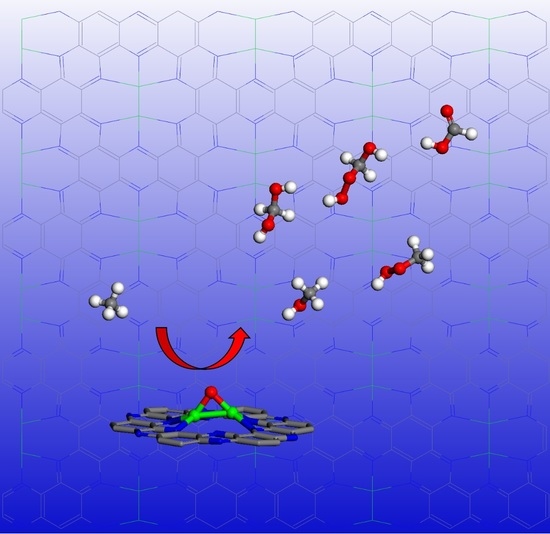
Methane Conversion over C2N-Supported Fe2 Dimers
Abstract
:1. Introduction
2. Results and Discussion
2.1. Geometric Structure and Electronic Properties of the Fe2@C2N Monolayer
2.2. Methane Activation over the Fe2@C2N Monolayer
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhao, Y.; Li, S.; Sun, Y. Theoretical study on the dissociative adsorption of CH4 on Pd-doped Ni surface. Chin. J. Catal. 2013, 34, 911–922. [Google Scholar] [CrossRef]
- Yuan, J.; Zhang, W.; Li, X.; Yang, J. A high performance catalysts for methane conversion to methanol: Grapheme supported single atom Co. Chem. Commun. 2018, 54, 2284–2287. [Google Scholar] [CrossRef] [PubMed]
- Sushkevich, V.; Palagin, D.; Ranocchiari, M.; Van Bokhoven, J. Selective anaerobic oxidation of methane enables direct synthesis of methanol. Science 2017, 356, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Schwach, P.; Pan, X.; Bao, X. Direct conversion of methane to value-added chemicals over heterogeneous catalysts: Challenges and prospects. Chem. Rev. 2017, 117, 8497–8520. [Google Scholar] [CrossRef]
- Narsimhan, K.; Iyoki, K.; Dinh, K.; Roman-Leshkov, Y. Catalytic oxidation of methane into methanol over copper exchanged zeolites with oxygen at low temperature. ACS Cent. Sci. 2016, 2, 424–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Research Grant Targeting “Holy Grail” of Catalysis. Available online: https://www.egr.uh.edu/news/201401/research-grant-targeting-holy-grail-catalysis (accessed on 13 January 2014).
- Impeng, S.; Khongpracha, P.; Warakulwit, C.; Jansang, B.; Sirijaraensre, J.; Masahiro Ehara, M.; Limtrakul, J. Direct oxidation of methane to methanol on Fe-O modified graphene. RSC Adv. 2014, 4, 12572–12578. [Google Scholar] [CrossRef]
- Tang, P.; Zhu, Q.; Wu, Z.; Ma, D. Methane activation: The past and future. Energy Environ. Sci. 2014, 7, 2580–2591. [Google Scholar] [CrossRef]
- Cui, X.; Li, H.; Wang, Y.; Hu, Y.; Hua, L.; Li, H.; Han, X.; Liu, Q.; Yang, F.; He, L.; et al. Room-temperature methane conversion by graphene-confined single iron atoms. Chem 2018, 4, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Bayatsarmadi, B.; Zheng, Y.; Vasileff, A.; Qiao, S. Recent advances in atomic metal doping of carbon-based nanomaterials for energy conversion. Small 2017, 13, 1700191. [Google Scholar] [CrossRef]
- Luo, Z.; Castleman, A.; Khanna, S. Reacitivy of metal clusters. Chem. Rev. 2016, 116, 14456–14492. [Google Scholar] [CrossRef]
- Tyo, E.; Vajda, S. Catalysis by clusters with precise numbers of atoms. Nat. Nanotechnol. 2015, 10, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, A.; Qiao, B.; Li, J.; Liu, J.; Zhang, T. Single-atom catalysts: A new frontier in heterogeneous catalysis. Acc. Chem. Res. 2013, 46, 1740–1748. [Google Scholar] [CrossRef] [PubMed]
- Impeng, S.; Khongpracha, P.; Sirijaraensre, J.; Jansang, A.; Ehara, M.; Limtrakul, J. Methane activation on Fe- and FeO-embedded graphene and boron nitride sheet: Role of atomic defects in catalytic activities. RSC Adv. 2015, 5, 97918–97927. [Google Scholar] [CrossRef]
- Sahoo, S.; Suib, S.; Pamir Alpay, S. Graphene supported single atom transition metal catalysts for methane activation. ChemCatChem 2018, 10, 3229–3235. [Google Scholar] [CrossRef]
- Solomon, E.; Heppner, D.; Johnston, E.; Ginsbach, J.; Cirera, J.; Qayyum, M.; Kieber-Emmons, M.; Kjaergaard, C.; Hadt, R.; Li, T. Copper active sites in biology. Chem. Rev. 2014, 114, 3659–3853. [Google Scholar] [CrossRef] [Green Version]
- Lieberman, R.; Rosenzweig, A. Crystal structure of a membrane-bound metalloenzyme that catalyses the biological oxidation of methane. Nature 2005, 434, 177–182. [Google Scholar] [CrossRef]
- Balasubramanian, R.; Smith, S.; Rawat, S.; Yatsunyk, L.; Stemmler, T.; Rosenzweig, A. Oxidation of methane by a biological dicopper centre. Nature 2010, 465, 115–119. [Google Scholar] [CrossRef] [Green Version]
- Panov, G.; Sobolev, V.; Dubkov, K.; Parmon, V.; Ovanesyan, N.; Shilov, A.; Shteinman, A. Iron complexes in zeolites as a new model of methane monooxygenase. React. Kinet. Catal. Lett. 1997, 61, 251–258. [Google Scholar] [CrossRef]
- Ovanesyan, N.; Shteinman, A.; Dubkov, K.; Sobolev, V.; Panov, G. The state of iron in the Fe-ZSM-5-N2O system for selective oxidation of methane to methanol from data of Mössbauer spectroscopy. Kinet. Catal. 1998, 39, 792–797. [Google Scholar]
- Narsimhan, K.; Michaelis, V.; Mathies, G.; Gunther, W.; Griffin, R.; Román-Leshkov, Y. Methane to acetic acid over Cu-exchanged zeolites: Mechanistic insights from a site-specific carbonylation reaction. J. Am. Chem. Soc. 2015, 137, 1825–1832. [Google Scholar] [CrossRef] [Green Version]
- Wannakao, S.; Nongnual, T.; Khongpracha, P.; Maihom, T.; Limtrakul, J. Reaction mechanisms for CO catalytic oxidation by N2O on Fe-embedded graphene. J. Phys. Chem. C 2012, 116, 16992–16998. [Google Scholar] [CrossRef]
- Li, F.; Chen, Z. Cu dimer anchored C2N Monolayer: Low-cost and efficient catalyst for CO oxidation. Nanoscale 2018, 10, 15696–15705. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhao, J.; Li, F.; Chen, Z. Copper dimer supported on C2N-layer as an efficient electrocatalyst for CO2 reduction reaction: A computational study. J. Phys. Chem. C 2018, 122, 19712–19721. [Google Scholar] [CrossRef]
- Li, F.; Liu, X.; Chen, Z. 1+1ʹ > 2: Heteronuclear bi-atom catalyst outperforms its homonuclear counterparts for CO oxidation. Small Methods 2019, 3, 1800480. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, L.; Yang, C.; Jiang, Q. Atomic (single, double, and triple atoms) catalysis: Frontiers, opportunities, and challenges. J. Mater. Chem. A 2019, 7, 3492–3515. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, C.; Liu, Z.; Chen, C.; Li, Y. Structural regulation with atomic-level precision: From single-atomic site to diatomic and atomic interface catalysis. Matter 2020, 2, 78–110. [Google Scholar] [CrossRef]
- Mahmood, J.; Lee, E.; Jung, M.; Shin, D.; Jeon, I.; Jung, S.; Choi, H.; Seo, J.; Bae, S.; Sohn, S.; et al. Nitrogenated holey two-dimensional structure. Nat. Commun. 2015, 6, 6486–6492. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.; Li, W.; Li, G.; Ao, Z.; An, T. Density functional theory investigation of the enhanced adsorption mechanism and potential catalytic activity for formaldehyde degradation on Al-decorated C2N monolayer. Chin. J. Catal. 2019, 40, 664–673. [Google Scholar] [CrossRef]
- He, B.; Shen, J.; Tian, Z. Iron-embedded C2N monolayer: A promising low-cost and high-activity single-atom catalyst for CO oxidation. Phys. Chem. Chem. Phys. 2016, 18, 24261–24269. [Google Scholar] [CrossRef]
- Li, X.; Zhong, W.; Cui, P.; Li, J.; Jiang, J. Design of efficient catalysts with double transition metal atoms on C2N layer. J. Phys. Chem. Lett. 2016, 7, 1750–1755. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, S.; Zhao, J. Selective C-C coupling by spatially confined dimeric metal centers. iScience 2020, 23, 101051. [Google Scholar] [CrossRef] [PubMed]
- Hammond, C.; Forde, M.; Rahim, M.; Thetford, A.; He, Q.; Jenkins, R.; Dimitratos, N.; Lopez-Sanchez, J.; Dummer, N.; Murphy, D.; et al. Direct catalytic conversion of methane to methanol in an aqueous medium by using copper-promoted Fe-ZSM-5. Angew. Chem. Int. Ed. 2012, 51, 5129–5133. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, K.; Shiota, Y.; Yumura, T.; Yamabe, T. Direct methane-methanol and benzene-phenol conversions on Fe-ZSM-5 zeolite: Theoretical predictions on the reaction pathways and energetics. J. Phys. Chem. B 2000, 104, 734–740. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, A.; Zhang, Z.; Jiao, M.; Zhou, Z. Transition metal anchored C2N monolayers as efficient bifunctional electrocatalysts for hydrogen and oxygen evolution reactions. J. Mater. Chem. A 2018, 6, 11446–11452. [Google Scholar] [CrossRef]
- Pantu, P.; Pabchanda, S.; Limtrakul, J. Theoretical investigation of the selective oxidation of methane to methanol on nanostructured Fe-ZSM-5 by the ONIOM method. ChemPhysChem 2004, 5, 1901–1906. [Google Scholar] [CrossRef] [PubMed]
- Sirijaraensre, J.; Limtrakul, J. Structures and mechanisms of the dehydration of benzaldoxime over Fe-ZSM-5 zeolites: A DFT study. Struct. Chem. 2013, 24, 1307–1318. [Google Scholar] [CrossRef]
- Schlichting, I.; Berendzen, J.; Chu, K.; Stock, A.; Maves, S.; Benson, D.; Sweet, R.; Ringe, D.; Petsko, G.; Sligar, S. The catalytic pathway of cytochrome P450cam at atomic resolution. Science 2000, 287, 1615–1622. [Google Scholar] [CrossRef]
- Snydera, B.; Böttgera, L.; Bolsb, M.; Yana, J.; Rhodaa, H.; Jacobsa, A.; Huc, M.; Zhao, J.; Alpc, E.; Hedmand, B.; et al. Structural characterization of a non-heme iron active site in zeolites that hydroxylates methane. Proc. Natl. Acad. Sci. USA 2018, 115, 4565–4570. [Google Scholar] [CrossRef] [Green Version]
- Kresse, G.; Furthmüller, F. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Blöch, P. Projector augmented-wave method. Phy. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [Green Version]
- Perdew, J.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monkhorst, H.; Pack, J. Special points for brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Latimer, A.; Aljama, H.; Kakekhani, A.; Yoo, J.; Kulkarni, A.; Tsai, C.; Melchor, M.; Abild-Pedersen, F.; Nørskov, J. Mechanistic insights into heterogeneous methane activation. Phys. Chem. Chem. Phys. 2017, 19, 3575–3581. [Google Scholar] [CrossRef] [PubMed]
- Latimer, A.; Kulkarni, A.; Aljama, H.; Montoya, J.; Yoo, J.; Tsai, C.; Abild-Pedersen, F.; Studt, F.; Nørskov, J. Understanding trends in C-H bond activation in heterogeneous catalysis. Nat. Mater. 2017, 16, 225–229. [Google Scholar] [CrossRef]
- Arvidsson, A.A.; Zhdanov, V.P.; Carlsson, P.-A.; Grönbecka, H.; Hellman, A. Metal dimer sites in ZSM-5 zeolite for methane-to-methanol conversion from first-principles kinetic modelling: Is the [Cu–O–Cu]2+ motif relevant for Ni, Co, Fe, Ag, and Au? Catal. Sci. Technol. 2017, 7, 1470–1477. [Google Scholar] [CrossRef] [Green Version]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, H.; Han, B.; Li, F.; Zhao, J. Methane Conversion over C2N-Supported Fe2 Dimers. Catalysts 2020, 10, 973. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10090973
Meng H, Han B, Li F, Zhao J. Methane Conversion over C2N-Supported Fe2 Dimers. Catalysts. 2020; 10(9):973. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10090973
Chicago/Turabian StyleMeng, Haihong, Bing Han, Fengyu Li, and Jingxiang Zhao. 2020. "Methane Conversion over C2N-Supported Fe2 Dimers" Catalysts 10, no. 9: 973. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10090973