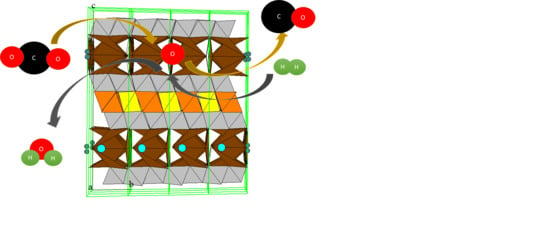
Reverse Water Gas Shift by Chemical Looping with Iron-Substituted Hexaaluminate Catalysts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structure, Texture, and H2-Reduction Behavior of Fe-Substituted Ba-Hexaaluminates
2.2. Factors Affecting the Performance of Fe-Substituted Ba-Hexaaluminates
3. Materials and Methods
3.1. Catalysts Preparation
3.2. Catalysts Characterization
3.3. Catalysts Testing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhu, M.; Ge, Q.; Zhu, X. Catalytic Reduction of CO2 to CO via Reverse Water Gas Shift Reaction: Recent advances in the Design of Active and Selective Supported Metal Catalysts. Trans. Tianjin Univ. 2020, 26, 172–187. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, D.U.; Hu, X.-M.; Daasbjerg, K.; Skrydstrup, T. Chemically and electrochemically catalyzed conversion of CO2 to CO with follow-up utilization to value-added chemicals. Nat. Catal. 2018, 1, 244–254. [Google Scholar] [CrossRef]
- Daza, Y.A.; Kuhn, J.N. CO2 conversion by reverse water gas shift catalysis: Comparison of catalysts, mechanisms and their consequences for CO2 conversion to liquid fuel. RSC Adv. 2016, 6, 49675–49691. [Google Scholar] [CrossRef]
- Saeidi, S.; Najari, S.; Fazlollahi, F.; Nikoo, M.K.; Sefidkon, F.; Klemeš, J.J.; Baxter, L.L. Mechanisms and kinetics of CO2 hydrogenation to value-added products: A detailed review on current status and future trends. Renew. Sust. Energy Rev. 2017, 80, 1292–1311. [Google Scholar] [CrossRef]
- Kaiser, P.; Unde, R.B.; Kern, C.; Jess, A. Production of Liquid Hydrocarbons with CO2 as Carbon Source based on Reverse Water-Gas Shift and Fischer-Tropsch Synthesis. Chem. Ing. Tech. 2013, 85, 489–499. [Google Scholar] [CrossRef]
- Vázquez, F.V.; Pfeifer, P.; Lehtonen, J.; Piermartini, P.; Simell, P.; Alopaeus, V. Catalyst Screening and Kinetic Modeling for CO Production by High Pressure and Temperature Reverse Water Gas Shift for Fischer-Tropsch Applications. Ind. Eng. Chem. Res. 2017, 56, 13262–13272. [Google Scholar] [CrossRef]
- Lyngfelt, A. Oxygen Carriers for Chemical Looping Combustion—4000 h of Operational Experience. Oil Gas Sci. Technol. 2011, 66, 161–172. [Google Scholar] [CrossRef] [Green Version]
- Bayham, S.; McGiveron, O.; Tong, A.; Chung, E.; Kathe, M.; Wang, D.W.; Zeng, L.; Fan, L.S. Parametric and dynamic studies of an iron-based 25-kWth coal direct chemical looping unit using subbituminous coal. Appl. Energy 2015, 145, 354–363. [Google Scholar] [CrossRef] [Green Version]
- Tong, A.; Bayham, S.; Kathe, M.V.; Zeng, L.; Luo, S.; Fan, L.-S. Iron-based syngas chemical Looping process and coal-direct chemical looping process development at Ohio State University. Appl. Energy 2014, 113, 1836–1845. [Google Scholar] [CrossRef]
- Galvita, V.; Hempel, T.; Lorenz, H.; Rihko-Struckmann, L.K.; Sundmacher, K. Deactivation of modified iron oxide materials in the cyclic water gas shift process for CO-free hydrogen production. Ind. Eng. Chem. Res. 2008, 47, 303–310. [Google Scholar] [CrossRef]
- Datta, P.; Rihko-Struckmann, L.K.; Sundmacher, K. Influence of molybdenum on the stability of iron oxide materials for hydrogen production with cyclic water gas shift process. Mater. Chem. Phys. 2011, 129, 1089–1095. [Google Scholar] [CrossRef]
- Galvita, V.V.; Poelman, H.; Bliznuk, V.; Detavernier, C.; Marin, G.B. CeO2-Modified Fe2O3 for CO2 Utilization via Chemical Looping. Ind. Eng. Chem. Res. 2013, 52, 8416–8426. [Google Scholar] [CrossRef]
- Meledina, M.; Turner, S.; Galvita, V.V.; Poelman, H.; Marin, G.B.; Van Tendeloo, G. Local environment of Fe dopants in nanoscale Fe: CeO2−x oxygen storage material. Nanoscale 2015, 7, 3196–3204. [Google Scholar] [CrossRef] [PubMed]
- Mattisson, T.; Lyngfelt, A.; Cho, P. The use of iron oxide as an oxygen carrier in chemical-looping combustion of methane with inherent separation of CO2. Fuel 2001, 80, 1953–1962. [Google Scholar] [CrossRef]
- Galvita, V.V.; Poelman, H.; Marin, G.B. Hydrogen Production from Methane and Carbon Dioxide by Catalyst-Assisted Chemical Looping. Top. Catal. 2011, 54, 907–913. [Google Scholar] [CrossRef]
- Corbella, B.M.; Palacios, J.M. Titania-supported iron oxide as oxygen carrier for chemical-looping combustion of methane. Fuel 2004, 86, 113–122. [Google Scholar] [CrossRef]
- Rihko-Struckmann, L.K.; Datta, P.; Wenzel, M.; Sundmacher, K.; Dharanipragada, N.V.R.A.; Poelman, H.; Galvita, V.V.; Marin, G.B. Hydrogen and Carbon Monoxide Production by Chemical Looping over Iron-Aluminum Oxides. Energy Technol. 2016, 4, 304–313. [Google Scholar] [CrossRef]
- Daza, Y.A.; Kent, R.A.; Yung, M.M.; Kuhn, J.N. Carbon Dioxide Conversion by Reverse Water−Gas Shift Chemical Looping on Perovskite-Type Oxides. Ind. Eng. Chem. Res. 2014, 53, 5828–5837. [Google Scholar] [CrossRef]
- Daza, Y.A.; Maiti, D.; Kent, R.A.; Bhethanabotla, V.R.; Kuhn, J.N. Isothermal reverse water gas shift chemical looping on La0.75Sr0.25Co(1−y)FeyO3 perovskite-type oxides. Catal. Today 2015, 258, 691–698. [Google Scholar]
- Hare, B.J.; Maiti, D.; Daza, Y.A.; Bhethanabotla, V.R.; Kuhn, J.N. Enhanced CO2 Conversion to CO by Silica-Supported Perovskite Oxides at Low Temperatures. ACS Catal. 2018, 8, 3021–3029. [Google Scholar] [CrossRef]
- Maiti, D.; Hare, B.J.; Daza, Y.A.; Ramos, A.E.; Kuhn, J.N.; Bhethanabotla, V.R. Earth abundant perovskite oxides for low temperature CO2 conversion. Energy Environ. Sci. 2018, 11, 648–659. [Google Scholar] [CrossRef]
- Ramos, A.E.; Maiti, D.; Daza, Y.A.; Kuhn, J.N.; Bhethanabotla, V.R. Co, Fe, and Mn in La-perovskite oxides for low temperature thermochemical CO2 conversion. Catal. Today 2019, 338, 52–59. [Google Scholar] [CrossRef]
- Hare, B.J.; Maiti, D.; Meier, A.J.; Bhethanabotla, V.R.; Kuhn, J.N. CO2 Conversion Performance of Perovskite Oxides Designed with Abundant Metals. Ind. Eng. Chem. Res. 2019, 58, 12551–12560. [Google Scholar] [CrossRef]
- Hare, B.J.; Maiti, D.; Ramani, S.; Ramos, A.E.; Bhethanabotla, V.R.; Kuhn, J.N. Thermochemical conversion of carbon dioxide by reverse water-gas shift chemical looping using supported perovskite oxides. Catal. Today 2019, 323, 225–232. [Google Scholar] [CrossRef]
- Hu, J.; Galvita, V.V.; Poelman, H.; Detavernier, C.; Marin, G.B. A core-shell structured Fe2O3/ZrO2@ZrO2 nanomaterial with enhanced redox activity and stability for CO2 conversion. J. CO2 Util. 2017, 17, 20–31. [Google Scholar] [CrossRef]
- Wenzel, M.; Dharanipragada, N.V.R.A.; Galvita, V.V.; Poelman, H.; Marin, G.B.; Rihko-Struckmann, L.; Sundmacher, K. CO production from CO2 via reverse-water–gas shift reaction performed in a chemical looping mode: Kinetics on modified iron oxide. J. CO2 Util. 2017, 17, 60–68. [Google Scholar] [CrossRef] [Green Version]
- Dharanipragada, N.V.R.A.; Meledina, M.; Galvita, V.V.; Poelman, H.; Turner, S.; Van Tendeloo, G.; Detavernier, C.; Marin, G.B. Deactivation Study of Fe2O3−CeO2 during Redox Cycles for CO Production from CO2. Ind. Eng. Chem. Res. 2016, 55, 5911–5922. [Google Scholar] [CrossRef]
- Hu, J.; Buelens, L.; Theofanidis, S.-A.; Galvita, V.V.; Poelman, H.; Marin, G.B. CO2 conversion to CO by auto-thermal catalyst-assisted chemical looping. J. CO2 Util. 2016, 16, 8–16. [Google Scholar] [CrossRef]
- Galvita, V.V.; Poelman, H.; Detavernier, C.; Marin, G.B. Catalyst-assisted chemical looping for CO2 conversion to CO. Appl. Catal. B Environ. 2015, 164, 184–191. [Google Scholar] [CrossRef]
- Ma, L.; Qiu, Y.; Li, M.; Cui, D.; Zhang, S.; Zeng, D.; Xiao, R. Spinel-Structured Ternary Ferrites as Effective Agents for Chemical Looping CO2 Splitting. Ind. Eng. Chem. Res. 2020, 59, 6924–6930. [Google Scholar] [CrossRef]
- Kim, S.; Lee, D.; Lee, J.Y.; Eom, H.; Lee, H.J.; Cho, I.; Lee, K. Catalytic combustion of methane in simulated PSA offgas over Mn-substituted La–Sr-hexaaluminate (LaxSr1−xMnAl11O19). J. Mol. Catal. A Chem. 2011, 335, 60–64. [Google Scholar] [CrossRef]
- Forzatti, P.; Groppi, G. Catalytic combustion for the production of energy. Catal. Today 1999, 54, 165–180. [Google Scholar] [CrossRef]
- Huang, F.; Wang, X.; Li, L.; Liu, X.; Xu, J.; Huang, C.; Zhang, T. Effect of magnesium substitution into Fe-based La-hexaaluminates on the activity for CH4 catalytic combustion. Catal. Sci. Tech. 2016, 6, 7860–7867. [Google Scholar] [CrossRef]
- Sidwell, R.W.; Zhu, H.; Kee, R.J.; Wickham, D.T. Catalytic combustion of premixed methane-in-air on a high-temperature hexaaluminate stagnation surface. Combust. Flame 2003, 134, 55–66. [Google Scholar] [CrossRef]
- Chu, W.; Yang, W.; Lin, L. Selective Oxidation of Methane to Syngas over NiO/Barium Hexaaluminate. Catal. Lett. 2001, 74, 139–144. [Google Scholar] [CrossRef]
- Chu, W.; Yang, W.; Lin, L. The partial oxidation of methane to syngas over the nickel-modified hexaaluminate catalysts BaNiyAl12−yO19−δ. Appl. Catal, A Gener. 2002, 235, 39–45. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, Z.; Cheng, T.; Zhou, G.; Wang, J.; Li, W.; Bi, Y.; Zhen, K. Studies on Carbon Deposition on Hexaaluminate LaNiAl11O19 Catalysts during CO2 Reforming of Methane. Kinet. Catal. 2002, 43, 522–527. [Google Scholar] [CrossRef]
- Ikkour, K.; Sellam, D.; Kiennemann, A.; Tezkratt, S.; Cherifi, O. Activity of Ni Substituted Ca-La-hexaaluminate Catalyst in Dry Reforming of Methane. Catal. Lett. 2009, 132, 213–217. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Lee, S.J.; Song, K.S. Performance of Ni catalyst supported on La-hexaaluminate in CO2 reforming of CH4. Korean J. Chem. Eng. 2007, 24, 477–480. [Google Scholar] [CrossRef]
- Gardner, T.H.; Spivey, J.J.; Kugler, E.L.; Campos, A.; Hissam, J.C.; Roy, A.D. Structural Characterization of Ni-Substituted Hexaaluminate Catalysts Using EXAFS, XANES, XPS, XRD, and TPR. J. Phys. Chem. C 2010, 114, 7888–7894. [Google Scholar] [CrossRef]
- Iyi, N.; Takekawa, S.; Kimura, S. Crystal Chemistry of Hexaaluminates: β-Alumina and Magnetoplumbite Structures. J. Solid State Chem. 1989, 83, 8–19. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Zhu, Y.; Liu, X.; Zhang, T. Thermal Evolution Crystal Structure and Fe Crystallographic Sites in LaFexAl12–xO19 Hexaaluminates. J. Phys. Chem. C 2014, 118, 10792–10804. [Google Scholar] [CrossRef]
- Santiago, M.; Pérez-Ramírez, J. Decomposition of N2O over Hexaaluminate Catalysts. Environ. Sci. Technol. 2007, 41, 1704–1709. [Google Scholar] [CrossRef] [PubMed]
- Zwinkels, M.F.; Järås, S.G.; Menon, P.G.; Griffin, T.A. Catalytic Materials for High-Temperature Combustion. Catal. Rev. Sci. Eng. 1993, 35, 319–358. [Google Scholar] [CrossRef]
- Laassiri, S.; Duprez, D.; Royer, S.; Alamdari, H. Solvent free synthesis of nanocrystalline Hexaaluminate type mixed oxides with high specific surface areas for CO oxidation reaction. Catal. Sci. Technol. 2011, 1, 1124–1127. [Google Scholar] [CrossRef]
- Gao, J.; Jia, C.; Zhang, M.; Gu, F.; Xu, G.; Zhong, Z.; Su, F. Template preparation of high-surface-area barium hexaaluminate as nickel catalyst support for improved CO methanation. RSC Adv. 2013, 3, 18156–18163. [Google Scholar] [CrossRef]
- Santiago, M.; Groen, J.C.; Pérez-Ramírez, J. Carbon-templated hexaaluminates with enhanced surface area and catalytic performance. J. Catal. 2008, 257, 152–162. [Google Scholar] [CrossRef]
- Mleczko, L.; Duff, D.G.; Karpenko, A.; Kockrick, E.; Gepret, V.; Tulke, A.; Vichmann, D. Method for reducing carbon dioxide at high temperatures on mixed metal oxide catalysts in the form of hexaaluminates. PCT International Application WO 2013135656, 19 September 2013. [Google Scholar]
- Utsis, N.; Vidruk-Nehemya, R.; Landau, M.V.; Herskowitz, M. Novel bifunctional catalysts based on crystalline multi-oxide matrices containing iron ions for CO2 hydrogenation to liquid fuels and chemicals. Faraday Discuss. 2016, 188, 545–563. [Google Scholar] [CrossRef]
- Utsis, N.; Landau, M.V.; Erenburg, A.; Vidruk-Nehemya, R.; Herskowitz, M. Performance of Reverse Water Gas Shift on Coprecipitated and C-Templated BaFe-Hexaaluminate: The Effect of Fe Loading, Texture, and Promotion with K. ChemCatChem 2018, 10, 3795–3805. [Google Scholar] [CrossRef]
- Landau, M.V.; Meiri, N.; Utsis, N.; Vidruk-Nehemya, R.; Herskowitz, M. Conversion of CO2, CO, and H2 in CO2 Hydrogenation to Fungible Liquid Fuels on Fe-Based Catalysts. Ind. Eng. Chem. Res. 2017, 56, 13334–13355. [Google Scholar] [CrossRef]
- Groppi, G.; Bellotto, M.; Cristiam, C.; Forzatti, P.; Villa, P. Preparation and characterization of hexaaluminate-based materials for catalytic combustion. Appl. Catal. A General 1993, 104, 101–108. [Google Scholar] [CrossRef]
- Groppi, G.; Cristiani, C.; Forzatti, P. BaFeXAl(12−x)O19 system for high-temperature catalytic combustion: Physico-chemical characterization and catalytic activity. J. Catal. 1997, 168, 95–103. [Google Scholar] [CrossRef]
- Artizzu-Duart, P.; Millet, J.; Guilhaume, N.; Garbowski, E.; Primet, M. Catalytic combustion of methane on substituted barium hexaaluminates. Catal. Today 2000, 59, 163–177. [Google Scholar] [CrossRef]
- Sandiumenge, F.; Gali, S.; Rodriguez, J. X-ray profile analysis of cation substitution in SrAlxFe12−xO19 solid solution. Mater. Res. Bull. 1988, 23, 685–692. [Google Scholar] [CrossRef]
- Amoyal, M.; Vidruk-Nehemya, R.; Landau, M.V.; Herskowitz, M. Effect of potassium on the active phases of Fe catalysts for carbon dioxide conversion to liquid fuels through hydrogenation. J. Catal. 2017, 348, 29–39. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E.J. Adsorption of Gases in Multimolecular Layers. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar]










 —Fe1;
—Fe1;  —Fe2;
—Fe2;  —Fe3;
—Fe3;  —Fe4;
—Fe4;  —Fe5;
—Fe5;  —Ba.
—Ba.
 —Fe1;
—Fe1;  —Fe2;
—Fe2;  —Fe3;
—Fe3;  —Fe4;
—Fe4;  —Fe5;
—Fe5;  —Ba.
—Ba.





| Catalysts | Preparation Method | Chemical Formula | Surface Area, m2g−1 | Pore Volume, cm3g−1 | Average Pore Diameter, nm |
|---|---|---|---|---|---|
| BaFeHAl | CP | Ba0.9Al5.7Fe5.3O17.1 | 5 | 0.02 | 14 |
| BaFeHAl-20 | CT | Ba0.8Al5.1 Fe5.3O17.8 | 20 | 0.09 | 17 |
| BaFeHAl-30 | CP-HEM | Ba0.9Al5.7Fe5.3O17.1 | 30 | 0.14 | 19 |
| 6%K/BaFeHAl | CP | 6%K/Ba0.9Al5.7Fe5.3 O17.1 | 4 | 0.02 | 20 |
| 6%K/BaFeHAl-20 | CT | 6%K/Ba0.8 Al5.1 Fe5.3O17.8 | 13 | 0.05 | 17 |
| 6%K/BaFeHAl-30 | CP-HEM | 6%K/Ba0.9Al5.7Fe5.3O17.1 | 20 | 0.12 | 36 |
| BaFeHF | CP | Ba0.9Fe11.0O17.4 | 2 | 0.01 | 18 |
| BaFeHF-18 | CP-HEM | Ba0.9Fe11.0O17.4 | 18 | 0.07 | 16 |
| 6%K/BaFeHF | CP | 6%K/Ba0.9Fe11.0O17.4 | 2 | 0.01 | 20 |
| 6%K/BaFeHF-18 | CP-HEM | 6%K/Ba0.9Fe11.0O17.4 | 8 | 0.03 | 16 |
| Catalyst | Phase Composition, wt % of As-Prepared Catalysts after H2-Treatment at Temperature, °C | |||||
|---|---|---|---|---|---|---|
| 6%K/BaFeHAl-30 | 350 | 400 | 450 | 500 | 550 | 600 |
| BaFeHAl | 100 | 100 | 100 | 38 | 0 | 0 |
| BaFeHFr | - | - | - | 46 | 73 | 71 |
| BaCO3 | - | - | - | 8 | 9 | 12 |
| Fe3O4 | - | - | - | - | 7 | 6 |
| Al2O3 | - | - | - | 8 | 11 | 11 |
| 6%K/BaFeHF-18 | 350 | 400 | 500 | 500 | 550 | 600 |
| BaFeHF | 100 | 88 | 50 | 26 | 0 | 0 |
| Fe3O4 | - | 9 | 41 | 55 | 80 | 41 |
| FeO | - | 3 | 5 | 13 | 11 | 49 |
| BaCO3 | - | - | 4 | 6 | 9 | 6 |
| BaO | - | - | - | - | - | 4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Utsis, N.; Landau, M.V.; Erenburg, A.; Herskowitz, M. Reverse Water Gas Shift by Chemical Looping with Iron-Substituted Hexaaluminate Catalysts. Catalysts 2020, 10, 1082. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10091082
Utsis N, Landau MV, Erenburg A, Herskowitz M. Reverse Water Gas Shift by Chemical Looping with Iron-Substituted Hexaaluminate Catalysts. Catalysts. 2020; 10(9):1082. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10091082
Chicago/Turabian StyleUtsis, Natalie, Miron V. Landau, Alexander Erenburg, and Moti Herskowitz. 2020. "Reverse Water Gas Shift by Chemical Looping with Iron-Substituted Hexaaluminate Catalysts" Catalysts 10, no. 9: 1082. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10091082