1. Introduction
The term biomass refers to plants that synthesize organic matter by exploiting solar energy and biological organisms, such as animals and microorganisms, which use plants as food. Biomass is abundantly present in nature, and, because of its eco-friendly properties, it is increasingly attracting the attention of researchers as a promising sustainable energy source [
1,
2,
3,
4,
5,
6,
7,
8,
9]. In particular, carbohydrates derived from biomass are the focus of considerable research interest as a resource that can be converted into valuable compounds [
10,
11,
12,
13]. The most abundant and inexpensive of carbohydrates, glucose, can be obtained by acid-saccharification or enzymatic saccharification of starchy and cellulosic. Glucose can in turn be converted into the expensive carbohydrate fructose through an isomerization assisted by a base catalyst. Fructose is easily switchable not only with dietary sugar for food applications, but with useful substances such as 5-hydroxymethylfurfural (HMF) and levulinic acid [
14]. Therefore, a reaction for producing fructose from glucose has been in the limelight, and studies on this transformation have been actively promoted [
15,
16,
17,
18,
19,
20].
The isomerization of glucose to fructose is known to be possible using biological enzymes or chemically inorganic catalysts [
21,
22]. In practice, catalyzing the mentioned isomerization with enzymes requires high-purity glucose as a reactant, and it has a disadvantage that its process operating temperature needs to remain in a narrow window [
14]. However, glucose isomerization effected via chemical means using an inorganic catalyst has multiple advantages: its operating temperature is not limited in comparison with the corresponding enzymatic process and has a long life. Indeed, base catalysts such as hydrotalcite, NaOH, and KOH are well known to be effective catalysts of the chemical isomerization of glucose to produce fructose. For this reason, studies on glucose isomerization using the base catalysts have actively been conducted [
23,
24,
25,
26]. Therefore, in this study, we examined the use of base catalysts for glucose isomerization.
Glucose isomerization over base catalysts has been reported to generally follow the mechanism (
Figure 1). Initially, glucose ring-opening takes place, so that the substrate assumes an open-chain structure [
27]. Afterwards, glucose isomerization occurs, whereby fructose is generated via the Lobry de Bruyn-Alberda van Eksenstein (LdB-AvE) mechanism [
28,
29]. According to this mechanism, C2 protons are first extracted from glucose by a base catalyst to produce an intermediate. Subsequently, a proton is transferred from O2 to O1, and fructose is produced due to the protonation of C1. In some studies, the ring-opening of glucose has been influenced by the solvent [
30,
31]. In addition, the basic properties of the catalyst used in the reaction have been reported to play an important role in the glucose isomerization [
32,
33]. Therefore, many studies have been conducted on the effect of the basic properties of the catalyst on glucose isomerization; a number of reports have also been published on the effects of individual properties of the solvent on the said reaction [
31,
34,
35]. In fact, most of the published studies have focused either on the effect of the solvent or on those of the catalyst on the reaction whereby fructose is obtained from glucose isomerization. However, the proposed overall mechanism of glucose isomerization suggests that the effect of the solvent on glucose ring-opening and the correlation between the basic properties of the catalyst by the solvent for the isomerization should be evaluated concomitantly. Therefore, we suggested the validity of investigating the interaction between the solvent and the catalyst, and not simply the individual characteristics of the solvent and the catalyst, as an approach to increasing the efficiency of glucose isomerization.
Hydrotalcite (HT) has been widely studied as catalyst of glucose isomerization, and we selected it as a model catalyst in the present study. HT is characterized by a layered structure, and it has been widely used as an efficient catalyst for various base catalytic reactions [
36,
37,
38,
39]. In general, HT is known to have a chemical composition of [
][
] [
40,
41,
42]. Mg–Al HT, whereby the M
2+ and M
3+ sites are filled by Mg and Al cations, respectively, is a representative HT. The basic properties of Mg–Al HT can be controlled through various methods [
43,
44]. In particular, an easy way to control such properties is to tune the atomic Mg/Al atomic ratio in Mg–Al HT, and many studies have been published in which the Mg/Al ratio was made to vary [
44,
45].
In this study, we prepared four Mg–Al HT catalysts characterized by different Mg/Al atomic ratios. We then used these species to catalyze glucose isomerization, which was conducted in different reaction solvents (two protic solvents and two aprotic solvents). We observed that catalysts with different Mg/Al atomic ratios displayed different basicity and that they exhibited different catalytic activities in the four solvents. Based on our results, we would like to propose the validity of considering the effect of the interaction between the solvent and catalyst in the preparation of efficient Mg–Al HT catalysts for the isomerization of glucose to fructose.
2. Results and Discussion
2.1. Formation of HTX Catalysts (X = 1.5, 2, 3, and 4)
We performed XRD measurements to confirm the structural properties of Mg–Al HT catalysts prepared with different Mg/Al atomic ratios. As can be evinced from
Figure 2, the HTX catalysts included peaks corresponding to the (003), (006), (009), and (110) planes (JCPDS #14-0191) [
46]. These characteristic peaks indicated that the prepared HTX catalysts maintained a well-developed layered double hydroxide structure. In addition, data confirmed that the XRD peaks for the (003) and (110) planes gradually widened as the atomic ratio of Mg/Al decreased, indicating that smaller crystallite size had formed. Therefore, XRD analysis results indicated that Mg–Al HT catalysts characterized by different Mg/Al atomic ratios had been successfully prepared. Notably, furthermore, according to literature, the basic properties of the catalyst are expected to depend on the crystallite sizes [
24,
38,
44].
Table 1 showed the measured values for the crystallite size, BET surface area, and Mg/Al atomic ratio of the HTX catalysts. Crystallite sizes were calculated via the Scherrer’s equation and using the XRD peaks of the samples for the (003) and (110) planes. As expected based on the XRD measurement results, the crystallite size values of the prepared HTX catalysts displayed a tendency to decrease as the Mg/Al atomic ratio decreased. As previously reported, the decrease in crystallite size as the Mg/Al atomic ratio decreases could be explained by Al centers interrupting the growth of a brucite-like layer structure; please note that brucite is a mineral form of Mg(OH)
2. In other words, HTX catalysts characterized by a low Mg/Al atomic ratio included a relatively high amount of Al
3+. As a result, Al
3+ ions filled sites that would have otherwise been occupied by Mg
2+ ions, which limited the formation of the layered structure, resulting in the observed decrease in crystallite size. The tendency of the crystallite size to decrease as the Mg/Al atomic ratio decreased was also consistent with the observation of a gradual increase in the BET surface area of the samples.
We also performed ICP-AES measurements to confirm the successful formation of HTX catalysts, and the relevant results are shown in
Table 1. As can be evinced from this, the observed Mg/Al atomic ratios of the prepared samples were slightly higher or similar to the target values. However, it was judged to be somewhat consistent, because all Al species were incompletely integrated into a layered structure. Evidence thus strongly supported the successful formation of HTX (X = 1.5, 2, 3, and 4) catalysts characterized by different Mg/Al atomic ratios. Notably, the basic properties of the catalysts were expected to be controllable through this change in composition [
44].
FTIR and FE-SEM analyses were also performed to further confirm the successful formation of HTX catalysts.
Figure 3 showed the FTIR spectra of all the catalyst samples. All spectra included the typical peaks of Mg–Al HT, at 3440–3482, 1639–1650, and 1365–1380 cm
−1 [
44]. Among these peaks, the one at about 3450 cm
−1 shifted to lower wavenumber values as the Mg/Al atomic ratio decreased. This peak appeared to be due to a variety factors, such as interlayer water molecules, stretching vibrations of –OH in the hydroxide later, positively charged hydroxide layers, and hydrogen bonds between water molecules and anionic species [
46,
47,
48]. According to this, the peak shifted to lower wavenumber values as the Al content increased (and the Mg/Al atomic ratio decreased), because strong hydrogen bonds were formed [
46,
47,
48]. These results also corroborated the conclusion that the desired samples had been successfully prepared.
Result of FE-SEM analysis is presented in
Figure 4. As can be evinced from this figure, the HTX catalysts maintained their unique double-layer structure. These appeared to be limited to the growth of the layered structure, because Mg
2+ ions were replaced with Al
3+ ions due to the increase in the amount of Al
3+ cations as the Mg/Al atomic ratio decreased. These results were consistent with those of XRD, crystallite size, FT-IR, and BET surface area analyses.
2.2. Properties of Various Solvents Used for Glucose Isomerization
We used four polar solvents (water, MeOH, DMSO, and DMF) for the glucose isomerization. In particular, water and MeOH are polar protic solvents, whereas DMSO and DMF are polar aprotic solvents. Literature data indicated that the polarity, basicity, and acidity of the solvent affect the efficiency of glucose conversion to fructose [
17,
31,
49]. Polarity is the property that allows the solvent to stabilize charges or dipoles as a result of dielectric polarization. Basicity represents the ability of the solvent to accept protons, whereas acidity identifies the solvent’s ability to provide protons in solvent-to-solvent hydrogen bonding. The effect of solvent has been extensively studied for glucose isomerization [
17,
28]. In some studies, it has been reported that the role of solvent in the ring-opening is very important and that the barrier of the reaction is a little higher from acidic solution than basicity solution [
30]. Other studies have reported that aprotic solvents suppress the overall conversion of glucose, but significantly increase the selectivity of dehydration products during glucose degradation. On the other hand, protic solvents have been reported to have little effect on this decomposition [
31].
Table 2 listed the values for the polarity, basicity, and acidity of the four solvents used in this study [
50]. As can be evinced, water displayed the highest polarity and acidity among the four solvents. On the other hand, DMSO displayed the highest basicity. We expect that various properties of the solvent influence the basic properties of the catalyst, thus affecting the efficiency of glucose isomerization. Therefore, we performed the glucose isomerization in various solvents over the different prepared HTX catalysts.
2.3. Glucose Isomerization to Fructose Over HTX Catalysts (X = 1.5, 2, 3, and 4)
Glucose isomerization was performed to probe the activity of HTX catalyst in the different solvents. The said reaction was carried out for 5 h in a batch-type reactor at 100 °C.
Figure 5 presented values for the glucose conversion, fructose selectivity, and fructose yield of the various reaction with different solvents. The catalytic activities were calculated using the equations from
Section 3.4.
Results thus confirmed that the activity of each HTX catalyst were different depending on the solvents used in the reaction. When water was used as the solvent, HT4 afforded the highest fructose yield, whereas use of HT1.5 afforded the lowest yield. Additionally, the fructose yield and glucose conversion decreased as the Mg/Al atomic ratio of the catalyst decreased. By contrast, when MeOH, DMSO, and DMF were used as the solvents, HT1.5 afforded the highest fructose yield, whereas HT4 afforded the lowest fructose yield. Contrary to the results obtained in water, when MeOH, DMSO, and DMF were used as solvents in the glucose isomerization, fructose yield and glucose conversion increased as the Mg/Al atomic ratio of the catalyst decreased. As described above, each HTX catalyst displayed a different activity for glucose isomerization. As mentioned earlier, the described activity was expected to depend on the basic properties of the catalysts, which in turn are affected by the solvents used for the isomerization. In order to evaluate this assumption, we determined the base properties of the catalysts, implementing the Hammett indicator method; we compared the base strength exhibited by the HTX catalysts in each solvent.
2.4. The Basic Properties of HTX Catalysts (X = 1.5, 2, 3, and 4) in Various Solvents
Among the available methods for analyzing the basic properties of a catalyst, CO
2-TPD analysis allows to determine a catalyst base strength by adsorbing CO
2 gas on the catalyst and then comparing the temperature at which CO
2 is desorbed while increasing the temperature. However, since carbonate ions present in the intermediate layer of hydrotalcite are released during the described experiment, it is difficult to accurately determine the base strength of HTX catalysts via CO
2-TPD analysis [
43]. In addition, we decided that CO
2-TPD analysis was not suitable for this study, because we wanted to analyze the change in basic properties of the catalysts in various solvents. Therefore, we implemented the Hammett indicator method to qualitatively determine the base strengths that the various catalysts displayed in the different solvents.
The results of the Hammett indicator method performed on the various catalysts in the different solvents are presented in
Table 3 and
Supplementary Materials Figure S1. The data in columns 2–4 of
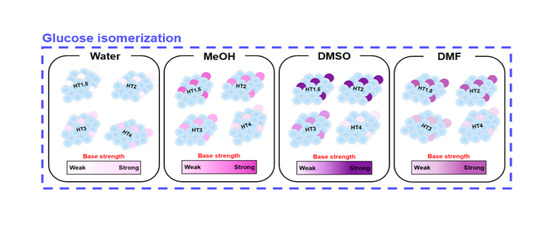
Table 3 provide information on whether the solution characterized by a particular solvent/indicator pair underwent a color change (“O”) or maintained its original color or colorless status (“X”) when a specific HTX catalyst was added to it. In particular, the solvents tested included water, MeOH, DMSO, and DMF, whereas the two indicators utilized were B.T.B and P.P, which have different values of the base strength, H_ = 7.3 and H_ = 9.5, respectively. Notably, the ‘H_’ parameter is indicative of the relative basic strength of the indicator: A high H_ value means that the indicator has a relatively high base strength. When using B.T.B, a change in color of the solution from yellow to green or blue means that the catalyst has a base strength of 7.3 or higher in the utilized solvent. Similarly, when P.P is used, a change in appearance of the solution from colorless to pink means that the catalyst has a base strength of 9.5 or more in the utilized solvent. Moreover, when using the same solvent/indicator pair, the darker the color of the solution following a color change, the higher the catalyst base strength.
Based on the results of the Hammett indicator method, it could be confirmed that the basic properties of HTX catalysts changed depending on the solvent used. First, in the case of the B.T.B/water of indicator/solvent pair, upon the addition of all four catalysts, the solution’s color changed from yellow to green or blue. However, only when HT3 and 4 were added to the solutions characterized by the P.P/water of indicator/solvent pair did the said solutions switch from colorless to pink. This observation indicates that HT3 and 4 had relatively high base strength in water. In addition, the solutions comprising the indicator B.T.B dissolved in MeOH, DMSO, or DMF also changed in color from yellow to green or blue as all four catalysts were added to them. However, in contrast to the results obtained using water as the solvent, the solutions containing indicator P.P dissolved in MeOH, DMSO, or DMF switched from colorless to pink only when catalysts HT1.5 and 2 were added to them. Notably, when HT3 and HT4 were added to these solutions, although the catalysts themselves turned pink, no clear change in the color of the solution was observed. This observation descends from the fact that more than 10% of the indicator should be adsorbed on the base site of the catalyst, but for HT3 and 4 catalysts in MeOH, DMSO, and DMF solvents, there were not enough bases for the indicator to adsorb [
51]. So, we determined that the change in color of the solutions containing HT3 and 4 solutions was not substantial enough to be observable. By contrast, HT1.5 and 2 displayed a relatively high base strength in MeOH, DMSO, and DMF.
These results indicated that the prepared HTX catalysts displayed different basic properties in different solvents. Therefore, evidence strongly supported our suggestion that it is not just the individual properties of the solvent and catalyst that should be considered when aiming to improve the efficiency of glucose isomerization to fructose, but also the interaction between solvent and catalyst. We also confirmed together the basic properties of the catalyst with the solvent and the activity of the glucose isomerization that were the result of the Hammett indicator method from the following.
2.5. Relationship between the Fructose Yield and the Catlayst Basic Properties in Various Solvents Used in the Glucose Isomerization
Figure 6 showed the fructose yields and the results of Hammett indicator method performed to investigate the glucose isomerization. The
x-axis of each graph presents the catalyst utilized, whereas the fructose is yield reported in the
y-axis. For the fructose yield, the average values were used after conducting the glucose isomerization three times. The standard deviation of the experimental values was shown by the red line. The color of the graph reflected the color of the solution when P.P was used in the Hammett indicator method. Additionally, the bar in the right hand side of
Figure 6 provided visual information on how the color of the solution reflected the relative base strength: The higher the position of the color in the bar, the higher the base strength of the catalyst.
As mentioned earlier, different HTX catalysts displayed significantly different activities in the glucose isomerization in the same solvent. Furthermore, the activity of a specific catalyst was strongly influenced by the identity of the solvent used for the reaction. In addition, the results of the Hammett indicator method confirmed that the base strength of the catalysts varied depending on the solvent. When water was used as the solvent for the glucose isomerization, HT1.5 displayed relatively weaker base strength than HT2, 3, and 4, and with HT1.5 as the catalyst, the value of fructose yield was the lowest. By contrast, HT4 catalyst displayed the strongest base strength and the highest fructose yield. On the other hand, HT1.5 displayed relatively strong base strength, and its use was associated with the highest fructose yield in MeOH, DMSO, and DMF. Notably, in the mentioned solvents, HT4 displayed relatively weak base strength and its use was associated with low fructose yield. All in all, evidence thus indicates that the fructose yield of the glucose isomerization increased with the base strength of the catalyst in the reaction solvent, and it decreased as the catalyst base strength decreased. Moreover, results strongly supported our suggestions that the basic properties of the catalyst were strongly influenced by the solvent utilized and that these basic properties could in turn affect the yield of the glucose isomerization.
In summary, when used in the same solvent, the prepared HTX catalysts displayed different base properties. In other words, the basic properties of Mg–Al HT catalysts could be controlled tuning their Mg/Al atomic ratio. In addition, the catalytic activities and the basic properties of the catalysts varied significantly in different solvents used for the glucose isomerization. Our results confirmed that the basic properties of the catalyst depend on the solvent and that changes in the basic properties of the catalyst affect the yield of fructose from glucose isomerization. Therefore, we suggested the validity of evaluating the relationship between the changes in the basic properties of the catalyst caused by the solvent and the solvent, rather than the properties of the catalyst and the solvent considered in isolation, as an approach to increasing the efficiency of the process leading to fructose production via glucose isomerization.
3. Materials and Methods
3.1. Catalyst Preparation
Mg–Al HT catalysts with Mg/Al atomic ratios ranging from 1.5 to 4 were prepared by a co-precipitation method. We used Mg(NO
3)
2∙6H
2O (Sigma-Aldrich, St. Louis, MO, USA) and Al(NO
3)
3∙9H
2O (Sigma-Aldrich) as metal precursors. To form a metal precursor solution, known amounts of these precursors were dissolved in 100 mL of distilled water [
44]. Moreover, in order to obtain a basic solution, known amounts of NaOH (Sigma-Aldrich) and Na
2CO
3 (Sigma-Aldrich) were dissolved in 75 mL of distilled water. This basic solution was used as a precipitator, with its pH acting as a precipitation control agent; notably, the Na
2CO
3 solution was also used as a source of interlayer anions. Thereafter, co-precipitation was performed by pooling together the mentioned two solutions under stirring while the pH was maintained at 9.5 in 100 mL of distillation at room temperature. The mixture was stirred for 18 h, and the precipitate obtained as a result was collected by filtration and washed with distilled water to remove Na
+ and NO
3− ions. The precipitate thus isolated was dried at 100 °C overnight. The samples were named HTX (X = 1.5, 2, 3, and 4), where X represents the Mg/Al atomic ratio in the sample.
3.2. Catalyst Characterization
To determine the crystalline structures of the prepared catalysts, X-ray diffraction (XRD) patterns were recorded using a X’pert-Pro PAN-analytical diffractometer with Cu-Kα radiation (λ = 1.54056 Å). Notably, the diffraction patterns were recorded within the 2θ range 10°–90°. FTIR spectra were obtained using a Jasco FTIR 460 spectrometer in the 4500–1000 cm−1 wavenumber range employing the KBr pellet technique. Field emission scanning electron microscope (FE-SEM) images were obtained using a JSM-6700FE-SEM instrument operating at 15 kV. Prior to the FE-SEM analysis, the samples were treated by conducting resin and coating with platinum. Inductively coupled plasma atomic emission spectroscopy (ICP-AES) analyses were conducted to confirm the Mg/Al atomic ratios in the samples using an OPTIMA 4300DV (Perkin-Elmer) instrument. In order to determine the specific surface areas of the catalyst samples via the Brunauer–Emmett–Teller (BET) method, N2 adsorption and desorption measurements were conducted using a TriStar II (Micromeritics Corp.) instrument.
3.3. Hammett Indicator Method
Several methods exist to analyze the basic properties of catalysts. Among them, CO2 temperature-programmed desorption (CO2-TPD) is widely used to analyze the basic properties of heterogeneous catalysts. However, in the case of the Mg–Al HT catalysts used in this study, it is difficult to obtain accurate data from CO2-TPD experiments, given the presence of carbonate ions in the catalyst’s intermediate layer. Therefore, we opted to qualitatively analyze the base properties of the HTX catalysts in various solvents employing the Hammett index titration method, which is widely utilized to macroscopically analyze the acid or base properties of catalysts relying on pH-dependent color changes of the solution.
In order to qualitatively analyze the strength of the basic sites of the samples, two Hammett indicators were utilized: bromothymol blue (B.T.B, H_ = 7.3) and phenolphthalein (P.P, H_ = 9.5). These indicators were added to the four solvents utilized in this study to obtain 1.0 × 10−5 M solutions. Notably, the four solvents used to carry out the glucose isomerization were water, methanol (MeOH), Dimethyl sulfoxide (DMSO), and N,N-dimethylformamide (DMF). In detail, 0.02 g of the catalyst were added to a 2 mL indicator/solvent pair, and the thus obtained solution was stirred for 2 h until equilibrium was reached. Thereafter, the color of the solution was observed to qualitatively infer the strength of the base site.
3.4. Isomerization of Glucose to Fructose and Product Analysis
In this study, isomerization of glucose to fructose was used as a model reaction, which was conducted in a batch-type reactor [
24,
38,
43,
44]. In a typical reaction, glucose (0.3 g, Sigma-Aldrich), catalyst (0.1 g), and solvent (10 mL) were introduced into the reactor, which was then sealed. The reaction was carried out in four solvents: water, MeOH (DUKSAN), DMSO (JUNSEI), and DMF (Sigma-Aldrich). The reactor was placed in a pre-heated oil bath at 100 °C for 5 h, during which the contents of the reactor were subjected to vigorous stirring. After the catalytic reaction, the reactor was immersed in cold water and allowed to cool to room temperature, a process that triggered the precipitation of the catalyst, which was subsequently removed by filtration using a syringe filter. The filtrate was analyzed using high performance liquid chromatography (HPLC) with a refractive index detector (YL9100, Biorad Aminex HPX87H column). The flow rate of the mobile phase (0.005 M H
2SO
4 solution) was fixed at 0.5 mL/min and the temperature of the column (Biorad Aminex HPX87H) was maintained at 50 °C. Glucose conversion, fructose selectivity, and fructose yield are calculated using the following equations.
4. Conclusions
In this study, we prepared a series of Mg–Al HT catalysts (HTX, X = 1.5, 2, 3, and 4) characterized by different Mg/Al atomic ratios (X) implementing the co-precipitation method; we then used the prepared species to catalyze the fructose-producing glucose isomerization. We utilized four different solvents with different properties (water, MeOH, DMSO, and DMF) to perform the said reaction in a batch-type reactor. It indicated that the catalytic activities of HTX catalysts were substantially affected by the reaction solvent utilized. In water, use of HT4 provided the highest fructose yield: by contrast, use of HT1.5 was associated with the highest fructose yields in the other three solvents. In fact, these results were expected based on the different basic properties of the Mg–Al HT catalysts in the different solvents. In other words, the properties of the reaction solvent strongly affected the base properties of HTX catalysts, which in turn affected their catalytic activity in the glucose isomerization. We went on to implement the Hammett indicator method to determine the basic properties of the catalysts in various solvents. We thus confirmed that basic properties of the HTX catalysts were dramatically different in different solvents. HT4 displayed the strongest base strength in water, whereas HT1.5 displayed the strongest relative base strength in the other three solvents. Ultimately, fructose yield increased alongside the base strength of the catalyst, which was heavily dependent on the chosen reaction solvent. It means that the basic properties of the HTX catalysts can vary depending on the solvent, and the changed basic properties eventually affect their catalytic activity in the glucose isomerization. Therefore, we came to the conclusion that the interaction between the solvent and the catalyst should be taken into careful consideration when designing efficient Mg–Al base catalysts of the glucose isomerization.