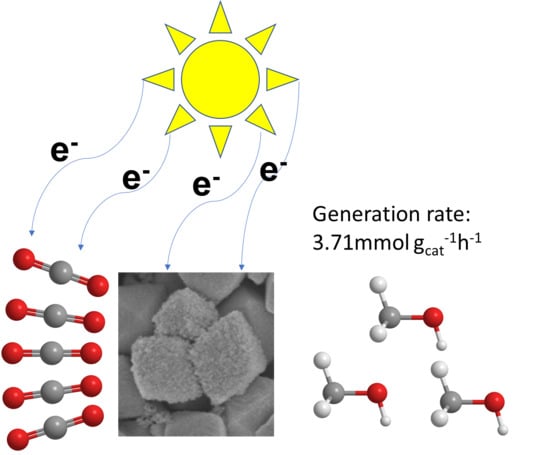
Porous Copper/Zinc Bimetallic Oxides Derived from MOFs for Efficient Photocatalytic Reduction of CO2 to Methanol
Abstract
:Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lashgari, M.; Soodi, S. CO2 conversion into methanol under ambient conditions using efficient nanocomposite photocatalyst/solar-energy materials in aqueous medium. RSC Adv. 2020, 10, 15072–15078. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Fang, S.; Xie, S.; Zheng, Y.; Hu, Y.H. Thermo-Photo catalytic CO2 hydrogenation over Ru/TiO2. J. Mater. Chem. A 2020, 8, 7390–7394. [Google Scholar] [CrossRef]
- Iqbal, F.; Mumtaz, A.; Shahabuddin, S.; Abd Mutalib, M.I.; Shaharun, M.S.; Trinh Duy, N.; Khan, M.R.; Abdullah, B. Photocatalytic reduction of CO2 to methanol over ZnFe2O4/TiO2 (p-n) heterojunctions under visible light irradiation. J. Chem. Technol. Biotechnol. 2020, 95, 2208–2221. [Google Scholar] [CrossRef]
- Liu, J.-Y.; Gong, X.-Q.; Li, R.; Shi, H.; Cronin, S.B.; Alexandrova, A.N. (Photo)Electrocatalytic CO2 Reduction at the Defective Anatase TiO2 (101) Surface. ACS Catal. 2020, 10, 4048–4058. [Google Scholar] [CrossRef]
- Tahir, M. Well-Designed ZnFe2O4/Ag/TiO2 nanorods heterojunction with Ag as electron mediator for photocatalytic CO2 reduction to fuels under UV/visible light. J. CO2 Util. 2020, 37, 134–146. [Google Scholar] [CrossRef]
- Gu, F.; Wang, Y.; Meng, Z.; Liu, W.; Qiu, L. A coupled photocatalytic/enzymatic system for sustainable conversion of CO2 to formate. Catal. Commun. 2020, 136, 105903. [Google Scholar] [CrossRef]
- Madhusudan, P.; Wageh, S.; Al-Ghamdi, A.A.; Zhang, J.; Cheng, B.; Yu, Y. Graphene-Zn0.5Cd0.5S nanocomposite with enhanced visible-light photocatalytic CO2 reduction activity. Appl. Surf. Sci. 2020, 506, 144683. [Google Scholar] [CrossRef]
- Sarkar, P.; Riyajuddin, S.; Das, A.; Chowdhury, A.H.; Ghosh, K.; Islam, S.M. Mesoporous covalent organic framework: An active photo-catalyst for formic acid synthesis through carbon dioxide reduction under visible light. Mol. Catal. 2020, 484, 110730. [Google Scholar] [CrossRef]
- Manzoor, N.; Sadiq, M.; Naqvi, M.; Sikandar, U.; Naqvi, S.R. Experimental Study of CO2 Conversion into Methanol by Synthesized Photocatalyst (ZnFe2O4/TiO2) Using Visible Light as an Energy Source. Catalysts 2020, 10, 163. [Google Scholar] [CrossRef] [Green Version]
- Moura Torquato, L.D.; Pastrian, F.A.C.; Lima Perini, J.A.; Irikura, K.; Batista, A.P.D.L.; de Oliveira-Filho, A.G.S.; Cordoba de Torresi, S.I.; Boldrin Zanoni, M.V. Relation between the nature of the surface facets and the reactivity of Cu2O nanostructures anchored on TiO2NT@PDA electrodes in the photoelectrocatalytic conversion of CO2 to methanol. Appl. Catal. B Environ. 2020, 261, 118221. [Google Scholar] [CrossRef]
- Zhang, L.; Cao, H.; Lu, Y.; Zhang, H.; Hou, G.; Tang, Y.; Zheng, G. High-Efficiency and sustainable photoelectric conversion of CO2 to methanol over CuxO/TNTs catalyst by pulse potential method. J. Solid State Electrochem. 2020, 24, 447–459. [Google Scholar] [CrossRef]
- Al-Rowaili, F.N.; Jamal, A.; Shammakh, M.S.B.; Rana, A. A Review on Recent Advances for Electrochemical Reduction of Carbon Dioxide to Methanol Using Metal-Organic Framework (MOF) and Non-MOF Catalysts: Challenges and Future Prospects. ACS Sustain. Chem. Eng. 2018, 6, 15895–15914. [Google Scholar] [CrossRef]
- Li, D.; Kassymova, M.; Cai, X.; Zang, S.-Q.; Jiang, H.-L. Photocatalytic CO2 reduction over metal-organic framework-based materials. Coord. Chem. Rev. 2020, 412, 213262. [Google Scholar] [CrossRef]
- Liu, C.; Wang, W.; Liu, B.; Qiao, J.; Lv, L.; Gao, X.; Zhang, X.; Xu, D.; Liu, W.; Liu, J.; et al. Recent Advances in MOF-based Nanocatalysts for Photo-Promoted CO2 Reduction Applications. Catalysts 2019, 9, 658. [Google Scholar] [CrossRef] [Green Version]
- Maina, J.W.; Schutz, J.A.; Grundy, L.; Ligneris, E.D.; Yi, Z.; Kong, L.; Pozo-Gonzalo, C.; Ionescu, M.; Dumee, L.F. Inorganic Nanoparticles/Metal Organic Framework Hybrid Membrane Reactors for Efficient Photocatalytic Conversion of CO2. ACS Appl. Mater. Interfaces 2017, 9, 35010–35017. [Google Scholar] [CrossRef]
- Mosier, A.M.; Larson, H.L.W.; Webster, E.R.; Ivos, M.; Tian, F.; Benz, L. Low-Temperature Adsorption and Diffusion of Methanol in ZIF-8 Nanoparticle Films. Langmuir 2016, 32, 2947–2954. [Google Scholar] [CrossRef]
- Muthukumaran, M.; Gnanamoorthy, G.; Prasath, P.V.; Abinaya, M.; Dhinagaran, G.; Sagadevan, S.; Mohammad, F.; Oh, W.C.; Venkatachalam, K. Enhanced photocatalytic activity of Cuprous Oxide nanoparticles for malachite green degradation under the visible light radiation. Mater. Res. Express 2020, 7, 015038. [Google Scholar] [CrossRef]
- Xia, H.; Zhang, Y.; Zhou, P.; Li, C.; Yang, D. Oxidative degradation of organic pollutants using cuprous oxide in acidic solution: Hydroxyl radical generation. Desalin. Water Treat. 2019, 171, 262–269. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Li, J.; Li, W.; Chen, D.; Qin, Q. Formation of Cu2O Solid Solution via High-Frequency Electromagnetic Field-Assisted Ball Milling: The Reaction Mechanism. Materials 2020, 13, 618. [Google Scholar] [CrossRef] [Green Version]
- Abdullah, M.I.; Eek, E. Automatic photocatalytic method for the determination of dissolved organic carbon (DOC) in natural waters. Water Res. 1996, 30, 1813–1822. [Google Scholar] [CrossRef]
- Julkapli, N.; Bagheri, S.; Abd Hamid, S.B. Recent Advances in Heterogeneous Photocatalytic Decolorization of Synthetic Dyes. Sci. World J. 2014, 2014, 692307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, M.; Ma, Y.; Zhang, H.; Ye, B.; Dong, X. Enhanced Photocatalytic activity of graphitic carbon nitride/cadmium sulfide heterojunctions by protonating treatment. J. Phys. Chem. Solids 2018, 116, 50–57. [Google Scholar] [CrossRef]
- Wang, G.; Sun, Q.; Liu, Y.; Huang, B.; Dai, Y.; Zhang, X.; Qin, X. A Bismuth-Based Metal-Organic Framework as an Efficient Visible-Light-Driven Photocatalyst. Chem. A Eur. J. 2015, 21, 2364–2367. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, H.; Zeng, Z.; Zeng, G.; Xu, P.; Xiao, R.; Huang, D.; Chen, X.; He, L.; Zhou, C.; et al. Metal-Organic frameworks derived Bi2O2CO3/porous carbon nitride: A nanosized Z-scheme systems with enhanced photocatalytic activity. Appl. Catal. B Environ. 2020, 267, 118700. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, Y.; Wang, Z.; Wang, P.; Zheng, Z.; Qin, X.; Zhang, X.; Dai, Y.; Whangbo, M.-H.; Huang, B. Selective photocatalytic conversion of alcohol to aldehydes by singlet oxygen over Bi-based metal-organic frameworks under UV-vis light irradiation. Appl. Catal. B Environ. 2019, 254, 463–470. [Google Scholar] [CrossRef]
- Celaya, C.A.; Delesma, C.; Valades-Pelayo, P.J.; Andres Jaramillo-Quintero, O.; Castillo-Araiza, C.O.; Ramos, L.; Sebastian, P.J.; Muniz, J. Exploring the potential of graphene oxide as a functional material to produce hydrocarbons via photocatalysis: Theory meets experiment. Fuel 2020, 271, 117616. [Google Scholar] [CrossRef]
- Song, H.; Meng, X.; Wang, S.; Zhou, W.; Wang, X.; Kako, T.; Ye, J. Direct and Selective Photocatalytic Oxidation of CH4 to Oxygenates with O-2 on Cocatalysts/ZnO at Room Temperature in Water. J. Am. Chem. Soc. 2019, 141, 20507–20515. [Google Scholar] [CrossRef]
- Taraka, T.P.Y.; Gautam, A.; Jain, S.L.; Bojja, S.; Pal, U. Controlled addition of Cu/Zn in hierarchical CuO/ZnO p-n heterojunction photocatalyst for high photoreduction of CO2 to MeOH. J. CO2 Util. 2019, 31, 207–214. [Google Scholar] [CrossRef]
- Tountas, A.A.; Peng, X.; Tavasoli, A.V.; Duchesne, P.N.; Dingle, T.L.; Dong, Y.; Hurtado, L.; Mohan, A.; Sun, W.; Ulmer, U.; et al. Towards Solar Methanol: Past, Present, and Future. Adv. Sci. 2019, 6, 1801903. [Google Scholar] [CrossRef] [Green Version]
- Tzompantzi-Flores, C.; Castillo-Rodriguez, J.C.; Gomez, R.; Perez Hernandez, R.; Santolalla-Vargas, C.E.; Tzompantzi, F. Photocatalytic Evaluation of the ZrO2:Zn5(OH)6(CO3)2 Composite for the H2 Production via Water Splitting. Top. Catal. 2020, 63, 575–585. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, W.; Qian, B.; Wang, Y.; Wang, B.; Li, S.; Liu, D.; Feng, D.; Ma, T.; Song, X.-M. Room-temperature photocatalytic methanol fuel cell based on one-dimension semiconductor photoanode: Intrinsic mechanism of photogenerated charge separation. Electrochim. Acta 2019, 318, 413–421. [Google Scholar] [CrossRef]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Jiao, X.; Chen, D.; Li, C.; Zhang, M. Porous Copper/Zinc Bimetallic Oxides Derived from MOFs for Efficient Photocatalytic Reduction of CO2 to Methanol. Catalysts 2020, 10, 1127. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10101127
Wang Z, Jiao X, Chen D, Li C, Zhang M. Porous Copper/Zinc Bimetallic Oxides Derived from MOFs for Efficient Photocatalytic Reduction of CO2 to Methanol. Catalysts. 2020; 10(10):1127. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10101127
Chicago/Turabian StyleWang, Zhenyu, Xiuling Jiao, Dairong Chen, Cheng Li, and Minghui Zhang. 2020. "Porous Copper/Zinc Bimetallic Oxides Derived from MOFs for Efficient Photocatalytic Reduction of CO2 to Methanol" Catalysts 10, no. 10: 1127. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10101127