Visible Light Responsive Strontium Carbonate Catalyst Derived from Solvothermal Synthesis
Abstract
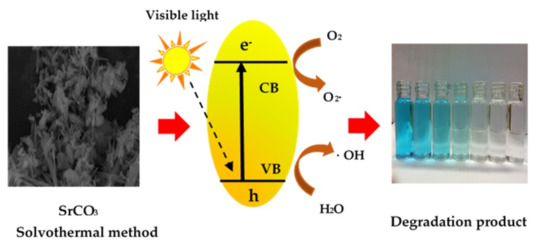
:1. Introduction
2. Results and Discussions
2.1. Effects of Synthesis Conditions
2.2. Optical Properties
2.3. Decolourisation of Methylene Blue (MB)
2.4. Degradation Products
3. Materials and Methods
3.1. Chemicals
3.2. Synthesis of Strontium Carbonate (SrCO3)
3.3. Materials Characterisation
3.4. Catalyst Performance Examinations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vosoughifar, A. Photodegradation of dye in waste water using CaWO4/NiO nanocomposites co-precipitation preparation and characterization. J. Mater. Sci. Mater. Electron. 2018, 29, 3194–3200. [Google Scholar] [CrossRef]
- Chanathaworn, J.; Bunyakan, C.; Wiyaratn, W.; Chungsiriporn, J. Photocatalytic decolurization of basic dye by TiO2 nanoparticle in photoreactor. Songklanakarin J. Sci. Technol. 2012, 34, 203–210. [Google Scholar]
- El-Shishtawy, R.M.; Al-Zahrani, F.A.M.; Afzal, S.M.; Razvi, M.A.N.; Al-Amshany, Z.M.; Bakry, A.H.; Asiri, A.M. Synthesis, linear and nonlinear optical properties of a new dimethine cyanine dye derived from phenothiazine. RSC Adv. 2016, 6, 91546–91556. [Google Scholar] [CrossRef]
- Gupta, V.K.; Mittal, A.; Gajbe, V.; Mittal, J. Adsorption of basic fuchsin using waste materials bottom ash and deoiled soya-as adsorbents. J. Colloid Interface Sci. 2008, 319, 30–39. [Google Scholar] [CrossRef]
- Hou, C.; Hu, B.; Zhu, J. Photocatalytic degradation of methylene blue over TiO2 pretreated with varying concentrations of NaOH. Catalysts 2018, 8, 575. [Google Scholar] [CrossRef] [Green Version]
- Sareen, D.; Garg, R.; Grover, N. A Study on removal of methylene blue dye from waste water by adsorption technique using fly ash briquette. IJERT 2014, 3, 610–613. [Google Scholar]
- Kumar, R.; El-Shishtawy, R.M.; Barakat, M.A. Synthesis and characterization of Ag-Ag2O/TiO2@ polypyrrole heterojunction for enhanced photocatalytic degradation of methylene blue. Catalysts 2016, 6, 76. [Google Scholar] [CrossRef]
- Rouhi, M.; Babamoradi, M.; Hajizadeh, Z.; Maleki, A.; Maleki, S.T. Design and performance of polypyrrole/halloysite nanotubes/Fe3O4/Ag/Co nanocomposite for photocatalytic degradation of methylene blue under visible light irradiation. Optik 2020, 212, 164721. [Google Scholar] [CrossRef]
- Pan, X.; Chena, X.; Yi, Z. Photocatalytic oxidation of methane over SrCO3 decorated SrTiO3 nanocatalysts via a synergistic effect. Phys. Chem. Chem. Phys. 2016, 18, 31400–31409. [Google Scholar] [CrossRef]
- Gharaei, S.K.; Abbasnejad, M.; Maezono, R. Bandgap reduction of photocatalytic TiO2 nanotube by Cu doping. Sci. Rep. 2018, 8, 14192. [Google Scholar] [CrossRef] [Green Version]
- Theerthagiri, J.; Salla, S.; Senthil, R.A.; Nithyadharseni, P.; Madankumar, A.; Arunachalam, P.; Maiyalagan, T.; Kim, H.S. A review on ZnO nanostructured materials: Energy, environmental and biological applications. Nanotechnology 2019, 30, 392001. [Google Scholar] [PubMed]
- Thermal Energy Radiation Spectrum. Available online: http://agron-www.agron.iastate.edu/courses/Agron541/classes/541/lesson09a/9a.3.html (assessed on 1 September 2020).
- Karaahmet, O.; Cicek, B. Waste recycling of cathode ray tube glass through industrial production of transparent ceramic frits. J. Air Waste Manag. 2019, 69, 1258–1266. [Google Scholar] [CrossRef] [PubMed]
- Alimohammadi, E.; Sheibani, S.; Ataie, A. Preparation of nano-structured strontium carbonate from Dasht-e kavir celestite ore via mechanochemical method. J. Ultrafine Grained Nanostruct. Mater. 2018, 151, 147–152. [Google Scholar]
- Owusu, G.; Litz, J.E. Water leaching of SrS and precipitation of SrCO3 using carbon dioxide as precipitating agent. Hydrometallurgy 2000, 57, 23–29. [Google Scholar] [CrossRef]
- Lu, P.; Hu, X.; Li, Y.; Zhang, M.; Liu, X.; He, Y.; Dong, F.; Fu, D.; Zhang, Z. One-step preparation of a novel SrCO3/g-C3N4 nanocomposite and its application in selective adsorption of crystal violet. RSC Adv. 2018, 8, 6315–6325. [Google Scholar] [CrossRef] [Green Version]
- Divya, A.; Mathavan, T.; Harish, S.; Archana, J.; Milton Franklin Benial, A.; Hayakawa, Y.; Navaneetha, M. Synthesis and characterization of branchlet-like SrCO3 nanorods using triethylamine as a capping agent by wet chemical method. Appl. Surf. Sci. 2019, 487, 1271–1278. [Google Scholar] [CrossRef]
- Suchanek, W.L.; Riman, R.E. Hydrothermal synthesis of advanced ceramic powders. ASTRJ 2006, 45, 184–193. [Google Scholar]
- Khan, S.A.; Shahid, S.; Kanwai, S.; Rizwan, K.; Mahmood, T.; Ayub, K. Synthesis of novel metal complexes of 2-((phenyl(2-(4-sulfophenyl)hydrazono)methyl)diazinyl)benzoic acid formazan dyes: Characterization, antimicrobial and optical properties studies on leather. J. Mol. Struct. 2018, 1175, 73–89. [Google Scholar] [CrossRef]
- Baybars Ali Fil, C.Ö.; Korkmaz, M. Cationic dye (methylene blue) removal from aqueous solution by montmorillonite. Bull. Korean Chem. Soc. 2012, 33, 3184–3190. [Google Scholar]
- Song, L.; Zhang, S.; Chen, B. A novel visible-light-sensitive strontium carbonate photocatalyst with high photocatalytic activity. Catal. Commun. 2009, 10, 1565–1568. [Google Scholar] [CrossRef]
- Moldovan, A.; Neag, E.; Băbălău-Fussa, V.; Cadar, O.; Micle, V.; Roman, C. Optimized removal of methylene blue from aqueous solution using a commercial natural activated plant-based carbon and Taguchi experimental design. Anal. Lett. 2018, 52, 1–13. [Google Scholar] [CrossRef]
- Suqin, L.; Li, W.; Gaopeng, D.; Qiufei, H. Fabrication of Ag2CO3/SrCO3 rods with highly efficient visible-light photocatalytic activity. Rare Metal Mat. Eng. 2017, 46, 0312–0316. [Google Scholar] [CrossRef]
- Jin, J.; Chen, S.; Wang, J.; Chen, C.; Peng, T. SrCO3-modified brookite/anatase TiO2 heterophase junctions with enhanced activity and selectivity of CO2 photoreduction to CH4. Appl. Surf. Sci. 2019, 476, 937–947. [Google Scholar] [CrossRef]
- Jin, S.; Dong, G.; Luo, J.; Ma, F.; Wang, C. Improved photocatalytic NO removal activity of SrTiO3 by using SrCO3 as a new co-catalyst. Appl. Catal. B-Environ. 2018, 227, 24–34. [Google Scholar] [CrossRef]
- Castillejos, A.H.E.; De la Cruz Del, F.P.B.; Uribe, A.S. The direct conversion of celestite to strontium carbonate in sodium carbonate aqueous media. Hydrometallurgy 1996, 40, 207–222. [Google Scholar] [CrossRef]
- Zoraga, M.; Kahruman, C. Kinetics of conversion of celestite to strontium carbonate in solutions containing carbonate, bicarbonate and ammonium ions and dissolved ammonia. J. Serb. Chem. Soc. 2014, 79, 345–359. [Google Scholar] [CrossRef] [Green Version]
- Zou, X.; Wang, Y.; Liang, S.; Duan, D. Facile synthesis of ultrafine and high purity spherical strontium carbonate via gas-liquid reaction. Mater. Res. Express 2020, 7, 025009. [Google Scholar] [CrossRef]
- Yang, L.; Chu, D.; Wang, L.; Ge, G.; Sun, H. Facile synthesis of porous flower-like SrCO3 nanostructures by integrating bottom-up and top-down routes. Mater. Lett. 2016, 167, 4–8. [Google Scholar] [CrossRef]
- Wang, Z.; He, G.; Yin, H.; Bai, W.; Ding, D. Evolution of controllable urchin-like SrCO3 with enhanced electrochemical performance via an alternative processing. Appl. Surf. Sci. 2017, 411, 197–204. [Google Scholar] [CrossRef]
- Chen, L.; Jiang, J.; Bao, Z.; Pan, J.; Xu, W.; Zhou, L.; Wu, Z.; Chen, X. Synthesis of barium and strontium carbonate crystals with unusual morphologies using and organic additive. Russ. J. Phys. Chem. 2013, 87, 2239–2245. [Google Scholar]
- Ni, S.; Yang, X.; Li, T. Hydrothermal synthesis and photoluminescence properties of SrCO3. Mater. Lett. 2011, 65, 766–768. [Google Scholar]
- Cao, M.; Wu, X.; He, X.; Hu, C. Microemulsion-mediated solvothermal synthesis of SrCO3 nanostructures. Langmuir 2015, 21, 6093–6096. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, J.; Zhang, H.; Yin, X.; Yang, D.; Qian, J.; Liu, L.; Liu, X. Chemical synthesis of SrCO3 microcrystals via a homogeneous precipitation method. Micro Nano Lett. 2011, 6, 119–121. [Google Scholar]
- Li, L.; Lin, R.; Tong, Z.; Feng, Q. Crystallization control of SrCO3 nanostructure in imidazolium-based temperature ionic liquids. Mater. Res. Bull. 2012, 47, 3100–3106. [Google Scholar] [CrossRef]
- Wang, G.; Wang, L. Different morphologies of strontium carbonate in water/ethylene glycol and their photocatalytic activity. Fuller. Nanotub. Carbon Nanostructures 2019, 27, 46–51. [Google Scholar]
- Alavi, M.A.; Morsali, A. Syntheses and characterization of Sr(OH)2 and SrCO3 nanostructures by ultrasonic method. Ultrason. Sonochem. 2010, 17, 132–138. [Google Scholar]
- Song, L.; Li, Y.; He, P.; Zhang, S.; Wu, X.; Fang, S.; Shan, J.; Sun, D. Synthesis and sonocatalytic property of rod-shape Sr(OH)28H2O. Ultrason. Sonochemistry 2014, 21, 1318–1324. [Google Scholar]
- Li, S.; Zhang, H.; Xu, J.; Yang, D. Hydrothermal synthesis of f lower-like SrCO3 nanostructures. Mater. Lett. 2005, 59, 420–422. [Google Scholar] [CrossRef]
- Neville, G.A.; Becksteadlf, H.D.; Cooney, J.D. Thermal analyses (TGA and DSC) of some spironolactone solvates. Fresenius J. Anal. Chem. 1994, 349, 746–750. [Google Scholar]
- Stranic, I.; Pang, G.A.; Hanson, R.K.; Golden, D.M.; Bowman, C.T. Shock tube measurements of the tert-Butanol +OH reaction rate and the tert-C4H8OH radical β- scission branching ratio using isotopic labelling. J. Phys. Chem. A 2013, 117, 4777–4784. [Google Scholar]
- Mehrvar, M.; Anderson, W.A.; Young, M.M. Photocatalytic degradation of aqueous organic solvents in the presence of hydroxyl radical scavengers. Int. J. Photoenergy 2001, 3, 187–191. [Google Scholar] [CrossRef]
- Patterson, D.A.; Metcalfe, I.S.; Livingston, F.; Livingston, A.G. Wet air oxidation of linear alkylbenzene sulfonate 2 effect of pH. Ind. Eng. Chem. Res. 2001, 40, 5517–5525. [Google Scholar] [CrossRef]
- Houas, A.; Lachheb, H.; Ksibi, M.; Elaloui, E.; Guillard, C.; Herrmann, J.M. Photocatalytic degradation pathway of methylene blue in water. Appl. Catal. B Environ. 2001, 31, 145–157. [Google Scholar] [CrossRef]
- Rauf, M.A.; Meetani, M.A.; Khaleel, A.; Ahmed, A. Photocatalytic degradation of Methylene Blue using a mixed catalyst and product analysis by LC/MS. Chem. Eng. J. 2010, 157, 373–378. [Google Scholar] [CrossRef]
- Technical Application Guide for PHILIPS LED Lamps. Available online: http://yunyangsh.com/pdf_files/PHILIPS/LED/LED_dengpao/MASTER%20MR16%20LED%2010-50W.pdf (assessed on 31 August 2010).











| Conditions | Reaction Time | Morphology | Band Gap | References |
|---|---|---|---|---|
| Celetine ore (SrSO4) (industrial scale) | Reductive calcination followed by Na2CO3(aq) assisted precipitation (the black ash method) | - | - | [26] |
| Celetine ore (SrSO4) (industrial scale) | Double decomposition in Na2CO3(aq) | - | - | [27] |
| Sr(OH)2 + CO2 + EDTA | 50 °C, 10 min | spherical shape | - | [28] |
| Sr(NO3)2 + TEA + NaOH | 100 °C, 12 h | branchlet-like SrCO3 nanorods | - | [17] |
| SrCl2 + H2O + DMF + glycerol | 120 °C, 8 h | flower shape | - | [29] |
| Sr(NO3)2 + urea | 120 °C, 24 h | urchin-like SrCO3 | - | [30] |
| Sr(NO3)2 + (NH4)2CO3 + HMT | Room temp, 7 days | branch-like, flower-like, capsicum-like | - | [31] |
| CH3COO2Sr + Na2SO4 + hexamethylene triamine | 160 °C, 24 h | spherical shape | 3.17 | [32] |
| Sr(NO3)2 + Na2CO3 | 120 °C, 8 h | various shapes such as rod shape ellipsoid shape sphere shape | - | [33] |
| 1. SrCl2 + Na2CO3 2. SrCl2 + CO(NH2)2 3. SrCl2 + CO(NH2)2 + SDS | 1. 110 °C, 12 h 2. 110 °C, 12 h 3. 110 °C, 12 h | rod shape rod shape flower shape | - | [34] |
| Sr(OH)2 + flowing CO2 gas | 1. 50 °C, 12 h 2. 60 °C, 12 h 3. 70 °C, 12 h | nanowhisker rod shape pherical shape | - | [35] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wichannananon, P.; Kobkeatthawin, T.; Smith, S.M. Visible Light Responsive Strontium Carbonate Catalyst Derived from Solvothermal Synthesis. Catalysts 2020, 10, 1069. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10091069
Wichannananon P, Kobkeatthawin T, Smith SM. Visible Light Responsive Strontium Carbonate Catalyst Derived from Solvothermal Synthesis. Catalysts. 2020; 10(9):1069. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10091069
Chicago/Turabian StyleWichannananon, Pornnaphat, Thawanrat Kobkeatthawin, and Siwaporn Meejoo Smith. 2020. "Visible Light Responsive Strontium Carbonate Catalyst Derived from Solvothermal Synthesis" Catalysts 10, no. 9: 1069. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10091069