Fe3 Cluster Anchored on the C2N Monolayer for Efficient Electrochemical Nitrogen Fixation
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Geometry, Stability and Electronic Properties of the Fe3@C2N
2.2. N2 Adsorption on Fe3@C2N
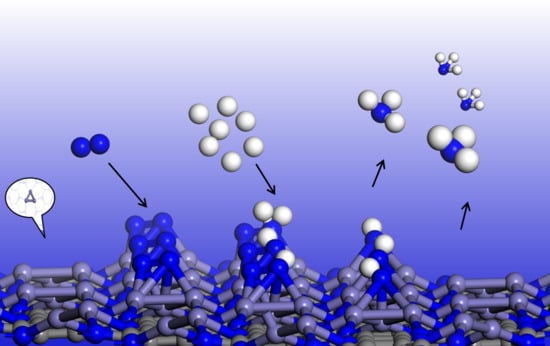
2.3. N2 Reaction on Fe3@C2N
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Guo, X.; Li, X.; Li, Y.; Yang, J.; Wan, X.; Chen, L.; Liu, J.; Liu, X.; Yu, R.; Zheng, L.; et al. Molecule template method for precise synthesis of Mo-based alloy clusters and electrocatalytic nitrogen reduction on partially reduced PtMo alloy oxide cluster. Nano Energy 2020, 78, 105211. [Google Scholar] [CrossRef]
- Guo, X.; Gu, J.; Lin, S.; Zhang, S.; Chen, Z.; Huang, S. Tackling the activity and selectivity challenges of electrocatalysts toward the nitrogen reduction reaction via atomically dispersed biatom catalysts. J. Am. Chem. Soc. 2020, 142, 5709–5721. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, N.; Kong, Z.; Ong, W.-J.; Zhao, X. Photocatalytic fixation of nitrogen to ammonia: State-of-the-art advancements and future prospects. Mater. Horiz. 2018, 5, 9–27. [Google Scholar] [CrossRef]
- Van der Ham, C.J.M.; Koper, M.T.; Hetterscheid, D.G. Challenges in reduction of dinitrogen by proton and electron transfer. Chem. Soc. Rev. 2014, 43, 5183–5191. [Google Scholar] [CrossRef]
- Shipman, M.A.; Symes, M.D. Recent progress towards the electrosynthesis of ammonia from sustainable resources. Catal. Today 2017, 286, 57–68. [Google Scholar] [CrossRef] [Green Version]
- Logadóttir, Á.; Nørskov, J.K. Ammonia synthesis over a Ru(0001) surface studied by density functional calculations. J. Catal. 2003, 220, 273–279. [Google Scholar] [CrossRef]
- Ertl, G.; Lee, S.B.; Weiss, M. Kinetics of nitrogen adsorption on Fe(111). Surf. Sci. 1982, 114, 515–526. [Google Scholar] [CrossRef]
- Montoya, J.H.; Tsai, C.; Vojvodic, A.; Nørskov, J.K. The challenge of electrochemical ammonia synthesis: A new perspective on the role of nitrogen scaling relations. ChemSusChem 2015, 8, 2180–2186. [Google Scholar] [CrossRef]
- Zhao, J.; Zhao, J.X.; Cai, Q.H. Single transition metal atom embedded into a MoS2 nanosheet as a promising catalyst for electrochemical ammonia synthesis. Phys. Chem. Chem. Phys. 2018, 20, 9248–9255. [Google Scholar] [CrossRef]
- Li, Q.Y.; He, L.Z.; Sun, C.H.; Zhang, X.W. Computational study of MoN2 monolayer as electrochemical catalysts for nitrogen reduction. J. Phys. Chem. C 2017, 121, 27563–27568. [Google Scholar] [CrossRef]
- Li, M.; Huang, H.; Low, J.; Gao, C.; Long, R.; Xiong, Y. Recent progress on electrocatalyst and photocatalyst design for nitrogen reduction. Small Methods 2019, 3, 1800388. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, Z. Single Mo atom supported on defective boron nitride monolayer as an efficient electrocatalyst for nitrogen fixation: A computational study. J. Am. Chem. Soc. 2017, 139, 12480–12487. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhao, J.X.; Cabrera, C.R.; Chen, Z.F. Computational screening of efficient single-atom catalysts based on graphitic carbon nitride (g-C3N4) for nitrogen electroreduction. Small Methods 2018, 2, 1800368. [Google Scholar]
- Li, Q.; Liu, C.; Qiu, S.; Zhou, F.; He, L.; Zhang, X.; Sun, C. Exploration of iron borides as electrochemical catalysts for nitrogen reduction reaction. J. Mater. Chem. A 2019, 7, 21507–21513. [Google Scholar] [CrossRef]
- Cheng, H.; Ding, L.-X.; Chen, G.-F.; Zhang, L.; Xue, J.; Wang, H. Molybdenum carbide nanodots enable efficient electrocatalytic nitrogen fixation under ambient conditions. Adv. Mater. 2018, 30, 1803694. [Google Scholar] [CrossRef]
- Li, X.-F.; Li, Q.-K.; Cheng, J.; Liu, L.; Yan, Q.; Wu, Y.C.; Zhang, X.Z.; Wang, Z.-Y.; Qiu, Q.; Luo, Y. Conversion of dinitrogen to ammonia by FeN3 embedded graphene. J. Am. Chem. Soc. 2016, 138, 8706–8709. [Google Scholar] [CrossRef]
- Azofra, L.M.; Sun, C.; Cavallo, L.; MacFarlane, D.R. Feasibility of N2 binding and reduction to ammonia on Fe-deposited MoS2 2D sheets: A DFT study. Chem. Eur. J. 2017, 23, 8275–8279. [Google Scholar] [CrossRef] [Green Version]
- Ling, C.; Ouyang, Y.X.; Li, Q.; Bai, X.; Mao, X.; Du, A.; Wang, J.L. A general two-step strategy–based high-through put screening of single atom catalysts for nitrogen fixation. Small Methods 2018, 3, 1800376. [Google Scholar] [CrossRef]
- Li, Q.; Qiu, S.; Liu, C.; Liu, M.; He, L.; Zhang, X.; Sun, C. Computational design of single molybdenum catalysts for nitrogen reduction reaction. J. Phys. Chem. C 2019, 123, 2347–2352. [Google Scholar] [CrossRef]
- Wang, M.; Liu, S.; Qian, T.; Liu, J.; Zhou, J.; Ji, H.; Xiong, J.; Zhong, J.; Yan, C. Over 56.55% faradaic efficiency of ambient ammonia synthesis enabled by positively shifting the reaction potential. Nat. Commun. 2019, 10, 341. [Google Scholar] [CrossRef]
- Liu, C.; Li, Q.; Zhang, J.; Jin, Y.; MacFarlane, D.R.; Sun, C. Conversion of dinitrogen to ammonia on Ru atoms supported on boron sheets: A DFT study. J. Mater. Chem. A 2019, 7, 4771–4776. [Google Scholar] [CrossRef]
- Chen, Z.; Yan, J.; Jiang, Q. Single or double: Which is the altar of atomic catalysts for nitrogen reduction reaction? Small Methods 2018, 3, 1800291. [Google Scholar] [CrossRef]
- Li, F.; Chen, L.; Liu, H.; Wang, D.; Shi, C.; Pan, H. Enhanced N2-fixation by engineering the edges of two-dimensional transition-metal disulfides. J. Phys. Chem. C 2019, 123, 22221–22227. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, A.; Zhang, Z.H.; Zhou, Z. Double-atom catalysts: Transition metal dimer anchored C2N monolayers as N2 fixation electrocatalysts. J. Mater. Chem. A 2018, 6, 18599–18604. [Google Scholar] [CrossRef]
- Liu, J.; Ma, X.; Li, Y.; Wang, Y.; Xiao, H.; Li, J. Heterogeneous Fe3 single-cluster catalyst for ammonia synthesis via an associative mechanism. Nat. Commun. 2018, 9, 1610. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.W.; Zeng, Z.; Liu, L.; Huang, X.; Jia, Y. Computational evaluation of electrocatalytic nitrogen reduction on TM single-, double-, and triple-atom catalysts (TM = Mn, Fe, Co, Ni) based on graphdiyne monolayers. J. Phys. Chem. C 2019, 123, 19066–19076. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Q.; Fan, H.-N.; Luo, W.-B.; Liu, H.-K.; Dou, S.-X. Atomically dispersed metal dimer species with selective catalytic activity for nitrogen electrochemical reduction. J. Mater. Chem. A 2019, 7, 22242–22247. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, L.; Jiang, M.; Chen, D.; Wang, Z.; Yao, X.; Singh, C.V.; Jiang, Q. Triple atom catalyst with ultrahigh loading potential for nitrogen electrochemical reduction. J. Mater. Chem. A 2020, 8, 15086–15093. [Google Scholar] [CrossRef]
- Li, X.; Zhong, W.; Cui, P.; Li, J.; Jiang, J. Design of efficient catalysts with double transition metal atoms on C2N layer. J. Phys. Chem. Lett. 2016, 7, 1750–1755. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, A.; Zhang, Z.H.; Jiao, M.G.; Zhou, Z. Transition metal anchored C2N monolayers as efficient bifunctional electrocatalysts for hydrogen and oxygen evolution reactions. J. Mater. Chem. A 2018, 6, 11446–11452. [Google Scholar] [CrossRef]
- Li, F.; Liu, X.; Chen, Z. 1 + 1′ > 2: Heteronuclear biatom aatalyst outperforms its homonuclear counterparts for CO oxidation. Small Methods 2019, 10, 1800480. [Google Scholar] [CrossRef]
- Pei, W.; Zhou, S.; Zhao, J.; Xu, X.; Du, Y.; Dou, S.X. Immobilized trimeric metal clusters: A family of the smallest catalysts for selective CO2 reduction toward multi-carbon products. Nano Energy 2020, 76, 105049. [Google Scholar] [CrossRef]
- Martyna, G.J.; Klein, M.L.; Tuckerman, M. Nosè-Hoover chains: The canonical ensemble via continuous dynamics. J. Chem. Phys. 1992, 97, 2635–2643. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, Z.; Zhao, J. Computational screening of single transition metal atom supported on C2N monolayer for electrochemical ammonia synthesis. Phys. Chem. Chem. Phys. 2018, 20, 12835–12844. [Google Scholar] [CrossRef] [PubMed]
- Strongin, D.; Carrazza, J.; Bare, S.R.; Somorjai, G. The importance of C7 sites and surface roughness in the ammonia synthesis reaction over iron. J. Catal. 1987, 103, 213–215. [Google Scholar] [CrossRef] [Green Version]
- Somorjai, G.; Materer, N. Surface structures in ammonia synthesis. Top. Catal. 1994, 1, 215–231. [Google Scholar] [CrossRef]
- Ling, C.; Zhang, Y.; Li, Q.; Bai, X.; Shi, J.; Wang, J. New mechanism for N2 reduction: The essential role of surface hydrogenation. J. Am. Chem. Soc. 2019, 141, 18264–18270. [Google Scholar] [CrossRef]
- Gao, Z.; Huang, H.; Xu, S.; Li, L.; Yan, G.; Zhao, M.; Yang, W.; Zhao, X. Regulating the coordination environment through doping N atoms for single-atom Mn electrocatalyst of N2 reduction with high catalytic activity and selectivity: A theoretical study. Mol. Catal. 2020, 493, 111091. [Google Scholar] [CrossRef]
- Ling, C.; Bai, X.; Ouyang, Y.; Du, A.; Wang, J. Single molybdenum atom anchored on N-doped carbon as a promising electrocatalyst for nitrogen reduction into ammonia at ambient conditions. J. Phys. Chem. C 2018, 122, 16842–16847. [Google Scholar] [CrossRef]
- Choi, C.; Back, S.; Kim, N.Y.; Lim, J.; Kim, Y.H.; Jung, Y. Suppression of hydrogen evolution reaction in electrochemical N2 reduction using single-atom catalysts: A computational guideline. ACS Catal. 2018, 8, 7517–7525. [Google Scholar] [CrossRef]
- Yang, X.; Shang, C.; Zhou, S.; Zhao, J. MBenes: Emerging 2D materials as efficient electrocatalysts for the nitrogen reduction reaction. Nanoscale Horiz. 2020, 5, 1106–1115. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmüller, F. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blöch, P.E. Projetor augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bucko, T.; Hafner, J.; Lebegue, S.; Aangyan, J.G. Improved description of the structure of molecular and layered crystals: Ab initio DFT calculations with van der Waals Corrections. J. Phys. Chem. A 2010, 114, 11814–11824. [Google Scholar] [CrossRef] [PubMed]
- Monkhorst, H.J.; Pack, D.J. Special points for Brillonin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Henkelman, G.; Uberuaga, B.P.; Jónsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 2000, 113, 9901. [Google Scholar] [CrossRef] [Green Version]
- Nørskov, J.K.; Rossmeisl, J.; Logadottir, A.; Lindqvist, L.; Kitchin, J.R.; Bligaard, T.; Jonsson, H. Origin of the overpotential for oxygen reduction at a fuel-cellcathode. J. Phys. Chem. B 2004, 108, 17886–17892. [Google Scholar] [CrossRef]
- Skulason, E.; Bligaard, T.; Gudmundsdottir, S.; Studt, F.; Rossmeisl, J.; Abild-Pedersen, F.; Vegge, T.; Jonsson, H.; Norskov, J.K. A theoretical evaluation of possible transition metal electro-catalysts for N2 reduction. Phys. Chem. Chem. Phys. 2012, 14, 1235–1245. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Chen, Z. Cu dimer anchored C2N monolayer: Low-cost and efficient catalyst for CO oxidation. Nanoscale 2018, 10, 15696–15705. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, B.; Meng, H.; Li, F.; Zhao, J. Fe3 Cluster Anchored on the C2N Monolayer for Efficient Electrochemical Nitrogen Fixation. Catalysts 2020, 10, 974. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10090974
Han B, Meng H, Li F, Zhao J. Fe3 Cluster Anchored on the C2N Monolayer for Efficient Electrochemical Nitrogen Fixation. Catalysts. 2020; 10(9):974. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10090974
Chicago/Turabian StyleHan, Bing, Haihong Meng, Fengyu Li, and Jingxiang Zhao. 2020. "Fe3 Cluster Anchored on the C2N Monolayer for Efficient Electrochemical Nitrogen Fixation" Catalysts 10, no. 9: 974. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10090974