlin, C.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. New synthetic route to a highly efficient photoredox catalyst by mechanosynthesis. ACS Omega 2018, 3, 10938–10944. [Google Scholar] [CrossRef]
- Wang, G.-W. Mechanochemical organic synthesis. Chem. Soc. Rev. 2013, 42, 7668–7700. [Google Scholar] [CrossRef] [PubMed]
- Šepelák, V.; Duüvel, A.; Wilkening, M.; Becker, K.-D.; Heitjans, P. Mechanochemical reactions and syntheses of oxides. Chem. Soc. Rev. 2013, 42, 7507–7520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rightmire, N.R.; Hanusa, T.P. Advances in organometallic synthesis with mechanochemical methods. Dalton Trans. 2016, 45, 2352–2362. [Google Scholar] [CrossRef]
- Friščić, T. Supramolecular concepts and new techniques in mechanochemistry: Cocrystals, cages, rotaxanes. open metal-organic frameworks. Chem. Soc. Rev. 2012, 41, 3493–3510. [Google Scholar] [CrossRef] [PubMed]
- Delori, A.; Friščić, T.; Jones, W. The role of mechanochemistry and supramolecular design in the development of pharmaceutical materials. CrystEngComm 2012, 14, 2350–2362. [Google Scholar] [CrossRef]
- Lazuen Garay, A.; Pichon, A.; James, S.L. Solvent-free synthesis of metal complexes. Chem. Soc. Rev. 2007, 36, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Baláž, P.; Achimovičová, M.; Baláž, M.; Billik, P.; Cherkezova-Zheleva, Z.; Criado, J.M.; Delogu, F.; Dutková, E.; Gaffet, E.; Gotor, F.J.; et al. Hallmarks of mechanochemistry: From nanoparticles to technology. Chem. Soc. Rev. 2013, 42, 7571–7637. [Google Scholar] [CrossRef] [Green Version]
- Howard, J.L.; Cao, W.; Browne, D.L. Mechanochemistry as an emerging tool for molecular synthesis: What can it offer? Chem. Sci. 2018, 9, 3080–3094. [Google Scholar] [CrossRef] [Green Version]
- Friščić, T.; Mottillo, C.; Titi, H.M. Mechanochemistry for Synthesis. Angew. Chem. Int. Ed. 2020, 59, 1018–1029. [Google Scholar] [CrossRef]
- Do, J.-L.; Friščić, T. Mechanochemistry: A force of synthesis. ACS Cent. Sci. 2017, 3, 13–19. [Google Scholar] [CrossRef] [Green Version]
- Štrukil, V.; Margetić, D.; Igrc, M.D.; Eckert-Maksić, M.; Friščić, T. Desymmetrisation of aromatic diamines and synthesis of nonsymmetrical thiourea derivatives by click-mechanochemistry. Chem. Commun. 2012, 48, 9705–9707. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.X.; Xu, K.; Clegg, J.K.; Ganguly, R.; Hirao, H.; Friščić, T.; Garcia, F. The first synthesis of the sterically encumbered adamantoid phosphazane P4((NtBu)6: Enabled by mechanochemistry. Angew. Chem. Int. Ed. 2016, 55, 12736–12740. [Google Scholar] [CrossRef] [PubMed]
- Katsenis, A.D.; Puškarić, A.; Štrukil, V.; Mottillo, C.; Julien, P.A.; Užarević, K.; Pham, M.-H.; Do, T.-O.; Kimber, S.A.J.; Lazić, P.; et al. In situ X-ray diffraction monitoring of a mechanochemical reaction reveals a unique topology metal-organic framework. Nat. Commun. 2015, 6, 6662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, G.-W.; Komatsu, K.; Murata, Y.; Shiro, M. Synthesis and X ray structure of dumb-bell-shaped C120. Nature 1997, 387, 583–586. [Google Scholar] [CrossRef]
- Tan, D.; Mottillo, C.; Katsenis, A.D.; Štrukil, V.; Friščić, T. Development of C-N coupling using mechanochemistry: Catalytic coupling of arylsulfonamides and carbodiimides. Angew. Chem. Int. Ed. 2014, 53, 9321–9324. [Google Scholar] [CrossRef]
- Garra, P.; Dumur, F.; Morlet-Savary, F.; Dietlin, C.; Fouassier, J.-P.; Lalevée, J. A new highly efficient amine-free and peroxide-free redox system for free radical polymerization under air with possible light activation. Macromolecules 2016, 49, 6296–6309. [Google Scholar] [CrossRef]
- Garra, P.; Dietlin, C.; Morlet-Savary, F.; Dumur, F.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Redox two-component initiated free radical and cationic polymerizations: Concepts, reactions and application. Prog. Polym. Sci. 2019, 94, 33–56. [Google Scholar] [CrossRef]
- Garra, P.; Dumur, F.; Nechab, M.; Morlet-Savary, F.; Dietlin, C.; Graff, B.; Doronina, E.P.; Sidorkin, V.F.; Gigmes, D.; Fouassier, J.-P.; et al. Peroxide-free and amine-free redox free radical polymerization: Metal acetylacetonates/stable carbonyl compounds for highly efficient synthesis of composites. Macromolecules 2018, 51, 6395–6404. [Google Scholar] [CrossRef]
- Miller, G.A.; Gou, L.; Narayanan, V.; Scranton, A.B. Modeling of photobleaching for the photoinitiation of thick polymerization systems. J. Polym. Sci. A Polym. Chem. 2002, 40, 793–808. [Google Scholar] [CrossRef]
- Cook, W.A.; Chen, F. Enhanced visible radiation photopolymerization of dimethacrylates with the three component thioxanthone (CPTXO)–amine–iodonium salt system. Polym. Chem. 2015, 6, 1325–1338. [Google Scholar] [CrossRef]
- He, Y.; Zhou, W.; Wu, F.; Li, M.; Wang, E. Photoreaction and photopolymerization studies on squaraines dyes/iodonium salts combination. J. Photochem. Photobiol. A Chem. 2004, 162, 463–471. [Google Scholar] [CrossRef]
- Bonardi, A.-H.; Bonardi, F.; Dumur, F.; Gigmes, D.; Lalevée, J. Fillers as heaters for photothermal polymerization upon NIR light. Macromol. Rapid. Commun. 2019, 40, 1900495. [Google Scholar] [CrossRef] [PubMed]
- Bonardi, A.-H.; Bonardi, F.; Noirbent, G.; Dumur, F.; Dietlin, C.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Different NIR dye scaffolds for polymerization reactions under NIR light. Polym. Chem. 2019, 10, 6505–6514. [Google Scholar] [CrossRef]
- Mokbel, H.; Dumur, F.; Lalevée, J. On demand NIR activated photopolyaddition reactions. Polym. Chem. 2020, 11, 4250–4259. [Google Scholar] [CrossRef]
- Kim, J.U.; Park, I.S.; Chan, C.Y.; Tanaka, M.; Tsuchiya, Y.; Nakanotani, H.; Adachi, C. Nanosecond-time-scale delayed fluorescence molecule for deep-blue OLEDs with small efficiency rolloff. Nat. Commun. 2020, 11, 1765. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Mao, Z.; Xie, Z.; Zhang, Y.; Liu, S.; Zhao, J.; Xu, J.; Chi, Z.; Aldred, M.P. Recent advances in organic thermally activated delayed fluorescence materials. Chem. Soc. Rev. 2017, 46, 915–1016. [Google Scholar] [CrossRef]
- Liang, X.; Tu, Z.-L.; Zheng, Y.-X. Thermally activated delayed fluorescence materials: Towards realization of high efficiency through strategic small molecular design. Chem. Eur. J. 2019, 25, 5623–5642. [Google Scholar] [CrossRef]
- Congrave, D.G.; Drummond, B.H.; Conaghan, P.J.; Francis, H.; Jones, S.T.E.; Grey, C.P.; Greenham, N.C.; Credgington, D.; Bronstein, H. A Simple Molecular Design Strategy for Delayed Fluorescence toward 1000 nm. J. Am. Chem. Soc. 2019, 141, 18390–18394. [Google Scholar] [CrossRef]
- Adachi, C. Third-generation organic electroluminescence materials. Jap. J. Appl. Phys. 2014, 53, 060101. [Google Scholar] [CrossRef]
- Parker, C.A.; Hatchard, C.G. Triplet-singlet emission in fluid solutions. Phosphorescence of eosin. Trans. Faraday Soc. 1961, 57, 1894–1904. [Google Scholar] [CrossRef]
- Nakanotani, H.; Masui, K.; Nishide, J.; Shibata, T.; Adachi, C. Promising operational stability of high-efficiency organic light-emitting diodes based on thermally activated delayed fluorescence. Sci. Rep. 2013, 3, 2127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al Mousawi, A.; Magaldi Lara, D.; Noirbent, G.; Dumur, F.; Toufaily, J.; Hamieh, T.; Bui, T.-T.; Goubard, F.; Graff, B.; Gigmes, D.; et al. Carbazole derivatives with thermally activated delayed fluorescence property as photoinitiators/photoredox catalysts for LED 3D printing technology. Macromolecules 2017, 50, 4913–4926. [Google Scholar] [CrossRef]
- Bonardi, A.-H.; Dumur, F.; Noirbent, G.; Lalevée, J.; Gigmes, D. Organometallic vs. organic photoredox catalysts for photocuring reactions in the visible region. Beilstein J. Org. Chem. 2018, 14, 3025–3046. [Google Scholar] [CrossRef] [PubMed]
- Bouzrati-Zerelli, M.; Noirbent, G.; Goubard, G.; Bui, T.-T.; Villotte, S.; Dietlin, C.; Morlet-Savary, F.; Gigmes, D.; Fouassier, J.-P.; Dumur, F.; et al. A novel class of photoinitiators with a thermally activated delayed fluorescence (TADF) property. New J. Chem. 2018, 42, 8261–8270. [Google Scholar] [CrossRef] [Green Version]
- Linfoot, C.-L.; Leitl, M.-J.; Richardson, P.; Rausch, A.-F.; Chepelin, O.; White, F.-J.; Yersin, H.; Robertson, N. Thermally activated delayed fluorescence (TADF) and enhancing photoluminescence quantum yields of [CuI(diimine)(diphosphine)]+ complexes-photophysical, structural, and computational studies. Inorg. Chem. 2014, 53, 10854–10861. [Google Scholar] [CrossRef] [PubMed] [Green Version]













































| Laser Diode (405 nm) | Laser Diode (457 nm) | Halogen Lamp | ε405nm (M−1cm−1) | ε457nm (M−1cm−1) | |
|---|---|---|---|---|---|
| Cu-4/Iod | 20% | 25% | 43% | 1500 | 440 |
| Cu-5/Iod | 37% | 49% | 46% | 3100 | 1000 |
| Cu-6/Iod | 32% | 36% | 22% | 4000 | 1200 |
| CQ/Iod | 18% * |
| Laser Diode (405 nm) | Laser Diode (457 nm) | Halogen Lamp | ε405nm (M−1cm−1) | ε457nm (M−1cm−1) | |
|---|---|---|---|---|---|
| Cu-10/Iod | 30% | 42% | 25% | 3100 | 400 |
| Cu-11/Iod | 21% | 38% | 21% | 2500 | 350 |
| Cu-12/Iod | 32% | <5% | 19% | 2600 | 150 |
| Cu-13/Iod | 36% | 18% | 8% | 2000 | 120 |
| Cu-14/Iod | 33% | 15% | 16% | 2500 | 180 |
| Cu-18/Iod | 36% | 51% | 50% | 5500 | 1200 |
| Cu-19/Iod | 34% | 52% | 49% | 4800 | 2000 |
| Cu-20/Iod | 30% | 48% | 46% | 6300 | 2600 |
| Cu-18/Iod/NVK | 59% | 57% | |||
| Cu-2/Iod | 41% | 41% | 48% | ||
| Cu-2/Iod/NVK | 56% | ||||
| Cu-3/Iod | 21% | 38% | 38% | ||
| Cu-3/Iod/NVK | 41% | 42% |
| LED (405 nm) | LED (455 nm) | Halogen Lamp | ε405 nm (M−1cm−1) | ε457 nm (M−1cm−1) | |
|---|---|---|---|---|---|
| Cu-18/R-Br/MDEA | 59% * | 55% * | 49% * | 5500 | 1200 |
| BAPO | 53% * | ||||
| CQ/MDEA | 35% * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noirbent, G.; Dumur, F. Recent Advances on Copper Complexes as Visible Light Photoinitiators and (Photo) Redox Initiators of Polymerization. Catalysts 2020, 10, 953. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10090953
Noirbent G, Dumur F. Recent Advances on Copper Complexes as Visible Light Photoinitiators and (Photo) Redox Initiators of Polymerization. Catalysts. 2020; 10(9):953. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10090953
Chicago/Turabian StyleNoirbent, Guillaume, and Frédéric Dumur. 2020. "Recent Advances on Copper Complexes as Visible Light Photoinitiators and (Photo) Redox Initiators of Polymerization" Catalysts 10, no. 9: 953. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10090953